Abstract
Ribonucleases, antibiotics, bacterial toxins, and viruses inhibit protein synthesis, which results in apoptosis in mammalian cells. How the BCL-2 family of proteins regulates apoptosis in response to the shutoff of protein synthesis is not known. Here we demonstrate that an Escherichia coli toxin, MazF, inhibited protein synthesis by cleavage of cellular mRNA and induced apoptosis in mammalian cells. MazF-induced apoptosis required proapoptotic BAK and its upstream regulator, the proapoptotic BH3-only protein NBK/BIK, but not BIM, PUMA, or NOXA. Interestingly, in response to MazF induction, NBK/BIK activated BAK by displacing it from anti-apoptotic proteins MCL-1 and BCL-XL that sequester BAK. Furthermore, NBK/BIK- or BAK-deficient cells were resistant to cell death induced by pharmacologic inhibition of translation and by virus-mediated shutoff of protein synthesis. Thus, the BH3-only protein NBK/BIK is the apical regulator of a BAK-dependent apoptotic pathway in response to shutoff of protein synthesis that functions to displace BAK from sequestration by MCL1 and BCL-XL. Although NBK/BIK is dispensable for development, it is the BH3-only protein targeted for inactivation by viruses, suggesting that it plays a role in pathogen/toxin response through apoptosis activation.
Keywords: NBK/BIK, BAK, mRNA interferase, apoptosis
Apoptosis is a genetically coordinated and conserved cell death process in organisms from Caenorhabditis elegans to vertebrates (Danial and Korsmeyer 2004). Apoptosis is not only essential for successful crafting of complex multicellular tissues during embryonic development and for maintenance of normal cellular homeostasis in adult organisms, but also is needed for elimination of cells damaged by stress or pathogen infection (White 2006). A critical point of apoptosis regulation is controlled by members of the BCL-2 family. The BCL-2 family of proteins can be divided into three different subclasses based on conservation of BCL-2 homology (BH1–4) domains: multidomain anti-apoptotic proteins (BCL-2, BCL-XL, MCL-1, BCL-W, and Bfl-1/A1), multidomain proapoptotic proteins (BAX and BAK), and BH3-only proapoptotic proteins (BID, BAD, BIM, PUMA, NOXA, and NBK/BIK) (Danial and Korsmeyer 2004; Gelinas and White 2005; Willis and Adams 2005). Notably, BH3-only proteins are not able to kill cells that lack BAX and BAK, indicating that BH3-only proteins function upstream of and are dependent on BAX and BAK (Zong et al. 2001).
The proapoptotic BH3-only proteins are the most apical mediators of death induced by cytokine deprivation, activated oncogenes, DNA damage, chemotherapy, and γ-irradiation. For example, BID is a critical mediator of apoptosis mediated by death receptor signaling (Luo et al. 1998), BIM is the determinant of taxane responsiveness (Bouillet et al. 1999; Tan et al. 2005), PUMA and NOXA are central mediators of p53-induced apoptosis (Jeffers et al. 2003; Shibue et al. 2003), and BAD regulates apoptosis mediated by growth factor/cytokine signaling (Datta et al. 2002). In contrast, the cellular responses to specifically trigger the NBK/BIK-mediated apoptotic pathway are poorly characterized.
As in mammalian cells, bacterial cells also regulate cell death. In Escherichia coli cells, growth inhibition and subsequent cell death are mediated through a unique genetic system called “addiction modules” or “toxin–antitoxin modules,” which consist of a pair of genes encoding two components, one for a stable toxin and the other for an unstable antitoxin (Gerdes et al. 2005). The antitoxin and toxin are coexpressed, and their expression and function are negatively autoregulated either by the complex of antitoxin and toxin or by antitoxin alone. When the coexpression of antitoxin and toxin is inhibited, the antitoxin is rapidly degraded by a specific protease, enabling the toxin to act on its target. Such a genetic system for bacterial cell growth inhibition has been reported in a number of E. coli extrachromosomal elements (Gerdes et al. 2005).
One of the addiction modules on the E. coli chromosome, the mazEF system, consists of two adjacent genes, mazE and mazF, located downstream from the relA gene (Aizenman et al. 1996). MazF is a stable toxin, whereas MazE is a labile antitoxin that is quickly degraded by ChpPA, an ATP-dependent serine protease (Aizenman et al. 1996). It has been recently demonstrated that MazF is a sequence-specific endoribonuclease that specifically cleaves E. coli mRNA at the ACA triplet sequence to block de novo protein synthesis, resulting in cell growth arrest and subsequent bacterial cell death (Zhang et al. 2003). Furthermore, it has been shown that MazE is responsible for antagonizing the endoribonuclease activity of MazF (Zhang et al. 2003). The purpose of this addiction module is to provide a competitive growth advantage to the bacteria that encode it. For example, although the mazEF action causes individual cells to die, upon phage infection this gives benefit to the bacterial population by enabling phage exclusion (Hazan and Engelberg-Kulka 2004).
As in bacteria, inhibition of protein synthesis in mammalian cells induced by ribonuclease RNA cleavage, translation silencing with antibiotics, or pathogen infection leads to programmed cell death. In response to viral infection, interferons activate RNaseL that cleaves 18S and 28S ribosomal RNA, which inhibits protein synthesis, eventually inducing apoptosis mediated by cytochrome c release and caspase-3 activation to eliminate virus-infected cells (Silverman 2003). Virus-produced double-stranded RNA (dsRNA) activates RNA-activated protein kinase (PKR), which phosphorylates eukaryotic initiation factor 2 (eIF-2) thereby inhibiting mRNA translation, leading to apoptosis (Gil and Esteban 2000). In turn, viruses have evolved mechanisms to evade these and other host defenses by enabling viral but not host protein synthesis (Barzilai et al. 2005) and by inhibiting apoptosis (Roulston et al. 1999; White 2006). Adenovirus, for example, encodes gene products that block interferon-mediated gene expression, inhibit PKR activation, and prevent apoptosis (Roulston et al. 1999; Cuconati and White 2002). This allows viral but not cellular protein synthesis without cell death. Finally, antibiotics such as cycloheximide (CHX), puromycin, and emetin are part of the bacterial arsenal to inhibit and kill pathogens by targeting protein synthesis by various mechanisms (Meijerman et al. 1999). Although inhibition of protein synthesis by various means is a common weapon to gain a selective advantage and is known to activate the apoptotic response in mammalian cells, the pathway utilized to activate apoptosis is not known.
In this study, we demonstrated that the bacterial toxin MazF, as in bacteria, induces striking degradation of cellular mRNA and inhibition of protein synthesis upstream of apoptosis induction in mammalian cells. MazF expression in mammalian cells causes caspase-3 activation and poly (ADP-ribose) polymerase (PARP) cleavage, which are hallmarks of apoptotic cell death, all of which were blocked by the antitoxin MazE. Interestingly, expression of MazF in immortalized baby mouse kidney (iBMK) cells deficient for bax and/or bak, or BH3-only proapoptotic genes (puma, bim, noxa, and nbk/bik) revealed that NBK/BIK and BAK were required for apoptosis induced by MazF. In addition, NBK/BIK bound to MCL-1 and BCL-XL, thereby promoting the release of BAK from the MCL-1 and BCL-XL inhibitory complexes in response to MazF induction. Moreover, BAX and BAK, BAK, or NBK/BIK-deficiency conferred resistance to cell death induced by pharmacologic inhibition of protein synthesis and by shutoff of protein synthesis induced by viral infection. As the shutoff of protein synthesis is often a cellular response to pathogens, this identifies NBK/BIK as an MCL-1 and BCL-XL antagonist that functions in a BAK-specific apoptotic pathway to control this process.
