Abstract
Tuberous sclerosis complex is a dominant genetic disorder produced by mutations in either of two tumor suppressor genes, TSC1 and TSC2; it is characterized by hamartomatous tumors, and is associated with severe neurological and behavioral disturbances. Mutations in TSC1 or TSC2 deregulate a conserved growth control pathway that includes Ras homolog enriched in brain (Rheb) and Target of Rapamycin (TOR). To understand the function of this pathway in neural development, we have examined the contributions of multiple components of this pathway in both neuromuscular junction assembly and photoreceptor axon guidance in Drosophila. Expression of Rheb in the motoneuron, but not the muscle of the larval neuromuscular junction produced synaptic overgrowth and enhanced synaptic function, while reductions in Rheb function compromised synapse development. Synapse growth produced by Rheb is insensitive to rapamycin, an inhibitor of Tor complex 1, and requires wishful thinking, a bone morphogenetic protein receptor critical for functional synapse expansion. In the visual system, loss of Tsc1 in the developing retina disrupted axon guidance independently of cellular growth. Inhibiting Tor complex 1 with rapamycin or eliminating the Tor complex 1 effector, S6 kinase (S6k), did not rescue axon guidance abnormalities of Tsc1 mosaics, while reductions in Tor function suppressed those phenotypes. These findings show that Tsc-mediated control of axon guidance and synapse assembly occurs via growth-independent signaling mechanisms, and suggest that Tor complex 2, a regulator of actin organization, is critical in these aspects of neuronal development.
Introduction
Mutations in TSC1 or TSC2 result in tuberous sclerosis, a human syndrome characterized by formation of benign tumors, or hamartomas, and a range of neurological and behavioral anomalies, including epilepsy and autism. While neurological dysfunction in patients with tuberous sclerosis is clearly linked to structural brain abnormalities in the central nervous system [1], recent work has provided evidence that TSC1/2 may affect neural development by altering neuronal morphology and function. Loss of TSC function produces changes in dendritic architecture of hippocampal neurons and altered synaptic properties [2]. Rats heterozygous for TSC2 mutations show disruption of hippocampal physiology, including long term potentiation, a measure of synaptic plasticity [3]. Mutations in the Drosophila ortholog of TSC2, gigas, have also been shown to produce ectopic axon terminations in addition to the normal projections of sensory neurons [4], [5]. It is unclear to what degree neurological deficits associated with tuberous sclerosis complex result from disruptions of cytoarchitecture in specific brain regions or alterations in synaptic function directly.
TSC1 and TSC2 encoded proteins form a complex that regulates a small GTP-binding protein, Ras homolog enriched in brain (Rheb), promoting its endogenous GTPase activity and thereby limiting Rheb signaling. Rheb in turn controls the activity of Target of Rapamycin (TOR), a serine-threonine kinase. The TSC-Rheb-TOR pathway is a critical determinant of growth during development, regulating a number of cellular functions including translation, mRNA turnover, protein stability, and actin organization [6]. It is responsive to growth factors, such as insulin and insulin-like growth factors (ILGFs), and also serves as a nutrient sensor, thus integrating numerous signals related to cell and tissue growth. TOR plays a pivotal role in this signaling pathway, receiving regulatory inputs from Rheb and affecting downstream targets via two distinct molecular complexes. Tor complex 1 (TORC1) includes Raptor and mLST8, and regulates translation via phosphorylation of S6 kinase (S6K) and 4E-binding protein (4EBP). Tor complex 2 (TORC2) includes Rictor in addition to Tor and mLST8; in both yeast and mammalian cells TORC2 influences the actin cytoskeleton. Tor complex 1, but not Tor complex 2, is inhibited by the anti-proliferative and immunosuppressant compound rapamycin, emphasizing that TORC1 and 2 are pharmacologically separable entities. The distinct molecular outputs of TORC1 and 2 have also suggested that TORC2 may be the primary regulator of cell polarity and morphology. It is not known which functions of TSC-Rheb-TOR in the nervous system are mediated by either or both of the two Tor kinase-containing complexes, and if pharmacological intervention in tuberous sclerosis complex patients should best be directed at TORC1, with agents such as rapamycin, or if TORC2-specific agents will also be important.
The fruit fly Drosophila has proven to be an important system for understanding the molecular mechanisms of Tsc-Rheb-Tor signaling during development [7]. As in vertebrates, this signaling cascade is a critical regulator of growth. All of the principal elements of this pathway are represented in Drosophila, including molecular components upstream of Tsc, such as phosphatidyinositol-3 kinase (Pi3K), Akt, Pten and the insulin receptor ortholog, InR. Likewise, molecules that convey the signal downstream of Tsc, including Rheb, Tor, and S6k serve critical roles in the fruit fly. Mutations affecting all these genes have been identified in Drosophila, as well as transgenes that can convey gain-of-function effects. We have used these molecular and genetic tools to explore the function of Tsc-Rheb-Tor signaling in two fundamental processes essential to nervous system development, synapse formation and axon guidance.
The Drosophila neuromuscular junction has served as a powerful model for identifying the molecular components required for assembly and plasticity of a defined synapse [8]. This glutamatergic synapse must respond to greater than a 100-fold increase in the size of the muscle target from first to third instar larval stages. Physiological responses of this synapse are well-characterized using single-cell recording techniques, and morphological development with specific molecular markers has been extensively described. We have used this synapse to determine the role of gain or loss of Tsc-Rheb-Tor signaling on synapse assembly and function.
The visual system of Drosophila is equally well described in both molecular and genetic terms [9]. Photoreceptors show stereotyped projections to the brain, and genes required for photoreceptor axon projection and termination have been identified in numerous screens. Methods for making somatic cell mosaics have proven particularly powerful in determining what molecules are required in photoreceptors or in cells along their trajectory into the brain. Previous studies have shown that retinal clones mutant for the Drosophila Tsc2 ortholog gigas generated enlarged ommatidia with increased numbers of synaptic contact sites in the optic lamina [10]. We have taken advantage of this system to examine Tsc-Rheb-Tor requirements for photoreceptor axon guidance and formation of functional synaptic contacts in the brain.
Our results establish that either gain or loss of signaling via the Tsc-Rheb-Tor pathway affects synapse development at the Drosophila NMJ. Ectopic activation of the Tsc-Rheb-Tor signaling pathway produced profound synaptic overgrowth with commensurate increases in synaptic function. We show that Rheb-mediated enhancement of synaptic function depends upon bone morphogenetic protein (BMP) signaling mediated by wishful thinking (wit), a type II receptor. In the visual system, increased Tsc-Rheb-Tor signaling produced cell autonomous defects in photoreceptor axon guidance. Both genetic and pharmacological evidence suggest that TORC2 serves critical functions in both synapse development and axon guidance in Drosophila. Axon guidance phenotypes produced by null mutations in Pten and Tsc1 are distinct, demonstrating that regulation of signaling by these two tumor suppressor genes are not functionally equivalent in the nervous system.
Results
Activation of Tor signaling produces synaptic growth and enhanced synaptic function
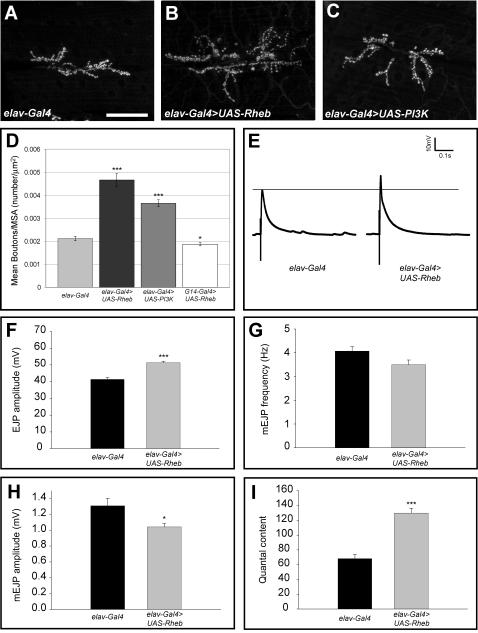
Tsc1/2 affect growth by inhibiting Rheb, a small GTP-binding protein that in turn governs Tor activity. Overexpression of Rheb activates the pathway independent of Tsc gene function [11]–[13]. We have used the Gal4-UAS system to overexpress Rheb in either the motoneuron or the muscle of the Drosophila third instar larval neuromuscular junction (NMJ), a well-characterized glutamatergic synapse [8] that shows dynamic growth during larval development. Ectopic expression of Rheb in the motoneuron of the third instar larval NMJ using a pan-neuronal Gal4 line (elav-Gal4) resulted in more than a doubling of synapse size, measured by the number of synaptic boutons/muscle area (Figure 1A–C, quantified in D). Similar results were seen using a motoneuron-specific OK6-Gal4 line (data not shown). We found no evidence of motoneuron axon misrouting at this level of Rheb activation; the motoneuron axon follows the normal trajectory and synapses at the correct location on muscle 6 and 7(data not shown). Indeed, elav-Gal4>UAS-Rheb animals are viable, indicating this degree of pathway activation is considerably more mild than loss of Tsc1 (see below). Expression of Rheb selectively in muscle (G14-Gal4>UAS-Rheb), while producing enlargement of muscle cells, did not increase the proportional size of the synapse (bouton number/muscle area, Figure 1D). Activation of Tor by overexpression of Pi3K in the motoneuron also produced an enlarged synapse, but to a lesser degree than overexpression of Rheb (Figure 1C, D).
Figure 1. Activation of the Tor pathway produces synaptic growth and enhanced physiological function.
The morphology of the third instar larval NMJ was visualized with the presynaptic marker anti-cysteine string protein (CSP) and confocal microscopy. Images shown are stacks of 20 or more optical sections. Neuronal (elav-Gal4) expression of either Rheb (B) or Pi3K (C) increased the size of the synapse compared to control animals bearing the elav-Gal4 transgene alone (A). Numbers of synaptic boutons/muscle area are quantified in D. Expression of either UAS-Rheb (n = 41) or UAS-Pi3K (n = 41) produced a significant increase in the number of boutons/muscle area compared to controls (n = 44), while expression of UAS-Rheb in the muscle (driven by G14-Gal4, n = 18) produced a reduction. Neuron-specific expression of Rheb also produced electrophysiological changes at the NMJ, determined by intracellular recordings from abdominal muscle 6 in third instar larvae. The amplitude of the EJP was significantly increased in animals expressing UAS-Rheb (n = 21) compared to controls with elav-Gal4 alone (n = 12). Examples of EJP voltage traces are shown in E, and mean EJP values are quantified in F. Quantal content, a measure of the number of synaptic quanta released in a single firing of the motoneuron, was nearly doubled by neuron-directed expression of Rheb compared to controls (I). Mini-EJP amplitude was decreased in these animals (H), while mEJP frequency showed no significant change (G). In this and all subsequent figures, *** denotes p-values less than 0.00005 using a student t-test comparison with controls, ** denotes p-values less than 0.005, and * denotes p-values less than 0.05. The scale bar is 50 microns in panel A.
Enlargement of the NMJ in Drosophila is not always associated with an electrophysiologically competent synapse. For example, highwire mutants display large NMJs but markedly compromised synaptic function [14], [15]. We therefore assessed the electrophysiological behavior of the NMJ in animals overexpressing Rheb in the motoneuron. This synapse showed nearly a doubling of the quantal content, a measure of the number of synaptic vesicles released per motoneuron firing (Fig 1I). The amplitude of the excitatory junctional potential (EJP), the voltage change in the muscle elicited by a suprathreshold stimulation of the motoneuron, also increased significantly compared to control synapses (Figure 1E, F). Mini-excitatory junctional potentials (mEJPs) are depolarizations of the muscle that result from spontaneous neurotransmitter release and provide a measure of vesicular fusion. While the mEJP frequencies of Rheb overexpressing animals showed no significant change (Figure 1G), the mEJP amplitudes were lower than matched controls (Figure 1H). In all, activation of Tor signaling via overexpression of Rheb produced an expanded synapse that was fully functional.
Reduction of Tor signaling produces a small synapse with compromised function
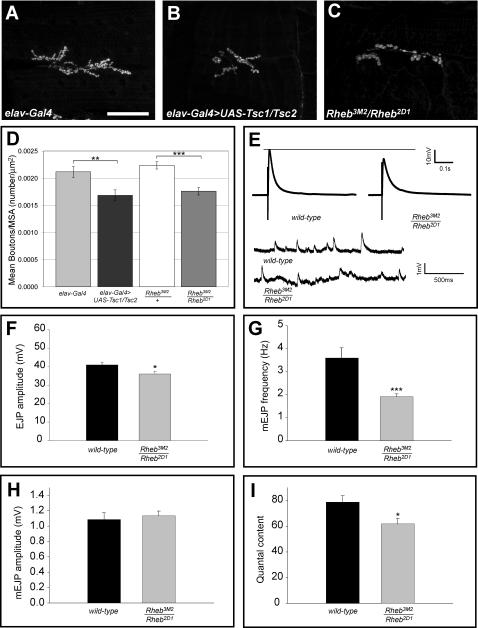
To determine if reduced Tsc-Rheb-Tor signaling compromises synapse growth and function we overexpressed Tsc1 and Tsc2 in the motoneuron, or compromised Rheb activity using a combination of hypomorphic Rheb alleles previously shown to cause reductions in cell size and number as well as S6k activity [11]. Overexpression of UAS-Tsc1/Tsc2 has been shown to limit growth mediated by Rheb [11]–[13], and we observed that Tsc1 and 2 overexpression in the motoneuron reduced synapse size compared to controls (Figure 2A, B, quantified in D). Consistent with this finding, Rheb hypomorphic mutant larvae showed a significantly reduced number of boutons per unit muscle area compared to heterozygous controls (Fig 2C, D). The NMJs of these animals also revealed significant changes in synaptic function. mEJP frequencies in Rheb mutant animals were half that of controls (Fig 2E, G), and EJP amplitudes were significantly reduced (Figure 2F). We also saw a reduction in the quantal content of Rheb mutants (Figure 2I), while mEJP amplitude showed no significant change (Figure 2H). Thus, reducing Tor activity by either of two mechanisms, overexpression of Tsc1/2 or partial loss-of-function mutations in Rheb, compromised synapse morphological development and function. Electrophysiology of hypomorphic Tor mutants showed a reduction in mEJP frequency similar to what we saw for Rheb mutants (data not shown).
Figure 2. Rheb activity is required for normal synapse assembly.
Panels A–C show anti-CSP staining of larval NMJs in a control animal (A, elav-Gal4 driver alone), an animal bearing elav-Gal4>UAS-Tsc1, UAS-Tsc2 (B), or a Rheb partial loss of function mutant (C). Reduction of Rheb function produced by either neuron-directed expression of Tsc1 and Tsc2 (n = 22) or mutation of Rheb (n = 40) significantly reduced synapse size compared to controls with elav-Gal4 alone (n = 44) or animals heterozygous for a Rheb mutation (n = 17), as measured by the number of synaptic boutons/muscle area (D). Panel E shows sample EJP traces for wild-type and Rheb mutant NMJs, as well as baseline recordings from these preparations showing the size and frequency of mini-EJPs. Panels F, G, and I show reductions in EJP amplitude, mini-EJP frequency, and quantal content for Rheb mutant synapses (n = 29) compared to wild-type controls (n = 10). Mini-EJP amplitude did not show a significant change (H). The scale bar in A is 50 microns.
Rapamycin does not inhibit synapse growth mediated by overexpression of Rheb
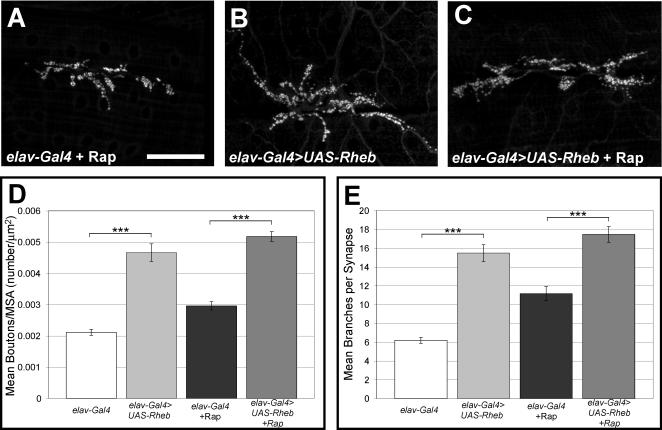
To evaluate if Rheb overexpression-mediated synapse expansion takes place via known growth regulatory pathways, we grew larvae from hatching to the third instar larval stage on rapamycin-containing food. Rapamycin has been shown to block growth mediated by TORC1 in Drosophila, and we used a concentration that produced clear developmental delay [16]. Culturing larvae bearing elav-Gal4 and UAS-Rheb+ transgenes on rapamycin reduced overall growth, including muscle size, but did not suppress the synaptic enlargement measured either by the number of synaptic boutons/muscle area or the number of motoneuron branches (Figure 3A–C, quantified in D, E). These findings show that Rheb-mediated synaptic growth did not require TORC1 activity, implicating TORC2 and its regulation of the actin cytoskeleton as serving critical functions in synaptic growth control. Interestingly, culturing elav-Gal4 control larvae (without the UAS-Rheb transgene) on rapamycin produced both an increase in the number of boutons per unit muscle area and an in increase in motoneuron branching (Figure 3D, E). This raises the possibility that blocking the activity of TORC1 with rapamycin indirectly influences the activity of other Tor complexes.