Results
MazF induces degradation of cellular mRNA in mammalian cells
In E. coli, MazF functions as an mRNA interferase to cleave cellular mRNA, while the antitoxin MazE antagonizes the endoribonuclease activity of MazF (Zhang et al. 2003). These findings led us to explore whether MazF functions as endoribonuclease in mammalian cells. To this end, a tetracycline (Tet)-inducible MazF expression system in T-Rex-293 cells stably expressing the Tet repressor was developed through stable cotransfection of the Tet-inducible MazF expression plasmid with pcDNA3 or the constitutive MazE expression plasmid. RT–PCR with specific primers for the MazF- or MazE-coding region demonstrated Tet-dependent expression of MazF mRNA and constitutive expression of MazE in established T-Rex-293 cell lines (data not shown). Using this system, we first examined whether cellular mRNAs were degraded in mammalian cells upon induction of MazF expression.
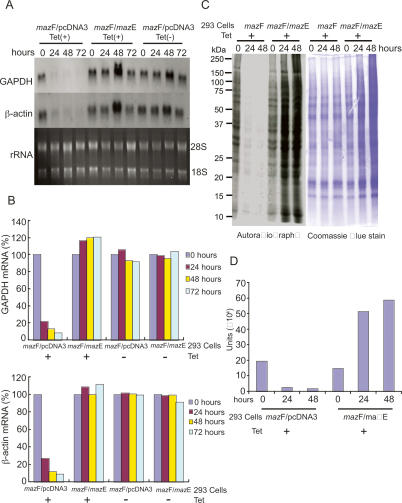
To assess cellular mRNA levels, total RNA was isolated from whole cells of Tet-treated or -untreated T-Rex-293 cells and subjected to Northern blot analysis using human GAPDH and β-actin cDNA probes. GAPDH and β-actin mRNAs were chosen as targets of MazF since GAPDH and β-actin genes are known to be housekeeping genes, both mRNAs exist abundantly, and both are stable under diverse conditions. In addition, GAPDH and β-actin mRNAs have 20 and 22 ACA sequences in their respective protein-coding regions, which are targets for MazF cleavage (Zhang et al. 2003). Data revealed that levels of both mRNAs were dramatically decreased in T-Rex-293 (mazF/pcDNA3) cells after 24 h, and were almost completely lost by 48 h post-induction of MazF (Fig. 1A). In contrast, levels of both mRNAs were maintained by coexpression of MazE with MazF throughout the time course of induction, similarly to the Tet-untreated control (Fig. 1A). The same results were obtained by real-time RT–PCR analysis using specific primers to amplify sequences of human GAPDH or β-actin cDNAs, each containing two ACA sequences (Fig. 1B). The data revealed a striking decrease (>90%) of both mRNAs upon MazF induction for 72 h, whereas constant levels of both mRNAs were maintained in cells expressing both MazE and MazF, similarly to the Tet-untreated control. Furthermore, it was notable that levels of 28S and 18S ribosomal RNA did not change even when MazF was expressed for 72 h (Fig. 1A), indicating that ribosomal RNA interacting with ribosomal proteins in cells may be protected from degradation by MazF. Taken together, the data indicate that MazF specifically eliminates ACA sequence-containing cellular mRNA but not ribosomal RNA, and that MazE neutralizes the MazF endoribonuclease function in mammalian cells, as was found for E. coli cells (Zhang et al. 2003).
Figure 1.
MazF inhibits protein synthesis through degradation of cellular mRNAs in mammalian cells. (A) Northern blot analysis of human GAPDH and β-actin. Total RNA from Tet-treated or -untreated T-Rex-293 cells at the indicated time points was probed with 32P-labeled human GAPDH and β-actin cDNA. 28S and 18S ribosomal RNAs were visualized by agarose-formaldehyde gel electrophoresis followed by ethidium bromide staining. (B) Quantification of mRNA levels. Human GAPDH and β-actin mRNA levels were quantified by real-time RT–PCR. Relative amounts of mRNA were calculated from the fluorescence signal in the 24-, 48-, and 72-h samples as compared with the corresponding 0-h sample. (C) 35S-methionine incorporation in T-Rex-293 cells. 35S-methionine-labeled total protein from Tet-treated T-Rex-293 cells at the indicated time points was subjected to SDS-PAGE and autoradiography (left) and stained with Coomassie blue (right). (D) Quantification of 35S-methionine-labeled proteins. Protein bands on the gel in C were scanned by PhosphorImager Storm 860 (Molecular Dynamics) and signal intensity was calculated.
MazF inhibits protein synthesis in mammalian cells
In E. coli cells, MazF induction causes protein synthesis to be inhibited through degradation of cellular mRNA, indicating that MazF is a general inhibitor for protein synthesis (Zhang et al. 2003). Thus, we investigated the effect of MazF on protein synthesis in T-Rex 293 cells. SDS-PAGE analysis of whole cellular protein from Tet-treated T-Rex-293 (mazF/pcDNA3) and T-Rex-293 (mazF/mazE) cells evaluated for 35S-methionine incorporation at 0, 24, and 48 h post-induction of MazF demonstrated that protein synthesis was strikingly inhibited as rapidly as 24 h (Fig. 1C), clearly indicating that all mRNAs with ACA sequences were degraded by MazF. In contrast, coexpression of MazE clearly prevented the inhibitory effect of MazF on protein synthesis (Fig. 1C,D). Furthermore, the loss of 35S-methionine incorporation was not due to an overall loss of cellular protein in MazF-expressing cells, as total protein levels remained constant over the 48-h induction period (Fig. 1C). The data indicate that MazF functions as an inhibitor of protein synthesis that is repressed by MazE in mammalian cells, as was found in E. coli cells (Zhang et al. 2003).
MazF induces apoptotic cell death in mammalian cells
In E. coli, sequence-specific mRNA interference by MazF leads to rapid cell growth arrest and eventual cell death (Zhang et al. 2003). Thus, we examined the impact of MazF expression, mRNA elimination, and inhibition of protein synthesis on mammalian cell proliferation and viability. Induction of MazF in T-Rex-293 abruptly halted cell accumulation and induced progressive cytopathic effect (CPE) during the time course of MazF induction (Fig. 2A). Notably, the number of attached cells was dramatically decreased at 72 h post-induction of MazF (Fig. 2A). In contrast, in cells where MazE was coexpressed with MazF, cell number and morphology were maintained similarly to cells without MazF induction (MazF Tet [−] and MazF/E Tet [−]) (Fig. 2A). Thus, MazE is capable of completely neutralizing the toxic effect of MazF on mammalian cells.
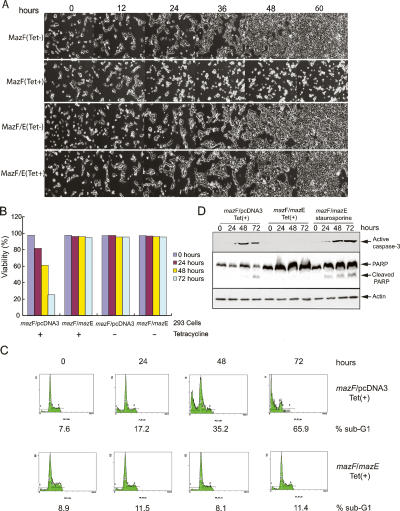
Figure 2.