Figure 3. Rapamycin does not block Rheb-mediated synapse growth.
Panels A–C show anti-CSP staining of NMJ synaptic boutons, demonstrating that the TORC1 inhibitor rapamycin does not block synapse growth in control animals or in larvae with neuron-directed expression of Rheb (elav-Gal4>UAS-Rheb). Panels D and E provide quantification of bouton numbers/muscle area and numbers of motoneuron branches, respectively, for elav-gal4 controls (n = 44), animals with neuronal expression of Rheb (n = 41), control animals treated with rapamycin (n = 26), and Rheb expressing animals treated with rapamycin (n = 29). The scale bar is 50 microns.
The Tsc-Rheb-Tor pathway regulates translation largely by controlling S6k [11]–[13]. Rheb activation of Tor produces phosphorylation and activation of S6k. The control of translation via S6k represents but one component of regulation affected by this pathway and is molecularly distinct from Tsc-Rheb-Tor-mediated control of the actin cytoskeleton. To evaluate the contribution of S6k to synapse growth we examined the NMJs of animals bearing a null mutation in S6k. S6k mutants are small, with a reduced muscle surface area compared to controls. The synapse size, however, as measured by the number of boutons per unit muscle area, is not reduced (data not shown). These findings contrast with the effects of Rheb mutations or Tsc1 and Tsc2 overexpression (Figure 2), but are consistent with the finding that rapamycin does not suppress synapse overgrowth (Figure 3). Together, these results suggest that TORC1 and S6k do not contribute significantly to the proportional growth of the NMJ.
Rheb-mediated changes in synapse function require the BMP-signaling receptor encoded by wishful thinking
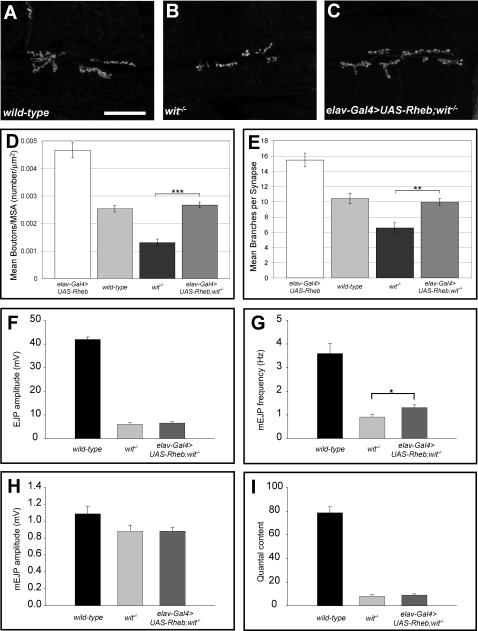
Growth factor-mediated signaling, including both Wingless (Wg) and BMP pathways, is important for normal NMJ growth in Drosophila. Animals bearing mutations in wishful thinking (wit), a gene encoding a BMP type II receptor, show a very small NMJ with dramatically compromised synaptic function [17]–[19]. To determine the relationship between Rheb-regulated synaptic growth and BMP-mediated synapse assembly we tested the ability of Rheb overexpression to rescue the synaptic growth defect of wit mutants. While Rheb+ expression in the motoneuron was able to restore the number of synaptic boutons to wild-type levels in wit mutant larvae, the number of boutons was significantly less than with Rheb overexpression alone (Figure 4A–E). Rheb overexpression modestly increased mini EJP frequency of wit mutants (Figure 4G), but showed no capacity to rescue either quantal content or EJP amplitudes (Figure 4F–I), therefore wit is clearly required for most Rheb-directed effects on synapse function. While these results do not establish the nature of the communication between Tor-Tsc signaling and the BMP pathway, it does demonstrate that an intact BMP system is necessary for Rheb-directed changes in synapse function.
Figure 4. Rheb-mediated synapse expansion and physiological function is BMP-signaling dependent.
Anti-CSP staining of synaptic boutons (panels A–C) shows the effects of wit on synapse growth (B), and the effects of neuron-directed expression of Rheb on wit mutant NMJs (C) compared to wild-type (A). Synapse size, measured by either the number of boutons/muscle area (D) or the number of motoneuron branches (E), is dramatically reduced in wit mutants (n = 20) compared to wild-type (n = 12), and is partially rescued by neuron-directed expression of Rheb (elav-Gal4>UAS-Rheb, n = 24). Reductions in EJP amplitudes (F), mini-EJP amplitudes (H), and quantal content (I) mediated by loss of wit (n = 8) are not rescued by neuron-directed expression of Rheb (n = 16) (n = 10 for wild-type). The decrease in mini-EJP frequency of wit mutants, a measure of spontaneous vesicle release, is rescued to a significant degree by expression of Rheb in the motoneuron (G). The scale bar represents 50 microns.
Tsc-Rheb-Tor signaling is critical for axon guidance in the visual system
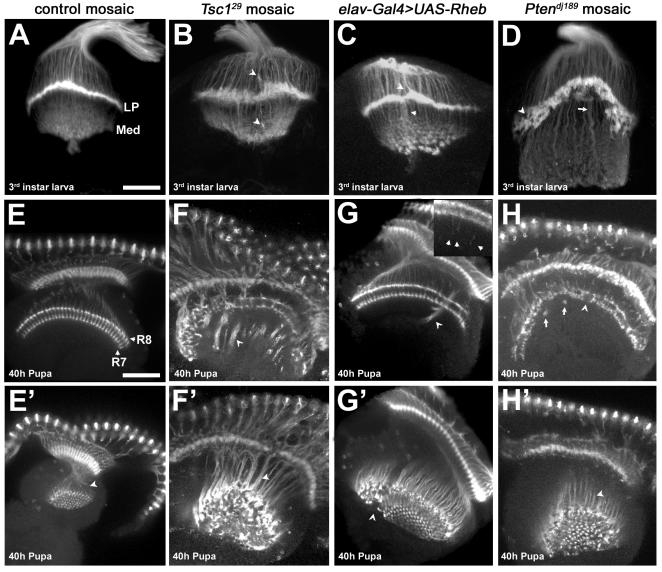
Another fundamental aspect of neural development is the correct specification of axon pathfinding and synapse formation with the correct targets. The Drosophila visual system offers a powerful experimental model for assessing the function of a signaling system in axon guidance. To evaluate the function of the Tsc-Rheb-Tor signaling pathway in axon guidance we generated genetic mosaic animals where mutant photoreceptor neurons project to a phenotypically wild-type brain. In the fruit fly Drosophila, each retinal sensory unit, or ommatidium, is comprised of eight photoreceptors, R1-8. In the third instar larval brain, R1-6 project to the first optic ganglion, the lamina, and terminate to form a discrete plexus where synapses will form later in development (Figure 5A). R7 and R8 project to a deeper level in the brain, the medulla, forming a discrete set of projections seen in both larval and pupal brains. In 40h pupae the R7 and R8 projections terminate in distinct layers in the medulla, producing a highly regular and stereotyped pattern (Figure 5E). Loss of Tsc1 function in the retina produces an enlarged eye disc with an increased number of photoreceptors [20]–[22]. Axon projections from Tsc1 mutant photoreceptors in the brains of third instar larvae and pupae showed severe axon guidance abnormalities (Figure 5B, F, F′, quantified in Table 1). In third instar larvae, R1-6 termination at the lamina plexus is disorganized, producing an irregular termination zone (compare Figure 5A to 5B). R7/R8 terminations within the larval medulla are also abnormal (Figure 5B, arrowhead). At the 40 hr pupal stage we observed gaps in the R7/R8 layers with large axon bundles, or fascicles, that projected past their appropriate termination points (Figure 5F, F′).
Figure 5. Photoreceptor axon projection defects associated with increased Tor signaling.
(A–D) Dorsal-posterior views of third instar optic lobes stained with MAb24B10 to visualize photoreceptor projections. (A) Mitotic clones in an FRT82B control background show proper termination of photoreceptor axons R1-6 at the lamina plexus (LP), and termination of photoreceptors R7 and R8 in the medulla (Med). (B) Tsc129 mutant axons terminate at incorrect positions above and below the lamina (arrowheads) and produce a broadened lamina plexus. (C) Neuronal expression of Rheb creates axon termination defects similar to those seen in Tsc1 mosaics (D) Ptendj189 mutant photoreceptors leave gaps and holes (arrowhead) in the lamina plexus, which is broader and noticeably “peaked.” The medulla contains axon projections which are thicker and much longer than in controls (arrow). (E–H′) Dorsal view of optic lobes from 40h pupae stained with MAb24B10. E′–H′ are lower optical planes of the optic lobes shown in E–H, respectively. (E, E′) Control photoreceptors R7 and R8 show two distinct layers of termination in the medulla (labels), and are arranged in a highly regular pattern (arrowhead). (F) Animals with Tsc129 mutant photoreceptors show severe disruption of the R7 and R8 termination layers. Instead of terminating at the correct positions, the axons fail to de-fasciculate, forming dense bundles (arrowheads) that project beyond the medulla. (G, G′) Neuron-directed expression of Rheb causes axon bundles to project beyond the medulla in a fashion similar to Tsc1 mosaics (arrowheads), but the phenotype is much less severe. (G, inset) Individual Rheb-overexpressing axons show an intermediate termination defect, stopping several microns beyond their normal targets (arrowheads in inset). (H) Ptendj189 mutant axons exhibit gaps and collapses in the R7/R8 termination zone (arrowhead). Thick axon bundles can be seen that bypass their usual stopping points and then loop back to terminate at other locations in the R7/R8 layers (arrows). (H′, F′) Axon bundles in Ptendj189 mosaics are not as densely packed as those of Tsc129 mosaics (arrowheads), but are still disorganized. All scale bars are 50 microns.
Table 1. Axon guidance defects in animals with altered Tsc-Rheb-Tor signaling.
| (Percent of optic lobes affected) | ||||||||
| Incorrect Terminations | ||||||||
| 3rd instar larvae | Thick LP | Gaps in LP | LP Peaked | Long R7/8 | Gaps in Med. | Above LP | Below LP | In Med. |
| Tsc129 (n = 58) | 70 | 41 | 7 | 5 | 31 | 45 | 52 | 72 |
| Ptendj189 (n = 38) | 24 | 100 | 79 | 63 | 29 | 58 | 68 | 95 |
| Rheb3M2/26.2 (n = 22) | 68 | 55 | 0 | 0 | 41 | 32 | 18 | 36 |
| TorA948V (n = 12) | 17 | 17 | 0 | 8 | 0 | 0 | 17 | 33 |
| S6kl-1 (n = 49) | 29 | 41 | 2 | 0 | 2 | 10 | 14 | 4 |
| TorA948V Tsc129 (n = 14) | 21 | 29 | 0 | 0 | 7 | 7 | 0 | 0 |
| S6kl-1 Tsc129 (n = 23) | 65 | 83 | 0 | 0 | 70 | 43 | 17 | 43 |
| wild-type +Rap (n = 80) | 19 | 13 | 0 | 0 | 9 | 21 | 14 | 13 |
| Tsc129 +Rap (n = 60) | 76 | 54 | 2 | 6 | 59 | 46 | 71 | 63 |
| 40-hour pupae | Pathfinding Defects | De-fasciculation Defects | Termination Layer Defects | |||||
| Tsc129 (n = 60) | 100 | 100 | 100 | |||||
| elav-Gal4>UAS-Rheb (n = 23) | 83 | 43 | 39 | |||||
| Ptendj189 (n = 73) | 25 | 30 | 8 | |||||
| Rheb26.2 (n = 80) | 36 | 3 | 19 | |||||
| S6kl-1 (n = 32) | 28 | 9 | 25 | |||||
| TorA948V (n = 20) | 35 | 0 | 10 | |||||
| TorA948V Tsc129 (n = 25) | 40 | 4 | 16 | |||||
*Tsc129, Ptendj189, and Rheb26.2 are eyFLP mosaics; all others are mutants. LP - lamina plexus; Med. - medulla; Rap - rapamycin
To evaluate the degree of pathway activation mediated by pan-neuronal expression of UAS-Rheb, which we used above to evaluate the role of Tor signaling at the NMJ, we examined photoreceptor pathfinding in elav-Gal4>UAS-Rheb larvae and pupae. These animals survive to adulthood and show disruptions in photoreceptor projections, but to a significantly lesser extent than found in Tsc1 mosaic animals (see Table 1). In elav-Gal4>UAS-Rheb pupal brains, abnormal bundles of axons that penetrate into deeper brain structures were found, but this phenotype was markedly less severe than in Tsc1 mosaic animals (Figure 5G, G′, Table 1). Close inspection of R7 and R8 endings in the medulla revealed individual photoreceptor axons growing past the correct termination site (Figure 5G inset). R1-6 endings in the larval brain also show irregularities, but the lamina plexus is less disrupted than in Tsc1 mosaics (Figure 5C). These findings indicate that the degree of pathway activation achieved with elav-Gal4>UAS-Rheb is markedly less than produced by loss of Tsc1. Moreover, these results suggest that there is a continuum of axon pathfinding abnormalities with different levels of pathway activation.
Pten, another negative regulator of cell growth and proliferation, encodes a phosphatase that converts the lipid signaling molecule phosphatidylinositol 3,4,5 triphosphate (PIP3) to PIP2, an inactive form, thus antagonizing PI3K activation of the TOR pathway. Like Tsc1, Pten retinal mosaics show eye overgrowth and precocious differentiation [23]–[27]. To determine if disruptions of Pten function affect axon guidance, we generated mosaic animals. Pten mutant photoreceptor projections showed disorganization of axon termini in the third instar larval brain and were notable for a misshapen and concave lamina plexus with a large number of gaps (Figure 5D, quantified in Table 1). At the pupal stage, Pten mutant projections showed significantly less severe defects than photoreceptors bearing a Tsc1 null mutation (Figure 5H, H′ and Table 1), with fewer projections failing to stop at the normal medulla termination sites. The penetrance of pathfinding, defasciculation, and termination defects in 40h pupae was lower in Pten than in Tsc1 null mutant photoreceptors projecting to a wild-type CNS (Table 1). In sum, Pten and Tsc1 mutant photoreceptor projections show distinct patterns of photoreceptor axon guidance defects, despite the fact that these two inhibitors of Tsc-Rheb-Tor signaling have similar influences on cell size, growth, and differentiation [20]–[22], [24]–[26], [28].
We also observed distinct effects of Tsc1 and Pten retinal mosaics on the differentiation of lamina neurons and visual system glia, detected with anti-Dachshund and anti-Repo antibodies, respectively (Figure S1). Pten mutant retinal projections produced an abnormally large lamina not seen in Tsc1 mosaics (Figure S1A–C). In both Pten and Tsc1 mosaics visual system glia were found in the brain in roughly normal positions (Figure S1D–F), although some disorganization was evident in brains receiving Tsc1 mutant photoreceptor projections. It is possible that this disruption of glial architecture may partially contribute to the axon projection defects observed in Tsc1 mutants.
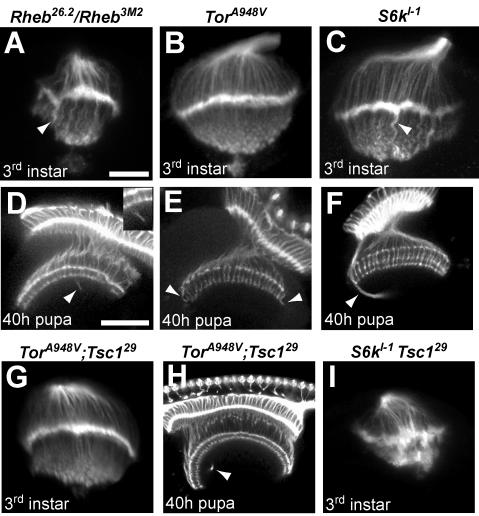
To evaluate the effects of reduced Tor signaling, we examined axon guidance in animals bearing hypomorphic mutations in Tor and Rheb, as well as a null allele of S6k, a key downstream target of TORC1. In all three of these mutants, mild axon projection defects were observed (Figure 6A–F, Table 1). Third instar larvae had irregular laminas and abnormally thick projections to the medulla (Figure 6A–C, arrowheads). In 40 h pupae, R7 and R8 terminations were largely normal, but there were projections which misrouted and failed to terminate correctly (Figure 6D–F, Table 1). Genetic mosaic analysis of Rheb mutant photoreceptor projections showed the same phenotypes, demonstrating that normal levels of Tor-Tsc signaling in the retina are required for proper photoreceptor targeting (data not shown). These findings establish that reductions in Tor-Tsc signaling also produce axon guidance defects, although quite mild in comparison to activation of the pathway achieved by loss of Tsc1 function. However, only the S6k mutants are null in these experiments, and we cannot therefore fully assess the contributions of Tor or Rheb to axon guidance compared to Tsc1.