MazF induces apoptotic cell death in mammalian cells. (A) Identical frames from 4-d time-lapse videos of Tet-treated or -untreated T-Rex-293 cells performed as described previously (Degenhardt et al. 2006). (B) Viability analysis of T-Rex-293 cells in A. Tet-treated or -untreated T-Rex-293 cells at the indicated time points were subjected to trypan blue exclusion. Viability from representatives of three independent experiments was presented as a percent of total cells at time 0. (C) Representative illustration of propidium iodide labeling measured by FACS in Tet-treated T-Rex-293 cells. (D) Western blot analysis with lysates from T-Rex-293 cells. Whole-cell lysates from Tet-treated or staurosporine-treated T-Rex-293 cells at the indicated time points were immunoblotted with an anti-active caspase-3 antibody (top), anti-PARP antibody (middle), and anti-actin antibody (bottom).
The viability of T-Rex-293 cells that express MazF was also quantified by trypan blue exclusion. When MazF expression was induced, the cell viability of T-Rex-293 (mazF/pcDNA3) cells dropped strikingly (Fig. 2B). In contrast, coexpression of MazE with MazF conferred resistance to MazF-mediated killing (Fig. 2B), and cells without MazF or MazE alone also remained viable (Fig. 2B). Viability was also analyzed by fluorescence-activated cytometry for DNA content, and these results showed the same trends as trypan blue exclusion with apoptotic cell death by MazF induction, indicated by accumulation of a sub-G1 peak. The sub-G1 peak in T-Rex-293 (mazF/pcDNA3) increased in a time-dependent manner following Tet treatment, up to 65.9% at 72 h of induction, whereas the sub-G1 peak in T-Rex-293 (mazF/mazE) cells remained low (11.4% at 72 h of induction) (Fig. 2C). These data demonstrate that the MazF toxin induces cell death and that the MazE antitoxin prevents MazF-dependent cell death in mammalian cells.
MazF-induced time-dependent induction of cell death was consistent with the occurrence of MazF-induced apoptosis in human 293 cells. To confirm that cell death induced by MazF was apoptosis, we examined whether caspase-3, one of the executioner caspases in the apoptosis pathway, was activated in T-Rex-293 cells expressing MazF. Western blot analysis using an antibody that recognizes cleaved and activated caspase-3 revealed the presence of the processed active form of caspase-3 in extracts from T-Rex-293 (mazF/pcDNA3) cells treated with Tet (Fig. 2D). Active caspase-3 was also detected in extracts from T-Rex-293 (mazF/mazE) cells treated with staurosporine, a known inducer of apoptosis (Fig. 2D). In contrast, activation of caspase-3 was inhibited by coexpression of MazE with MazF [Tet-treated T-Rex-293 (mazF/mazE) cells] (Fig. 2D). In addition, we examined cleavage of PARP, a substrate of activated caspase-3. Cleaved PARP was detected in extracts from T-Rex-293 (mazF/pcDNA3) cells treated with Tet for 48 h, and the levels further increased at 72 h post-induction of MazF, similarly to staurosporine-treated T-Rex-293 (mazF/mazE) cells. As expected, in extracts of cells expressing both MazE and MazF, where the processed active caspase-3 was not detected, cleaved PARP was also not present (Fig. 2D). Note that although 293 cells express the anti-apoptotic viral BCL-2 homolog E1B 19K that blocks apoptosis by binding to BAX and BAK (Cuconati et al. 2002), the levels are low and insufficient to block apoptosis by exogenous stimuli (also see Fig. 7, below). Taken together, the data clearly demonstrate that MazF toxin, a sequence-specific endoribonuclease from E. coli, induces apoptotic cell death in mammalian cells, which can be prevented by MazE antitoxin coexpression.
Figure 7.
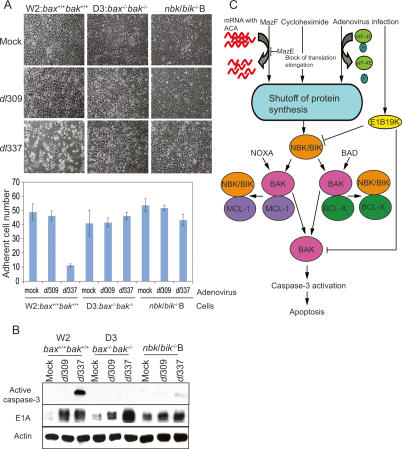
NBK/BIK mediates adenovirus-induced apoptosis. (A, top) Phase-contrast photographs of adenovirus-infected iBMK cells (magnification 100×). (Bottom) Adherent cell number was presented with error bars by counting adherent cells in three different images. (B) Western blot analysis with lysates from viral-infected iBMK cells. Whole-cell lysates from mock-, Ad5dl309-, or Ad5dl337-infected W2, D3, and nbk/bik −/− B cells were immunoblotted with an anti-active caspase-3 antibody (top), anti-E1A antibody (middle), and anti-actin antibody (bottom). (C) Apoptosis pathway induced by shutoff of protein synthesis. See text for details.
Levels of BCL-2 family proteins do not change during MazF-induced apoptosis
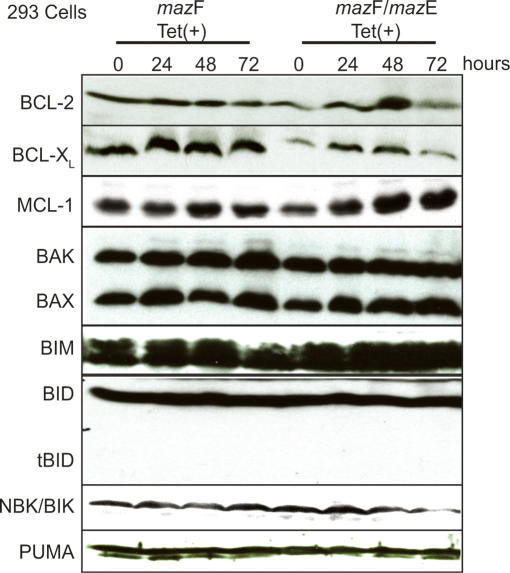
To gain insight into the mechanism of apoptosis induction by MazF upstream of caspase-3 activation, we examined anti-apoptotic (BCL-2, BCL-XL, and MCL-1) or proapoptotic (BAX, BAK, BID, BIM, NBK/BIK, and PUMA) protein levels for modulation by MazF-mediated mRNA cleavage. Cell lysates from Tet-treated T-Rex-293 (mazF/pcDNA3) and T-Rex-293 (mazF/mazE) cells for 0, 24, 48, and 72 h were subjected to Western blot analysis. The levels of BCL-2 family proteins remained unchanged, and truncated BID (tBID) was also undetectable during MazF-induced apoptosis (Fig. 3). These data suggest that loss of anti-apoptotic BCL-2 family members BCL-2, BCL-XL, and MCL-1 or up-regulation of proapoptotic BAX, BAK, BIM, BID, NBK/BIK, and PUMA proteins were not responsible for MazF-mediated apoptosis. Finally, the absence of tBID in MazF-expressing cells suggests that the apoptotic pathway mediated by the death receptor via tBID is also not involved.
Figure 3.
Levels of BCL-2 family proteins remain constant during MazF induction. Whole-cell lysates from Tet-treated T-Rex-293 cells were immunoblotted with antibodies that specifically recognize the anti-apoptotic and proapoptotic proteins indicated in the figure.