Figure 6. Effects of mutations that downregulate the Tor pathway on photoreceptor axon guidance, and genetic epistasis with Tsc1.
Optic lobes from third instar larvae (A–C) and 40h pupae (D–F) stained with MAb24B10. (A) Larvae heteroallelic for a hypomorphic combination of Rheb alleles show abnormal photoreceptor patterning and contain thick axon bundles that extend into the medulla (arrowhead). (D) At the 40 h pupal stage, Rheb mutants display axons that bypass their normal targets in the R7/R8 termination zones (arrowhead). (B) Larvae homozygous for a hypomorphic Tor allele show fairly normal photoreceptor patterning, but at the pupal stage (E) misrouted axons can be seen in the medulla (arrowheads). (C) S6k null homozygous larvae show thick axon bundles projecting past the lamina (arrowhead), while S6k pupae (F) display misrouted axons that initially bypass the R7/R8 termination zone (arrowhead). (G, H) Animals doubly mutant for Tor and Tsc1 do not show the severe photoreceptor defects seen when axons are mutant for Tsc1 alone (compare to Figure 5B, F, F′), although mild defects similar to those in Tor mutants are still apparent (arrowhead). (I) S6k-Tsc1 double homozygous mutants display a severe phenotype dissimilar to mutants for either S6k or Tsc1 alone. The scale bar is 25 microns in panel A, 50 microns in panel D.
To determine if the functional relationships critical for growth control are also in effect for axon guidance, we conducted genetic epistasis experiments between Tsc1 and both Tor and S6k. Tsc1 mosaic pupae show severe axon guidance abnormalities and Tsc1 mutant animals do not survive to the pupal stage; in contrast, animals bearing both a Tsc1 mutation and a hypomorphic Tor allele survived to pupal stages and showed only modest axon guidance abnormalities in larval and pupal brains (Figure 6G, H, Table 1). The gross disruptions of R7/R8 terminations in the medullas of 40h Tsc1 mosaic pupae were almost completely rescued by the presence of a hypomorphic allele of Tor. Genetic mosaics with Tsc1 Rheb double mutant chromosomes also showed dramatic rescue of photoreceptor axon guidance defects (data not shown). In contrast, S6k null mutations did not ameliorate the Tsc1 axon projection defects in the larval brain, and both the lamina plexus and medulla projections were highly disordered (Figure 6I, Table 1). These findings demonstrate that Tor and Rheb, but not S6k, are critical components of the photoreceptor axon guidance signaling system downstream of Tsc1.
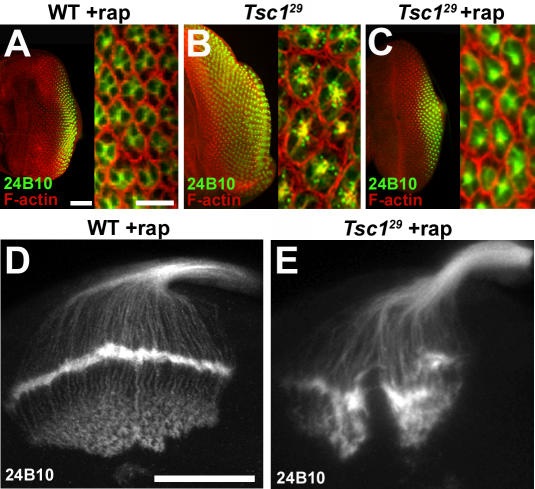
In order to evaluate if the growth control functions of Tsc-Rheb-Tor signaling are important for axon guidance, we used rapamycin to inhibit the abnormal growth produced by loss of Tsc1 function. Feeding animals with rapamycin between hatching and the third instar larval stage blocked the retinal cell growth and proliferation defects of Tsc1 mutant photoreceptor mosaics. This was evident in both the overall size of the developing retina and the size of the photoreceptor cell bodies (Figure 7A–C). While the growth defects of Tsc1 mosaics were rescued by rapamycin treatment, photoreceptors from these animals still showed severe axon guidance abnormalities in the third instar larval brain, with an irregular and disrupted lamina plexus, as well as disorganized projections to the medulla (Figure 7E, Table 1). Treatment of wild-type controls with rapamycin produced only mild defects in the lamina plexus (Figure 7D, Table 1) supporting the hypothesis that Tsc1-mediated regulation of axon guidance operates largely via a rapamycin-insensitive function of Tor. We noted that the excessive growth of Pten mutant retinas was not rescued by rapamycin treatment, in contrast to the effects of this TORC1 inhibitor on Tsc1 mosaics. While the growth and differentiation phenotypes of Pten and Tsc1 mutant retinas are comparable, the difference in their rapamycin responses highlights how disruption of signaling by these two regulators is distinct.
Figure 7. Axon guidance defects in Tsc1 mosaics are not suppressed by blocking growth.
(A–C) Third instar eye discs from wild type and Tsc1 mosaic larvae raised with or without rapamycin (rap). Ommatidial units, comprised of eight photoreceptors, were visualized with phalloidin (red) that detects F-actin, and MAb24B10 (green). Phalloidin staining is strongest at the perimeter of each ommatidium, outlining each sensory unit. Rapamycin treatment of Tsc1 mosaic eye discs (C) restored eye disk size and cell size compared to wild type (A). (D and E) Rapamycin treated third instar larval brains stained with MAb24B10. Rapamycin treatment blocked abnormal growth of the retina and the increase in photoreceptor cell size, but did not ameliorate the abnormal axon projections also characteristic to untreated Tsc129 mosaics. The scale bars in panel A represent 50 microns in the left image, 10 microns in the right image. The scale bar is 50 microns in panel D.
Discussion
Tsc-Rheb-Tor signaling in neural development
The Tsc-Rheb-Tor pathway is critical for integrating a variety of signals that govern cellular and organismal growth. Inappropriate activation of the pathway also leads to severe neurological and behavioral abnormalities, including mental retardation, autism, and epilepsy [1], [6]. While TSC mutations produce hamartomatous growths in the brain, recent evidence has suggested that these benign tumors may not be solely responsible for the nervous system dysfunction that is a hallmark of tuberous sclerosis complex. Loss of TSC2 in hippocampal neurons produces changes in neuronal morphology and synaptic transmission [2]. Heterozygosity for TSC2 in the rat compromises several measures of hippocampal long term potentiation [3]. Loss of Pten, an important upstream regulator of Tsc-Rheb-Tor signaling, in a limited set of neurons also affects neuronal morphology and socialization behavior [29]. These findings collectively provide evidence that Tsc-Rheb-Tor signaling is critical for the morphological and functional development of the nervous system. It is not clear, however, if the entire Tsc-Rheb-Tor signaling network is critical for nervous system development, or if neural function is strictly a consequence of altered growth regulation. It is also not known if loss of signaling is as detrimental to neuronal development as inappropriately elevated signaling, such as occurs with loss of TSC function. We have taken advantage of the genetic and molecular tools available in the fruit fly Drosophila to address these questions. Our findings demonstrate that appropriate levels of Tsc-Rheb-Tor signaling are critical for both NMJ development and for axon guidance in the visual system. In both these contexts, effects are independent of growth, implicating TORC2 rather than TORC1 as the complex mediating Tsc-Rheb-Tor signaling influences in the nervous system.
Tsc-Rheb-Tor effects on neural development are independent of growth regulation
Given the importance of Tsc-Rheb-Tor signaling in regulating cellular and tissue growth, it was important to determine if disruption of this pathway affects neural development via its effects on growth or through signaling components independent of those that govern cellular size and growth. To address this issue we used both pharmacological and genetic methods to block the increased growth produced by pathway activation. The immunosuppressant rapamycin is a TORC1-specific inhibitor that prevents activation of S6k and blocks growth mediated by loss of Tsc1. Rapamycin treatment retarded growth in larvae with pan-neuronal expression of Rheb, but failed to reduce the synapse expansion characteristic of these animals. Similarly, while rapamycin effectively reduced the retinal overgrowth of Tsc1 mosaic animals, it failed to suppress the photoreceptor axon guidance defects seen in the visual system. Loss of S6k function also failed to ameliorate axon guidance defects in Tsc1 mosaic animals. This contrasts with effects of Tor partial loss-of-function mutations, which effectively rescued axon guidance defects of Tsc1 mutants. Collectively, these findings demonstrate that the role of Tsc-Rheb-Tor signaling in synapse assembly and axon guidance is largely independent of TORC1, S6k, and their effects on growth. Indeed, while animals bearing null alleles of S6k have some axon pathfinding defects, the effects are relatively modest compared to Tsc1 mosaics, indicating that S6k does not provide the critical outputs affecting axon guidance.
Our findings parallel recent work in the mouse, where neuronal hypertrophy produced by loss of Pten in granule neurons of the cerebellum and dentate gyrus was not rescued by loss of S6k1 [30]. It is also of note that some but not all Tsc1/2-mediated changes in dendritic morphology of hippocampal neurons in organotypic cultures were suppressed by rapamycin treatment [2]. Our findings suggest that inhibition of growth regulatory components in tuberous sclerosis patients, such as achieved with rapamycin and related agents, may not affect all processes that are deranged in the nervous system.
Recent studies of Pi3 kinase, Akt and InR in Drosophila have shown that activation of signaling upstream of Tsc1/2 also produces increases in synapse size, both at the NMJ as well as central synapses [31]. Expression of these components in adult neurons demonstrated that Pi3 kinase-mediated synaptogenesis was age-independent, and therefore not a developmentally restricted phenomenon. In agreement with studies reported here, the expanded NMJs produced by activation of Pi3 kinase were functional, with increased stimulus-induced EJPs. Overexpression of the Drosophila ortholog of the epidermal growth factor receptor (EgfR) in central neurons increased neuronal cell size, without an increase in synapse number. These results are consistent with those reported here where we have been able to directly suppress growth mediated by Tsc-Rheb-Tor pathway activation without altering effects on synapse formation or axon guidance.
Recent studies have also demonstrated a link between Tsc1/Tsc2 and highwire, a gene known to effect synapse size and functionality in Drosophila [32]. The highwire ortholog Pam was shown to bind Tsc2 in pull-down assays, and it has been suggested that Pam may function as an E3 ubiquitin ligase to regulate the intracellular levels of the Tsc1/Tsc2 complex. This concept of Highwire as a negative regulator of Tsc levels is consistent with our findings, since highwire mutants have been shown to possess enlarged NMJs similar to what we see for Rheb overexpression [14]. Despite this, the enlarged synapses of highwire mutants display compromised synaptic function which is contrary to what we found when overexpressing Rheb, so Highwire is likely to have multiple functions at the synapse besides simply the regulation of Tsc.
Contributions of TORC1 versus TORC2 in synapse assembly and axon guidance
Tor has a number of molecular outputs that influence many cellular processes; notable among these are cellular growth and cellular morphology. TORC1, which contains Raptor and is sensitive to the anti-proliferative agent rapamycin, is a major contributor to the regulation of cellular growth, in large measure due to its effects on protein synthesis. TORC2, which includes Rictor, is implicated in the control of cell morphology mediated by regulation of the actin cytoskeleton [33]. Both pharmacological and genetic studies presented here argue in favor of Tor complex 2 providing an essential regulatory component of both synapse growth and axon guidance in Drosophila. Our results support recent work showing that changes in dendritic morphology of hippocampal neurons produced by loss of Tsc1 required regulation of the actin-depolymerizing factor Cofilin [2], implicating TORC2-mediated processes. There is a considerable body of work demonstrating that control of the actin cytoskeleton is critical for NMJ growth and function [34]–[36] and TORC2 may provide an important component of that control. Regulation of actin is also essential for axon guidance in the visual system (reviewed in [37], [38]), and disruption of Tor-mediated control of actin may be the underlying molecular deficit in Tsc1 mosaics.
Either gain or loss of Rheb signaling compromises neuromuscular junction assembly and axon guidance
A number of studies have suggested that TOR activation produced by loss of TSC1/2 affects neuronal morphology and synaptic function. Our findings support these observations; elevated Rheb activity produces synaptic enlargement and enhanced physiological function at the Drosophila NMJ. However, it was not evident from earlier studies whether loss of signaling through Rheb and Tor is also important for neural development. We provide evidence that this is the case. Partial loss-of-function mutations in Rheb compromise NMJ growth and function, as well as photoreceptor axon targeting in the visual system. Overexpression of Tsc1 and Tsc2 in the motoneuron also limited synaptic growth, supporting the conclusion that depressed levels of Rheb activity compromise synapse development.
Rheb-mediated synaptic development is dependent on a functional BMP signaling system
The capacity of Tsc-Rheb-Tor signaling to affect neuronal morphology and synapse function begs the question of whether these effects are dependent on signaling systems known to be critical for synapse development. At the Drosophila NMJ, BMP signaling is critical for normal growth and function. Mutations in wit, a gene encoding a type II BMP receptor, produce a small and poorly functioning NMJ [17], [19]. These deficits can be rescued by motoneuron expression of wit+, demonstrating that BMP signaling in the motoneuron is critical for synaptic expansion during larval growth. To determine if Rheb-mediated synaptic growth required BMP signaling, we placed elav-Gal4 and UAS-Rheb transgenes into a wit mutant background. While overexpression of Rheb and the accompanying activation of the Tor pathway partially rescued the defect in synapse growth produced by loss of wit function, it was unable to restore a normal EJP response or rescue quantal content. These findings establish that Tsc-Rheb-Tor mediated effects on synapse morphology are partially dependent on BMP signaling, and are fully dependent on BMP activity for a physiologically competent synapse. Our findings also establish that the functional deficits in wit mutants are not simply the result of reduced synapse size, since restoration of synapse size by expression of UAS-Rheb does not restore physiological function. Intersection of BMP, and Akt/PTEN/TOR signaling has been noted for other systems, and our results indicate the relationship between these pathways is important for synapse growth and plasticity as well [39].
Loss of function mutations in Tsc1 and Pten have different effects on axon guidance
Previous analysis of gigas/Tsc2 mutants demonstrated that loss of this gene in mechanoreceptors affects axon targeting, producing projections to novel areas in the CNS in addition to innervation of normal targets [5]. We have used genetic mosaics to evaluate the function of Tsc-Rheb-Tor signaling in photoreceptor axon guidance. Animals homozygous for Tsc1 in the retina showed grossly aberrant photoreceptor projections to both the lamina and medulla. R7 and R8 projections to the medulla in 40h pupae failed to terminate correctly and projected beyond normal targets to inappropriate regions within the brain. Somatic mosaics bearing retinal neurons mutant for Pten also showed photoreceptor axon guidance defects, but to a notably lesser degree. Since both Tsc1 and Pten alleles used for this analysis were nulls and show comparable effects on cellular growth and differentiation [23], it follows that Pten is not as critical for axon guidance as Tsc1. The distinctions between axon guidance phenotypes of Pten and Tsc1 null mutants indicate that altered timing of differentiation is not critical for axon guidance and that control of this pathway at the level of Pten or Tsc1 is not functionally equivalent. Our findings that rapamycin arrests retinal overgrowth produced by loss of Tsc1 but not Pten in the retina supports earlier work demonstrating that retinal overgrowth mediated by loss of Tsc1, but not Pten, can be suppressed by reductions in S6k activity [40]. Those results were interpreted as demonstrating that Pten is largely a regulator of Akt activity, whereas Tsc1/2 serves as a tumor suppressor and inhibitor affecting principally S6k. Our results support these relationships and emphasize that in the nervous system regulation of Tsc1/2 targets other than S6k are critical.
Graded activation of the Tsc-Rheb-Tor signaling axis produces graded effects on axon guidance
We have used two different genetic methods for activating the Tsc-Rheb-Tor pathway in the visual system; generating retinal mosaics with a loss of function allele of Tsc1, and pan-neuronal expression of Rheb using elav-Gal4 and UAS-Rheb. The comparison of these methods revealed that overexpression of Rheb produced milder axon guidance phenotypes in the visual system than complete loss of Tsc1 function. Of interest is that the degree of activation achieved with elav-Gal4>UAS-Rheb, a level that did not produce lethality, did result in discernable axon targeting defects in the visual system. This suggests that axon guidance controlled by Tsc-Rheb-Tor is sensitive to incremental changes in signaling. The range of neurological and behavioral phenotypes associated with loss of one copy of TSC1 or TSC2 is consistent with this model, where other environmental or genetic factors may affect signaling levels, producing a range of deficits. Our findings indicate that Drosophila can serve as a useful model for identifying how graded changes in signaling can produce a spectrum of defects in neural development.