MazF-induced apoptosis requires BAK but not BAX
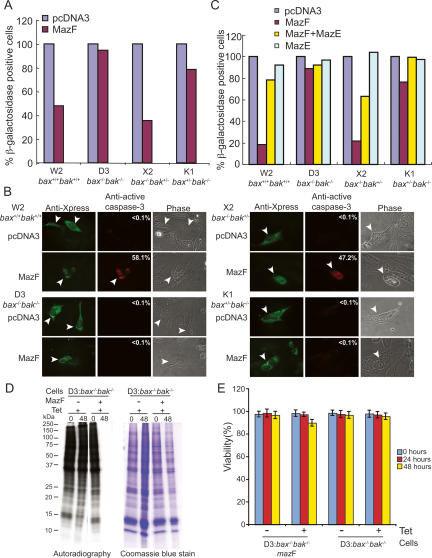
Proapoptotic members of the BCL-2 family, BAX and BAK, play crucial but predominantly functionally redundant roles in the mitochondria-dependent apoptosis pathway induced by numerous apoptotic stimuli downstream from BH3-only proteins (Danial and Korsmeyer 2004; Gelinas and White 2005; Willis and Adams 2005). To test the potential involvement of BAX and/or BAK in MazF-mediated apoptosis, we took advantage of W2 (bax+/−bak+/+), D3 (bax−/−bak−/−), X2 (bax−/−bak+/−), and K1 (bax+/−bak−/−) iBMK cells (Degenhardt et al. 2002a, b, 2006; Nelson et al. 2004; Degenhardt and White 2006). Tumor necrosis factor-α (TNF-α) induces apoptosis in W2, X2, and K1 iBMK cells, whereas D3 iBMK cells are resistant to TNF-α-induced apoptosis and that mediated by many other stimuli (Degenhardt et al. 2002b). First, the MazF expression plasmid pcDNA4/TO/mazF was transiently cotransfected with a LacZ expression plasmid, pcDNA6/His/lacZ, into W2, D3, X2, and K1 cells for 24 and 48 h, and then a β-galactosidase assay was performed to monitor the impact of MazF transient expression. MazF expression for 48 h (Fig. 4A) as well as for 24 h (data not shown) in W2 cells resulted in a significant decrease of β-galactosidase-positive cells. In contrast, little effect on β-galactosidase expression was observed in D3 cells expressing MazF for 24 h (data not shown) and 48 h (Fig. 4A). Interestingly, the number of β-galactosidase-positive cells in X2 cells was significantly decreased, similar to W2 cells, whereas K1 cells were preferentially resistant to MazF, suggesting that BAK deficiency was sufficient to tolerate MazF expression. Moreover, the preservation of β-galactosidase expression by MazF in K1 cells indicates that the loss of expression is due to apoptosis but not elimination of β-galactosidase mRNA.
Figure 4.
BAK function is required for MazF-induced apoptosis. (A) Viability of iBMK cells transiently expressing MazF. W2, D3, X2, and K1 cells transiently coexpressing LacZ and MazF were subjected to a β-galactosidase assay at 48 h post-transfection. β-Galactosidase-positive blue cells were calculated as a percentage of total cells. Data are representative of three independent experiments. (B) Immunofluorescence of activated caspase-3 in iBMK cells. W2, D3, X2, or K1 cells transiently coexpressing LacZ and MazF were costained with anti-Xpress and anti-active caspase-3 antibody. FITC (green) and Rhodamine (red) stains represent cells expressing LacZ and activated caspase-3, respectively. Numbers represent the percentage of activated caspase-3-positive cells. White arrows indicate the corresponding activated caspase-3-positive cells from the matching FITC-stained cells. (C) Viability of iBMK cells transiently coexpressing MazF and MazE. W2, D3, X2, and K1 cells transiently coexpressing LacZ and MazF and/or MazE were subjected to a β-galactosidase assay. β-Galactosidase-positive blue cells were calculated as described above. Data are representative of three independent experiments. (D) 35S-methionine incorporation upon MazF induction in BAX/BAK-deficient D3 cells. 35S-methionine-labeled total protein from Tet-treated D3/mazF and D3 cells at the indicated time points was subjected to SDS-PAGE and autoradiography (left) and stained with Coomassie blue (right). Note the pronounced inhibition of protein synthesis when MazF is expressed. (E) Viability analysis of D3/mazF and D3 cells. Tet-treated or -untreated D3/mazF and D3 cells at the indicated time points were subjected to trypan blue exclusion. Viability was presented with error bars as a percent of total cells. Despite the profound inhibition of 35S-methionine incorporation upon MazF induction (shown in D), defective apoptosis conferred by BAX/BAK deficiency maintained cell viability.
To test if the lack of β-galactosidase expression in W2 and X2 cells was due to apoptosis, cells expressing MazF for 48 h were examined for active caspase-3. Immunofluoresence using anti-active caspase-3 antibody showed the presence of active caspase-3-positive cells (red in Fig. 4B) in 58.1% of W2 cells and 47.2% of X2 cells transiently expressing MazF compared with that in <0.1% of pcDNA3-transfected W2 and X2 cells (Fig. 4B). However, D3 and K1 cells transiently expressing MazF had few cells with activated caspase-3 (<0.1%) (Fig. 4B), indicating that it was primarily the loss of BAK that prevented caspase-3 activation by MazF. Taken together, the data indicate that MazF induces a BAK- but not BAX-dependent mechanism to activate caspase-3 and apoptosis.
To investigate whether MazE can suppress BAK-mediated apoptosis induced by MazF, MazE was transiently coexpressed for 24 and 48 h in W2, D3, X2, or K1 cells with MazF. The result from the β-galactosidase assay showed that MazE expression for 48 h (Fig. 4C) as well as for 24 h (data not shown) in W2 and X2 cells with MazF significantly repressed MazF-induced cell death, similarly to W2 and X2 cells with pcDNA3 or MazE expression plasmid alone. Note that in D3 cells de novo protein synthesis was almost completely blocked by stable MazF induction, whereas viability of MazF-induced D3 cells remained high, similar to that of MazF-uninduced D3 and parental D3 cells (Fig. 4D,E). Thus, MazF expression induced the shutoff of protein synthesis and BAK-mediated apoptosis.
NBK/BIK is required for cell death induced by inhibition of protein synthesis
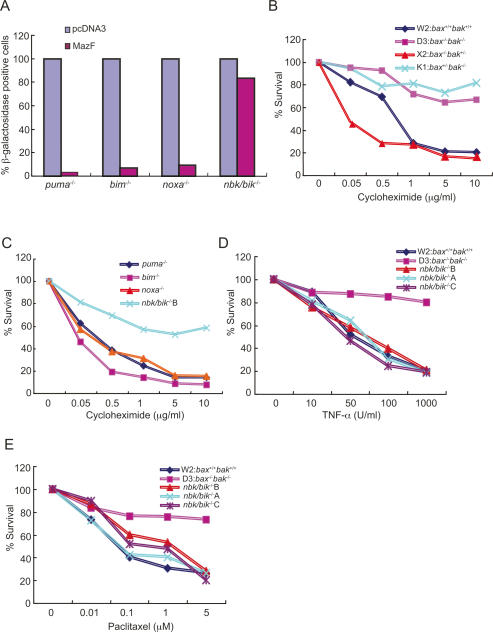
To identify the pathway by which inhibition of protein synthesis triggers BAK-mediated apoptosis, the functional requirement for upstream BH3-only proapoptotic proteins was examined. iBMK cell lines deficient for individual BH3-only proapoptotic proteins (PUMA, BIM, NOXA, and NBK/BIK) (Tan et al. 2005) were tested for resistance to MazF-mediated apoptosis. MazF was transiently coexpressed for 24 and 48 h with LacZ in puma−/−, bim−/−, noxa−/−, or nbk/bik−/− iBMK cells and monitored for β-galactosidase activity. The number of β-galactosidase-positive cells in puma−/−, bim−/−, and noxa−/− cells transiently expressing MazF for 48 h (Fig. 5A) as well as for 24 h (data not shown) significantly decreased (Fig. 5A), similarly to W2 and X2 cells (Fig. 4A). Interestingly, nbk/bik−/− cells were preferentially resistant to MazF-induced cell death (Fig. 5A), similarly to D3 and K1 cells (Fig. 4A). This suggests that MazF-mediated apoptosis requires NBK/BIK that signals through BAK.