Materials and Methods
Drosophila strains
UAS-RhebEP50.084/TM6B Tb [11], UAS-DP110WT [41], and y w hsFLP; UAS-Tsc1 UAS-Tsc2 [21] were crossed to elav-Gal4/CyO P[w+; ubi-GFP] [42] or OK6-Gal4 [17] for expression in neurons, and to G14-Gal4/CyO P[w+; ubi-GFP] (from C. Goodman) for expression in muscles. Stocks used for mutant analysis and genetic interaction studies were y w; Rheb3M2/TM6B Tb y+ [11], Rheb2D1/TM6B Tb [11], b w; witB11/TM6B Tb [19], w; witA12/TM6B Tb [19], S6kl-1/TM6B Tb [43], and Tor2.1/Ala948Val/CyO P[w+; ubi-GFP] [44]. Eye-specific mosaics were generated using the FLP-FRT technique [45] by crossing y w eyFLP GMR-lacZ; FRT82B I(3)cl-R3/TM6B Tb [45] or y w eyFLP GMR-lacZ; I(2)cl-L3 w+ FRT40A/CyO y+ [45] to w; FRT82B Tsc129/TM6B Tb [20], w; FRT82B Rheb26.2/TM6B Tb y+ [11], y w hsFLP; FRT40A Ptendj189/SM6-TM6B [24], y w eyFLP GMR lacZ; FRT82B [45], or y w eyFLP GMR lacZ; FRT40A [45]. Using this mosaic method, heterozygous cells have a growth disadvantage since they bear a Minute and cell-lethal mutation [l(3)cl-R3 or l(2)cl-L3]. For all retinal mosaics, we assessed the degree of mosaicism by examining the adult retina where mutant cells could be identified by loss of the w+ marker. In Tsc1, Pten and Rheb mosaics, mutant cells comprised the vast majority of the adult retina (>90%). Wild-type strains were y w, Oregon-R, or Canton-S.
Immunohistochemistry
For visualization of neuromuscular junction synapses, third instar larvae were filleted in PBS and fixed in 4% formaldehyde before staining with Anti-Cysteine String Protein mAB49 at 1∶1000 (a generous gift from Zinsmaier and Buchner) and FITC-conjugated Anti-HRP at 1∶50 (Jackson Labs). Bouton numbers were determined by a combination of CSP and HRP image data. Muscle surface area measurements were performed using ImageJ data analysis software (NIH) and represent the combined area of the second abdominal segment muscles 6 and 7. Third instar larvae and 40hr pupae were fixed, stained, and mounted as described [46] for photoreceptor analysis. Antibodies from the Developmental Studies Hybridoma Bank were used at 1∶25 for mouse anti-Chaoptin (MAb24B10), 1∶10 for mouse anti-Repo (MAb8D12), and 1∶25 for mouse anti-Dachshund (MabDac2-3). Secondary antibodies were from the AlexaFluor series (Invitrogen). Texas Red-phalloidin was used at 0.165 µM (Invitrogen). All images were acquired on a Nikon C1 upright laser confocal.
Rapamycin Treatment
Flies were raised on standard laboratory food supplemented with rapamycin (Sigma) to a final concentration of 3 µM for NMJ analysis or 2 µM for eye disks. Rapamycin treated Tsc129 mosaic animals with eye discs similar in size to control animals were selected for photoreceptor projection analysis.
Electrophysiology
Excitatory junctional potential (EJP) recordings were taken from muscle 6 in the second abdominal hemisegment of 3rd instar larvae. Dissections were done in Ca++-free saline and recordings were performed in HL3 following published protocols [47]. Recordings were acquired with an Axoclamp 2B amplifier and pClamp9 software (Axon Instruments). EJP amplitudes and mini-EJP amplitudes were measured with MiniAnalysis software from Synaptosoft.
Supporting Information
Patterning of lamina precursor cells and glia in Pten or Tsc1 mosaic animals. (A–F) Dorsal-posterior views of third instar larval optic lobes stained with anti-Dachshund (lamina precursor cell marker) or anti-Repo (glial cell marker). (A–C) Pten mosaic animals show a significantly larger lamina compared to control animals. This is not seen to the same extent in Tsc1 mosaics. (D–F) Glial cells successfully differentiate and migrate in both Pten and Tsc1 mosaics, however mild patterning defects are apparent and could possibly contribute to the photoreceptor patterning abnormalities observed. All scale bars are 50 microns.
(1.32 MB TIF)
Acknowledgments
We thank Hoda Pourhassan for assistance with data collection and analysis, the Bloomington Drosophila stock center for fly stocks, and the Developmental Studies Hybridoma Bank for antibodies.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by NIH contract grant number GM54832-09 to SBS, the Martin Lenz Harrison Endowment to SBS, and NIH grant RO1 GMO62509 to TN. MBO is an investigator with the Howard Hughes Medical Institute.
References
- 1.Ess KC. The neurobiology of tuberous sclerosis complex. Semin Pediatr Neurol. 2006;13:37–42. doi: 10.1016/j.spen.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 2.Tavazoie SF, Alvarez VA, Ridenour DA, Kwiatkowski DJ, Sabatini BL. Regulation of neuronal morphology and function by the tumor suppressors Tsc1 and Tsc2. Nat Neurosci. 2005;8:1727–1734. doi: 10.1038/nn1566. [DOI] [PubMed] [Google Scholar]
- 3.von der Brelie C, Waltereit R, Zhang L, Beck H, Kirschstein T. Impaired synaptic plasticity in a rat model of tuberous sclerosis. Eur J Neurosci. 2006;23:686–692. doi: 10.1111/j.1460-9568.2006.04594.x. [DOI] [PubMed] [Google Scholar]
- 4.Acebes A, Ferrus A. Increasing the number of synapses modifies olfactory perception in Drosophila. J Neurosci. 2001;21:6264–6273. doi: 10.1523/JNEUROSCI.21-16-06264.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Canal I, Acebes A, Ferrus A. Single neuron mosaics of the drosophila gigas mutant project beyond normal targets and modify behavior. J Neurosci. 1998;18:999–1008. doi: 10.1523/JNEUROSCI.18-03-00999.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Inoki K, Corradetti MN, Guan KL. Dysregulation of the TSC-mTOR pathway in human disease. Nat Genet. 2005;37:19–24. doi: 10.1038/ng1494. [DOI] [PubMed] [Google Scholar]
- 7.Neufeld TP. Genetic analysis of TOR signaling in Drosophila. Curr Top Microbiol Immunol. 2004;279:139–152. doi: 10.1007/978-3-642-18930-2_9. [DOI] [PubMed] [Google Scholar]
- 8.Prokop A, Meinertzhagen IA. Development and structure of synaptic contacts in Drosophila. Semin Cell Dev Biol. 2006;17:20–30. doi: 10.1016/j.semcdb.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 9.Mast JD, Prakash S, Chen PL, Clandinin TR. The mechanisms and molecules that connect photoreceptor axons to their targets in Drosophila. Semin Cell Dev Biol. 2006;17:42–49. doi: 10.1016/j.semcdb.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 10.Meinertzhagen IA. The early causal influence of cell size upon synaptic number: the mutant gigas of Drosophila. J Neurogenet. 1994;9:157–176. doi: 10.3109/01677069409167277. [DOI] [PubMed] [Google Scholar]
- 11.Stocker H, Radimerski T, Schindelholz B, Wittwer F, Belawat P, et al. Rheb is an essential regulator of S6K in controlling cell growth in Drosophila. Nat Cell Biol. 2003;5:559–565. doi: 10.1038/ncb995. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y, Gao X, Saucedo LJ, Ru B, Edgar BA, et al. Rheb is a direct target of the tuberous sclerosis tumour suppressor proteins. Nat Cell Biol. 2003;5:578–581. doi: 10.1038/ncb999. [DOI] [PubMed] [Google Scholar]
- 13.Saucedo LJ, Gao X, Chiarelli DA, Li L, Pan D, et al. Rheb promotes cell growth as a component of the insulin/TOR signalling network. Nat Cell Biol. 2003;5:566–571. doi: 10.1038/ncb996. [DOI] [PubMed] [Google Scholar]
- 14.Wan HI, DiAntonio A, Fetter RD, Bergstrom K, Strauss R, et al. Highwire regulates synaptic growth in Drosophila. Neuron. 2000;26:313–329. doi: 10.1016/s0896-6273(00)81166-6. [DOI] [PubMed] [Google Scholar]
- 15.McCabe BD, Hom S, Aberle H, Fetter RD, Marques G, et al. Highwire regulates presynaptic BMP signaling essential for synaptic growth. Neuron. 2004;41:891–905. doi: 10.1016/s0896-6273(04)00073-x. [DOI] [PubMed] [Google Scholar]
- 16.Zhang H, Stallock JP, Ng JC, Reinhard C, Neufeld TP. Regulation of cellular growth by the Drosophila target of rapamycin dTOR. Genes Dev. 2000;14:2712–2724. doi: 10.1101/gad.835000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aberle H, Haghighi AP, Fetter RD, McCabe BD, Magalhaes TR, et al. wishful thinking Encodes a BMP Type II Receptor that Regulates Synaptic Growth in Drosophila. Neuron. 2002;33:545–558. doi: 10.1016/s0896-6273(02)00589-5. [DOI] [PubMed] [Google Scholar]
- 18.Marques G, Haerry TE, Crotty ML, Xue M, Zhang B, et al. Retrograde Gbb signaling through the Bmp type 2 receptor wishful thinking regulates systemic FMRFa expression in Drosophila. Development. 2003;130:5457–5470. doi: 10.1242/dev.00772. [DOI] [PubMed] [Google Scholar]
- 19.Marques G, Bao H, Haerry TE, Shimell MJ, Duchek P, et al. The Drosophila BMP type II receptor Wishful Thinking regulates neuromuscular synapse morphology and function. Neuron. 2002;33:529–543. doi: 10.1016/s0896-6273(02)00595-0. [DOI] [PubMed] [Google Scholar]
- 20.Gao X, Pan D. TSC1 and TSC2 tumor suppressors antagonize insulin signaling in cell growth. Genes Dev. 2001;15:1383–1392. doi: 10.1101/gad.901101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Potter CJ, Huang H, Xu T. Drosophila Tsc1 functions with Tsc2 to antagonize insulin signaling in regulating cell growth, cell proliferation, and organ size. Cell. 2001;105:357–368. doi: 10.1016/s0092-8674(01)00333-6. [DOI] [PubMed] [Google Scholar]
- 22.Tapon N, Ito N, Dickson BJ, Treisman JE, Hariharan IK. The Drosophila tuberous sclerosis complex gene homologs restrict cell growth and cell proliferation. Cell. 2001;105:345–355. doi: 10.1016/s0092-8674(01)00332-4. [DOI] [PubMed] [Google Scholar]
- 23.Bateman JM, McNeill H. Temporal control of differentiation by the insulin receptor/tor pathway in Drosophila. Cell. 2004;119:87–96. doi: 10.1016/j.cell.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 24.Gao X, Neufeld TP, Pan D. Drosophila PTEN regulates cell growth and proliferation through PI3K-dependent and -independent pathways. Dev Biol. 2000;221:404–418. doi: 10.1006/dbio.2000.9680. [DOI] [PubMed] [Google Scholar]
- 25.Huang H, Potter CJ, Tao W, Li DM, Brogiolo W, et al. PTEN affects cell size, cell proliferation and apoptosis during Drosophila eye development. Development. 1999;126:5365–5372. doi: 10.1242/dev.126.23.5365. [DOI] [PubMed] [Google Scholar]
- 26.Goberdhan DC, Paricio N, Goodman EC, Mlodzik M, Wilson C. Drosophila tumor suppressor PTEN controls cell size and number by antagonizing the Chico/PI3-kinase signaling pathway. Genes Dev. 1999;13:3244–3258. doi: 10.1101/gad.13.24.3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scanga SE, Ruel L, Binari RC, Snow B, Stambolic V, et al. The conserved PI3'K/PTEN/Akt signaling pathway regulates both cell size and survival in Drosophila. Oncogene. 2000;19:3971–3977. doi: 10.1038/sj.onc.1203739. [DOI] [PubMed] [Google Scholar]
- 28.Ito N, Rubin GM. gigas, a Drosophila homolog of tuberous sclerosis gene product-2, regulates the cell cycle. Cell. 1999;96:529–539. doi: 10.1016/s0092-8674(00)80657-1. [DOI] [PubMed] [Google Scholar]
- 29.Kwon CH, Luikart BW, Powell CM, Zhou J, Matheny SA, et al. Pten regulates neuronal arborization and social interaction in mice. Neuron. 2006;50:377–388. doi: 10.1016/j.neuron.2006.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chalhoub N, Kozma SC, Baker SJ. S6k1 is not required for Pten-deficient neuronal hypertrophy. Brain Res. 2006;1100:32–41. doi: 10.1016/j.brainres.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 31.Martin-Pena A, Acebes A, Rodriguez JR, Sorribes A, de Polavieja GG, et al. Age-independent synaptogenesis by phosphoinositide 3 kinase. J Neurosci. 2006;26:10199–10208. doi: 10.1523/JNEUROSCI.1223-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murthy V, Han S, Beauchamp RL, Smith N, Haddad LA, et al. Pam and its ortholog highwire interact with and may negatively regulate the TSC1.TSC2 complex. J Biol Chem. 2004;279:1351–1358. doi: 10.1074/jbc.M310208200. [DOI] [PubMed] [Google Scholar]
- 33.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 34.Eaton BA, Fetter RD, Davis GW. Dynactin is necessary for synapse stabilization. Neuron. 2002;34:729–741. doi: 10.1016/s0896-6273(02)00721-3. [DOI] [PubMed] [Google Scholar]
- 35.Ang LH, Chen W, Yao Y, Ozawa R, Tao E, et al. Lim kinase regulates the development of olfactory and neuromuscular synapses. Dev Biol. 2006 doi: 10.1016/j.ydbio.2006.01.030. [DOI] [PubMed] [Google Scholar]
- 36.Coyle IP, Koh YH, Lee WC, Slind J, Fergestad T, et al. Nervous wreck, an SH3 adaptor protein that interacts with Wsp, regulates synaptic growth in Drosophila. Neuron. 2004;41:521–534. doi: 10.1016/s0896-6273(04)00016-9. [DOI] [PubMed] [Google Scholar]
- 37.Rao Y. Dissecting Nck/Dock Signaling Pathways in Drosophila Visual System. Int J Biol Sci. 2005;1:80–86. doi: 10.7150/ijbs.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luo L. Actin cytoskeleton regulation in neuronal morphogenesis and structural plasticity. Annu Rev Cell Dev Biol. 2002;18:601–635. doi: 10.1146/annurev.cellbio.18.031802.150501. [DOI] [PubMed] [Google Scholar]
- 39.He XC, Zhang J, Tong WG, Tawfik O, Ross J, et al. BMP signaling inhibits intestinal stem cell self-renewal through suppression of Wnt-beta-catenin signaling. Nat Genet. 2004;36:1117–1121. doi: 10.1038/ng1430. [DOI] [PubMed] [Google Scholar]
- 40.Radimerski T, Montagne J, Hemmings-Mieszczak M, Thomas G. Lethality of Drosophila lacking TSC tumor suppressor function rescued by reducing dS6K signaling. Genes Dev. 2002;16:2627–2632. doi: 10.1101/gad.239102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leevers SJ, Weinkove D, MacDougall LK, Hafen E, Waterfield MD. The Drosophila phosphoinositide 3-kinase Dp110 promotes cell growth. Embo J. 1996;15:6584–6594. [PMC free article] [PubMed] [Google Scholar]
- 42.Luo L, Liao YJ, Jan LY, Jan YN. Distinct morphogenetic functions of similar small GTPases: Drosophila Drac1 is involved in axonal outgrowth and myoblast fusion. Genes Dev. 1994;8:1787–1802. doi: 10.1101/gad.8.15.1787. [DOI] [PubMed] [Google Scholar]
- 43.Montagne J, Stewart MJ, Stocker H, Hafen E, Kozma SC, et al. Drosophila S6 kinase: a regulator of cell size. Science. 1999;285:2126–2129. doi: 10.1126/science.285.5436.2126. [DOI] [PubMed] [Google Scholar]
- 44.Zhang Y, Billington CJ, Jr, Pan D, Neufeld TP. Drosophila target of rapamycin kinase functions as a multimer. Genetics. 2006;172:355–362. doi: 10.1534/genetics.105.051979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Newsome TP, Asling B, Dickson BJ. Analysis of Drosophila photoreceptor axon guidance in eye-specific mosaics. Development. 2000;127:851–860. doi: 10.1242/dev.127.4.851. [DOI] [PubMed] [Google Scholar]
- 46.Blair SS. Imaginal Discs. In: Sullivan W, Ashburner M, Hawley RS, editors. Drosophila Protocols. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press; 2000. pp. 159–173. [Google Scholar]
- 47.Rawson JM, Lee M, Kennedy EL, Selleck SB. Drosophila neuromuscular synapse assembly and function require the TGF-beta type I receptor saxophone and the transcription factor Mad. J Neurobiol. 2003;55:134–150. doi: 10.1002/neu.10189. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Patterning of lamina precursor cells and glia in Pten or Tsc1 mosaic animals. (A–F) Dorsal-posterior views of third instar larval optic lobes stained with anti-Dachshund (lamina precursor cell marker) or anti-Repo (glial cell marker). (A–C) Pten mosaic animals show a significantly larger lamina compared to control animals. This is not seen to the same extent in Tsc1 mosaics. (D–F) Glial cells successfully differentiate and migrate in both Pten and Tsc1 mosaics, however mild patterning defects are apparent and could possibly contribute to the photoreceptor patterning abnormalities observed. All scale bars are 50 microns.
(1.32 MB TIF)