Figure 5.
NBK/BIK mediates MazF or CHXinduced cell death. (A) Viability of iBMK cells transiently expressing MazF. nbk/bik−/−, bim−/−, noxa−/−, or puma−/− iBMK cells coexpressing LacZ and MazF were subjected to a β-galactosidase assay. β-Galactosidase-positive blue cells were calculated as a percentage of total cells. Data are representative of three independent experiments. (B,C) Viability of CHX-treated iBMK cells. W2, D3, X2, and K1 cells (B), and nbk/bik −/−B, bim−/−, noxa−/−, or puma−/− cells (C) treated with CHX were subjected to an MTT assay. Data are representative of three independent experiments. (D,E) Viability of TNF-α/CHX- and paclitaxel-treated iBMK cells. W2, D3, and three independent nbk/bik−/− cell lines (A, B, and C) treated with TNF-α/CHX (0.05 μg/mL) (D) and paclitaxel (E) were subjected to an MTT assay. Data are representative of three independent experiments.
To test if apoptosis mediated by general translation inhibition was dependent on specific BH3-only proteins, we tested the apoptotic response of puma−/−, bim−/−, noxa−/−, and nbk/bik−/− iBMK cells to translation inhibition with CHX. In addition to W2, D3, X2, or K1 cells, puma−/−, bim−/−, noxa−/−, or nbk/bik−/− cells were treated with increasing concentrations of CHX (0.05, 0.5, 1.0, 5.0, and 10.0 μg/mL) for 24 h to induce translation inhibition, and viability was assessed. The survival of W2, X2, puma−/−, bim−/−, and noxa−/− cells treated with CHX decreased in a dose-dependent manner (Fig. 5B,C). In contrast, when D3, K1 (Fig. 5B), and nbk/bik−/− (Fig. 5C) cells were treated with CHX, cell viability remained high. In addition, W2 and three independent nbk/bik−/− iBMK cell lines were clearly sensitive to TNF-α- (Fig. 5D) and paclitaxel-induced cell death (Fig. 5E). In contrast, D3 cells were resistant to cell death induced by TNF-α and paclitaxel (Fig. 5D,E). These results clearly indicate that NBK/BIK is the BH3-only proapoptotic protein that is required for cell death incurred by inhibition of protein synthesis in response to MazF-induced mRNA degradation and CHX-induced translation inhibition upstream of BAK. Furthermore, NBK/BIK was not required for TNF-α-mediated apoptosis (Fig. 5D), which signals through tBID (Luo et al. 1998), nor was NBK/BIK required for apoptosis induced by taxanes (Fig. 5E), which is dependent on BIM (Bouillet et al. 1999; Tan et al. 2005). These findings support the role for specific BH3-only proteins controlling the response to discrete apoptotic stimuli.
NBK/BIK binds MCL-1 and BCL-XL, causing the release of BAK
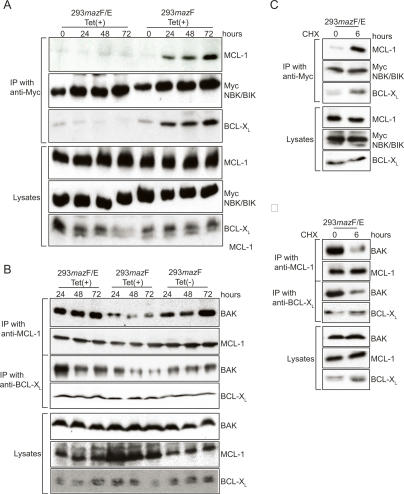
BAK is regulated through activation by BH3-only proteins and sequestered by anti-apoptotic proteins (Cuconati et al. 2003; Gelinas and White 2005; Willis and Adams 2005; Willis et al. 2005). Therefore, we examined how NBK/BIK regulates BAK-dependent apoptosis induced by MazF. 293mazF or 293mazF/mazE cells were transiently transfected with Myc-tagged NBK/BIK expression plasmid for 24 h and then treated with Tet to induce MazF. Immunoprecipitations were carried out using anti-Myc antibody. An anti-Myc antibody coimmunoprecipitated endogenous MCL-1 or BCL-XL with Myc-NBK/BIK in Tet-treated 293mazF cells (Fig. 6A), indicating that NBK/BIK interacts with MCL-1 and BCL-XL in response to apoptosis induced by MazF. In contrast, no NBK-BIK-MCL-1 or -BCL-XL interaction is observed in MazF/MazE-expressing cells and is observed only when MazF is expressed without its inhibitor MazE (Fig. 6A). Furthermore, immunoprecipitation with anti-MCL-1 and anti-BCL-XL antibody revealed that both endogenous MCL-1 and BCL-XL interact with endogenous BAK in cells without MazF and in those coexpressing MazE with MazF (Fig. 6B), whereas BAK is released from complex with both MCL-1 and BCL-XL upon MazF induction and activation of the apoptotic response (Fig. 6B). In addition, NBK/BIK interaction with both endogenous MCL-1 and BCL-XL (Fig. 6C) leads to displacement and release of BAK from these complexes in response to cell death induced by CHX in 293mazF/mazE cells (Fig. 6D). Note that in contrast to the apoptotic pathway stimulated by DNA damage where NOXA displaces BAK from MCL-1 (Cuconati et al. 2003; Nijhawan et al. 2003; Willis et al. 2005), the NBK/BIK-mediated displacement of BAK from MCL-1 does not involve degradation of MCL-1 (Fig. 3). Thus, these data clearly indicate that NBK/BIK activates BAK by displacing it from MCL-1 or BCL-XL when apoptosis is induced by MazF as well as by pharmacologic means.
Figure 6.
NBK/BIK interacts MCL-1 and BCL-XL to activate BAK in MazF- and CHX-induced apoptosis. (A) Immunoprecipitation (IP) with lysates from 293 cells transiently expressing Myc-NBK/BIK. Immunoprecipitation of MCl-1 and Myc-NBK/BIK, BCL-XL, and Myc-NBK/BIK from the soluble fraction of Tet-treated 293 cells transiently expressing Myc-NBK/BIK was carried out with anti-Myc antibody. Western blotting was carried out on precipitated samples and on lysates collected before immunoprecipitation with anti-MCL-1, anti-BCL-XL or anti-Myc antibody. (B) Immunoprecipitation (IP) with lysates from Tet-treated or untreated 293 cells. Immunoprecipitation of BAK, MCL-1, and BCL-XL from the soluble fraction of Tet-treated or -untreated 293 cells was carried out with anti-MCL-1 and anti-BCL-XL antibodies. Western blotting was carried out on precipitated samples and on lysates collected before immunoprecipitation with anti-MCL-1, anti-BCL-XL, and anti-BAK antibodies. (C,D) Immunoprecipitation (IP) with lysates from 1 μg/mL CHX-treated 293 cells transiently expressing Myc-NBK/BIK. Immunoprecipitation was carried out as indicated in A and B.
NBK/BIK is a mediator of apoptosis induced by adenovirus infection
Productive adenovirus infection abrogates host cell protein synthesis and triggers induction of apoptosis. To investigate whether NBK/BIK is required for adenovirus-induced apoptosis concomitant with shutoff of host cell protein synthesis, W2, bax−/−bak−/− D3, and nbk/bik−/− iBMK cells were infected with wild-type adenovirus type 5 (Ad5dl309) and an E1B 19K gene deletion (anti-apoptotic vBCL-2) mutant (Ad5dl337), and monitored for CPE. Cell morphology and adherent cell number of Ad5dl309-infected W2, D3, and nbk/bik−/− cells was maintained, similar to that of mock-infected W2, D3, and nbk/bik−/− cells, indicating that E1B 19K prevents adenovirus-induced apoptosis (Fig. 7A). Infection of W2 cells with Ad5dl337 cells resulted in almost complete destruction of the monolayer at 48 h post-infection, indicative of apoptosis leading to a decrease of adherent cell number (Fig. 7A), as expected (Cuconati et al. 2002). In contrast, Ad5dl337-infected D3 cells were resistant to adenovirus-induced apoptosis (Fig. 7A), as expected (Cuconati et al. 2002). Interestingly, apoptotic CPE was not observed in Ad5dl337-infected nbk/bik−/− cells, indicative of the preservation of cell viability (Fig. 7).
Apoptosis of infected iBMK cells was assessed by monitoring activation of caspase-3. As expected, in W2 and D3 cells infected with Ad5dl309, the processed active form of caspase-3 was undetectable (Fig. 7B). The activated caspase-3 was also not seen in nbk/bik−/− cells infected with Ad5dl309 (Fig. 7B). Abundant levels of activated caspase-3 were present in extracts from Ad5dl337-infected W2 cells, whereas activated caspase-3 did not appear in extracts from Ad5dl337-infected D3 cells (Fig. 7B), as expected (Cuconati et al. 2002). Interestingly, the amount of activated caspase-3 detected in nbk/bik−/− cells infected with Ad5dl337 was significantly less than that detected in Ad5dl337-infected W2 cells (Fig. 7B), which was consistent with the resistance of nbk/bik−/− cells to Ad5dl337-induced cell death (Fig. 7A). To ensure that iBMK cells were infected and expressing viral proteins, cell lysates were analyzed for E1A protein levels. Since E1A was used to immortalize the iBMK cells, low levels of E1A protein expression were detected in uninfected cells (Fig. 7B). E1A levels increased significantly in Ad5dl309-infected W2, D3, and nbk/bik−/− cells (Fig. 7B), indicating that virally infected cells expressed E1A from the viral genome. Higher levels of E1A were observed in Ad5dl337-infected D3 and nbk/bik−/− cells (Fig. 7B), indicating that production of E1A in Ad5dl337-infected D3 and nbk/bik−/− cells was due to absence of apoptosis by deficiency of BAX, BAK, and NBK/BIK. Thus, data indicate that NBK/BIK is the BH3-only protein that regulates apoptosis induced by adenovirus infection upstream of BAX and BAK.
Discussion
Protein synthesis, apoptosis, and the host response to pathogens
Degradation of mRNA and consequent shutoff of protein synthesis are evolutionally conserved mechanisms in the arsenal of defense responses shared among bacteria through mammals. Bacteria, for example, use the MazF/E addiction module such that sequence-specific mRNA cleavage and degradation inhibits protein synthesis to eliminate competitive bacteria to gain a selective growth advantage (Aizenman et al. 1996; Zhang et al. 2003; Gerdes et al. 2005). In mammals, virus infection activates the interferon response and RNaseL-mediated degradation of 28S and 18S ribosomal RNAs, which inhibits protein synthesis as part of the host antiviral response (Silverman 2003). Furthermore, PKR activated by virus-produced dsRNA phosphorylates eIF-2α, leading to inhibition of host protein translation, and eventually to apoptosis that eliminates virus-infected cells (Gil and Esteban 2000). Adenovirus prevents host cell protein synthesis in large part by causing the dephosphorylation and inactivation of the translation initiation factor cap-binding subunit elF-4E (Zhang et al. 1994). This favors translation of uncapped viral mRNA at the expense of host cellular mRNAs. This and other viral functions trigger both p53-dependent and -independent apoptosis that are inhibited by expression of a virally encoded anti-apoptotic BCL-2 homolog E1B 19K (Roulston et al. 1999; Cuconati and White 2002). Viruses, in turn, encode countermeasures circumventing PKR, interferons, mRNA degradation, and translation inhibition that preferentially enable the synthesis of viral proteins. In mammals, however, inhibition of host protein synthesis during infection also leads to apoptosis as the ultimate defense mechanism, and pathogens commonly encode elegant means to disable to this apoptotic response within their repertoire of countermeasures. Among the pathogen-encoded anti-apoptotic mechanisms are viral BCL-2 homologs, BH3-only protein antagonists, BAX and BAK inhibitors (Cuconati and White 2002; Goldmacher 2005), BH3-only protein degradation mechanisms (Fischer et al. 2004; Dong et al. 2005), viral inhibitor of apoptosis proteins (IAPs), and caspase inhibitors (Roulston et al. 1999). How inhibition of protein synthesis is functionally linked to apoptosis, however, has not been clear.
Role of NBK/BIK and BAK in the apoptotic response to protein synthesis inhibition
BH3-only protein’s function is linked to specific stimuli and/or specific cell types, and likely represents one of the control points that provide the specificity to apoptotic signaling pathways that ultimately converge on BAX and BAK. Despite being the first BH3-only protein to be identified (Boyd et al. 1995; Han et al. 1996), the apoptotic pathway where NBK/BIK, also known as BLK (Hedge et al. 1998), plays a role was not known. NBK/BIK is dispensable for murine development (Coultas et al. 2004), partly due to functional redundancy with other BH3-only proteins. Indeed, NBK/BIK or BIM is required for normal regulation of murine spermatogenesis (Coultas et al. 2005). Alternatively, NBK/BIK function may be more significant outside the context of normal development, instead playing a role in apoptosis in pathological conditions, perhaps in concert with other BH3-only proteins. NBK/BIK expression is associated with apoptosis in response to estrogen withdrawal in human breast cancer cells (Hur et al. 2004), and cell surface IgM ligation in human B cell lymphomas also induces NBK/BIK accumulation that is associated with apoptosis (Jiang and Clark 2001). Ectopic NBK/BIK expression sensitizes cancer cells to cell death by other agents (Radetzki et al. 2002; Zou et al. 2002; Lo et al. 2005), and its loss of expression is associated with renal cell carcinoma, suggesting a possible role in the acquisition of apoptosis resistance in tumor cells (Sturm et al. 2006). In vitro peptide binding and transient co-overexpression experiments have suggested that NBK/BIK binds to and can antagonize anti-apoptotic BCL-2, BCL-XL, and MCL-1 (Chen et al. 2005; Certo et al. 2006) but does not directly interact with or regulate BAX or BAK (Letai et al. 2002). However, the physiological context of these potential protein interactions was not known. Our observations suggest that NBK/BIK functions in response to protein synthesis inhibition to disrupt BAK–MCL-1 and BAK–BCL-XL interaction to activate BAK. It has recently become apparent that apoptosis activation requires inactivation of both the MCL-1 and BCL-XL inhibitory mechanisms. This is exemplified by the functional cooperation between NOXA, which antagonizes MCL-1, and BAD, which antagonizes BCL-XL and is required for BAK activation and apoptosis (Willis et al. 2005). By binding and antagonizing both MCL-1 and BCL-XL, NBK/BIK is sufficient for apoptosis induction (Fig. 7C). In addition to NBK/BIK, there is the possibility of an additional contribution of other BH3-only proteins to apoptosis induced by the inhibition of protein synthesis.
Function of NBK/BIK in the endoplasmic reticulum
Unlike other BH3-only proteins, NBK/BIK is not localized to mitochondria, but rather is integrated exclusively in the ER membrane, yet it regulates cytochrome c release and signaling of apoptosis from mitochondria (Han et al. 1996; Germain et al. 2002). One possibility is that the indirect regulation of mitochondrial apoptotic events arises from sequestration of anti-apoptotic BCL-2-like proteins away from mitochondria in the endoplasmic reticulum. Alternatively, NBK/BIK has been reported to regulate Ca++ release from endoplasmic reticulum stores in a BAX- and BAK-dependent fashion, which may indirectly affect mitochondrial apoptotic function (Mathai et al. 2005). The endoplasmic reticulum is a prominent location for cellular protein synthesis, placing NBK/BIK in an appropriate intracellular compartment for regulating the apoptotic response to the perturbation of protein synthesis.
How NBK/BIK is activated by mRNA degradation and inhibition of protein synthesis is still not known. Although NBK/BIK is regulated at the level of transcription and protein stability, no up-regulation of NBK/BIK by MazF was observed (Fig. 3). Evidence suggests that the proapoptotic activity of NBK/BIK is stimulated by phosphorylation (Verma et al. 2001). However, a slower migrating band corresponding to phosphorylated NBK/BIK was not detected during MazF-induced apoptosis (Fig. 3), suggesting that phosphorylation of NBK/BIK may not be involved in MazF-induced apoptosis. Alternatively, inhibition of protein synthesis could cause preferential loss of an anti-apoptotic NBK/BIK antagonist, resulting in the stimulation of its apoptotic activity. However, MazF did not reduce the levels of any apoptotic regulators examined (Fig. 3). Thus, the identity of a potential NBK/BIK-negative regulator remains to be determined.
Inhibition of NBK/BIK by viral homologs of BCL-2
Adenovirus infection induces E1A-mediated p53-dependent or -independent apoptosis (Teodoro et al. 1995; White 2006). The p53-dependent apoptotic pathway in infected cells is caused by E1A expression and interaction with RB and p300, and activation of a DNA damage response (Chiou and White 1997; Samuelson and Lowe 1997; Cuconati et al. 2003; White 2006). This triggers p53 accumulation and target gene expression, and BAX- and BAK-mediated apoptosis (White 2006). The E1B 55K protein binds directly to and inhibits p53, substantially mitigating both p53’s growth arrest and apoptosis activity, but the E1B 19K protein is still sufficient to inhibit all of the proapoptotic activity of p53 indirectly by binding to and inhibiting BAX and BAK (White 2006). These activities within E1B ensure inactivation of p53 function in infected cells.
The trigger for the p53-independent pathway in infected cells is likely due to both the p53-indepedent aspects of the DNA damage response and the shutoff of host cell protein synthesis. The p53-independent component of the DNA damage response causes the degradation of MCL-1 in proteasomes, resulting in the release of BAK from MCL-1, which is necessary but not sufficient for BAK activation (Cuconati et al. 2003). Normally, apoptosis activation has no consequence to virus-infected cells as adenovirus encodes an anti-apoptotic BCL-2 homolog, E1B 19K, to inhibit apoptosis. Following the release of BAK from MCL-1, E1B 19K binds to activated BAK, and by doing so blocks BAX activation and apoptosis, although BAX can functionally substitute for the absence of BAK (Cuconati et al. 2002). The capacity for E1B 19K to bind and inhibit both BAX and BAK is important due to their functional redundancy, but also because some antiviral pathways such as those mediated by TNF-α result in the generation of tBID, which binds and activates both BAX and BAK (Perez and White 2000; Wei et al. 2000). The specific targeting of activated BAX and BAK by E1B 19K may distinguish it from BCL-2 and BCL-XL, which has been reported to bind to BID and other BH3-only proteins upstream of BAX and BAK (Cheng et al. 2001). Interestingly, E1B 19K binds and inhibits NBK/BIK but not other BH3-only proteins, but the specific pathway this was important for was not known (Boyd et al. 1995; Han et al. 1996). The role of NBK/BIK in mediating apoptosis in response to the inhibition of protein synthesis may be the reason for E1B 19K singling out this specific BH3-only protein for inhibition. Interestingly, the viral BCL-2 homolog encoded by Epstein-Barr virus, BHRF1, also binds and inhibits NBK/BIK (Boyd et al. 1995; Elangovan and Chinnadurai 1997).
Utility of apoptosis-resistant cells able to tolerate MazF expression and mRNA elimination
In E. coli cells, MazF induction causes the “quasi-dormancy” state, under which the cells are still fully capable of protein synthesis if all ACA sequences in the mRNA for a specific protein are altered to MazF-uncleavable sequences, allowing synthesis of a single protein in cells (Suzuki et al. 2005). Because the single protein production (SPP) system can eliminate almost all background cellular protein synthesis (Suzuki et al. 2005), it is anticipated that it will enable NMR structural studies on proteins in living cells under truly physiological conditions. Due to the profound defect in apoptosis in response to mRNA degradation and inhibition of protein synthesis (Fig. 4D) by MazF, nbk/bik−/−, K1, or D3 cells that are capable of maintaining viability (Fig. 4E), this raises the possibility for SPP of ACA-less mRNAs in mammalian cells.
Materials and methods
Plasmids
The Tet-inducible MazF expression plasmid, pcDNA4/TO/mazF, or the MazE expression plasmid, pcDNA3/mazE, was constructed by insertion of the MazF- or MazE-coding region into pcDNA4/TO or pcDNA3 (Invitrogen), respectively. The pcDNA6/His/LacZ was purchased from Invitrogen. The Myc-NBK/BIK expression plasmid pcDNA3-myc-NBK/BIK was constructed as previously described (Han et al. 1996)
Cell lines and viral infection
Cell lines were maintained using standard tissue culture techniques with the exception that all iBMK cell lines were grown as previously described (Degenhardt et al. 2002b). Human T-Rex-293 cell lines that stably express MazF alone (mazF/pcDNA3) and that coexpress MazE with MazF (mazF/mazE), and BAX/BAK-deficient D3/mazF iBMK cells that stably express MazF alone were established through stable cotransfection with the Tet repressor expression plasmid (pcDNA6/TR) and either pcDNA4/TO/mazF and pcDNA3 or pcDNA4/TO/mazF and pcDNA3/mazE by PolyFect Transfection Reagent (Qiagen, Inc.) according to the manufacturer’s instructions, and were selected as follows: pcDNA6/TR, 5 μg/mL blasticidin; pcDNA4TO/mazF, 40 μg/mL zeocin; pcDNA3/mazE, 0.5 μg/mL geneticin. Infection of iBMK cells with the wild-type adenovirus (Ad5dl309) or the E1B 19K gene deletion mutant (Ad5dl337) was performed as previously described (Cuconati et al. 2002).
Viability assays
The viability of T-Rex-293 cell lines was determined by trypan blue exclusion and FACS analysis as previously described (Degenhardt et al. 2002b). β-Galactosidase assays to determine the viability of iBMK cells coexpressing LacZ and MazF were preformed as previously described (Han et al. 1996). β-Galactosidase-positive blue cells and the total number of cells (∼200) were independently counted. 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay to measure the viability of iBMK cells treated with paclitaxel, CHX, or both mouse TNF-α and CHX was performed as previously described (Ioffe et al. 2004).
Western blotting, immunofluorescence, and immunoprecipitation
Western blotting, immunofluorescence, and immunoprecipitation were performed as previously described (Han et al. 1996; Perez and White 2000) with the following antibodies: anti-active caspase-3 rabbit polyclonal antibody (Cell Signaling Technology); anti-BAX rabbit polyclonal antibody, anti-BAK rabbit polyclonal antibody (NT) (Upstate Biotechnology, Inc.); anti-BID goat polyclonal antibody (R&D Systems, Inc.); anti-BIM rabbit polyclonal antibody (Alexis Biochemical); anti-BCL-2 hamster monoclonal antibody; anti-PARP monoclonal antibody (PharMingen); anti-BCL-XL mouse monoclonal antibody (Trevigen); anti-MCL-1 rabbit polyclonal antibody (Stressgen Biotechnologies); anti-PUMA rabbit polyclonal antibody (Nelson et al. 2004); anti-E1A monoclonal antibody; anti-actin monoclonal antibody (Oncogene Research Products); anti-Xpress monoclonal antibody, anti-Myc monoclonal antibody (Invitrogen). An antibody directed toward human NBK/BIK was generated by the expression of a GST-tagged human NBK/BIK fusion protein encoding an N-terminal 78-amino-acid region in bacteria and immunization of rabbit (Cocalico).
RNA analysis and 35S-methionine incorporation
Northern blot analysis and real-time RT–PCR were preformed as previously described (Cuconati et al. 2003; Zhang et al. 2003). For Northern analysis, GAPDH or β-actin mRNA was visualized with 32P-labeled human GAPDH or β-actin cDNA. Real-time RT–PCR utilized human GAPDH primers and probe obtained from Applied Biosystems. β-Actin primer and probe sequences were 5′-GGGAAATCGTGCGTGACATT-3′ and 5′-CGGATGTCCACGTCACACTT-3′, and 5′-ATCACCATTGG CAATGAGCGGTTCC-3′, respectively. 35S-methionine incorporation in Tet-treated T-Rex-293 and D3/mazF cells was performed by incubation for 1 h in fresh methionine-free DMEM containing 10 μCi/mL 35S-methionine.
Acknowledgments
We thank Dr. Danny Reinberg and Alejandro Vaquero for their assistance in constructing Tet-inducible T-Rex-293 cell lines, and Chandreyee Mukherjee for production of the rabbit polyclonal antibody directed against NBK/BIK. This work was partially supported by Takara Bio, Inc. (to M.I.), and by a grant from the National Cancer Institute (R37CA53370) (to E.W.).
Footnotes
Article published online ahead of print. Article and publication date are online at http://www.genesdev.org/cgi/doi/10.1101/gad.1522007
References
- Aizenman E., Engelberg-Kulka H., Glaser G., Engelberg-Kulka H., Glaser G., Glaser G. An Escherichia coli chromosomal “addiction molecule” regulated by 3′, 5′-bisspyrophosphate: A model for programmed bacterial cell death. Proc. Natl. Acad. Sci. 1996;93:6059–6063. doi: 10.1073/pnas.93.12.6059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzilai A., Zivony-Elbom I., Sarid R., Noah E., Frenkel N., Zivony-Elbom I., Sarid R., Noah E., Frenkel N., Sarid R., Noah E., Frenkel N., Noah E., Frenkel N., Frenkel N. The herpes simplex virus type 1 vhs-UL41 gene secures viral replication by temporarily evading apoptotic cellular response to infection: Vhs-UL41 activity might require interactions with elements of cellular mRNA degradation machinery. J. Virol. 2005;80:505–513. doi: 10.1128/JVI.80.1.505-513.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouillet P., Metcalf D., Huang D.C.S., Tarlinton D.M., Kay T.W.H., Kontgen F., Adams J.M., Strasser A., Metcalf D., Huang D.C.S., Tarlinton D.M., Kay T.W.H., Kontgen F., Adams J.M., Strasser A., Huang D.C.S., Tarlinton D.M., Kay T.W.H., Kontgen F., Adams J.M., Strasser A., Tarlinton D.M., Kay T.W.H., Kontgen F., Adams J.M., Strasser A., Kay T.W.H., Kontgen F., Adams J.M., Strasser A., Kontgen F., Adams J.M., Strasser A., Adams J.M., Strasser A., Strasser A. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science. 1999;286:1735–1738. doi: 10.1126/science.286.5445.1735. [DOI] [PubMed] [Google Scholar]
- Boyd J.M., Gallo G.J., Elangovan B., Houghton A.B., Malstorm S., Avery B.J., Ebb R.G., Subramanian T., Chittenden T., Lutz R.J., Gallo G.J., Elangovan B., Houghton A.B., Malstorm S., Avery B.J., Ebb R.G., Subramanian T., Chittenden T., Lutz R.J., Elangovan B., Houghton A.B., Malstorm S., Avery B.J., Ebb R.G., Subramanian T., Chittenden T., Lutz R.J., Houghton A.B., Malstorm S., Avery B.J., Ebb R.G., Subramanian T., Chittenden T., Lutz R.J., Malstorm S., Avery B.J., Ebb R.G., Subramanian T., Chittenden T., Lutz R.J., Avery B.J., Ebb R.G., Subramanian T., Chittenden T., Lutz R.J., Ebb R.G., Subramanian T., Chittenden T., Lutz R.J., Subramanian T., Chittenden T., Lutz R.J., Chittenden T., Lutz R.J., Lutz R.J., et al. Bik, a novel death-inducing protein shares a distinct sequence motif with Bcl-2 family proteins and interacts with viral and cellular survival-promoting proteins. Oncogene. 1995;11:1921–1928. [PubMed] [Google Scholar]
- Certo M., Del Gaizo V., Nishino M., Wei G., Korsmeyer S., Armstrong S.A., Letai A., Del Gaizo V., Nishino M., Wei G., Korsmeyer S., Armstrong S.A., Letai A., Nishino M., Wei G., Korsmeyer S., Armstrong S.A., Letai A., Wei G., Korsmeyer S., Armstrong S.A., Letai A., Korsmeyer S., Armstrong S.A., Letai A., Armstrong S.A., Letai A., Letai A. Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer Cell. 2006;9:351–365. doi: 10.1016/j.ccr.2006.03.027. [DOI] [PubMed] [Google Scholar]
- Chen L., Willis S.N., Wei A., Smith B.J., Fletcher J.I., Hinds M.G., Colman P.M., Day C.L., Adams J.M., Huang D.C., Willis S.N., Wei A., Smith B.J., Fletcher J.I., Hinds M.G., Colman P.M., Day C.L., Adams J.M., Huang D.C., Wei A., Smith B.J., Fletcher J.I., Hinds M.G., Colman P.M., Day C.L., Adams J.M., Huang D.C., Smith B.J., Fletcher J.I., Hinds M.G., Colman P.M., Day C.L., Adams J.M., Huang D.C., Fletcher J.I., Hinds M.G., Colman P.M., Day C.L., Adams J.M., Huang D.C., Hinds M.G., Colman P.M., Day C.L., Adams J.M., Huang D.C., Colman P.M., Day C.L., Adams J.M., Huang D.C., Day C.L., Adams J.M., Huang D.C., Adams J.M., Huang D.C., Huang D.C. Differential targeting of pro-survival Bcl-2 proteins by BH3-only ligands allows complementary apoptotic function. Mol. Cell. 2005;17:393–403. doi: 10.1016/j.molcel.2004.12.030. [DOI] [PubMed] [Google Scholar]
- Cheng E.H., Wei M.C., Weiler S., Flavell R.A., Mak T.W., Lindsten T., Korsmeyer S.J., Wei M.C., Weiler S., Flavell R.A., Mak T.W., Lindsten T., Korsmeyer S.J., Weiler S., Flavell R.A., Mak T.W., Lindsten T., Korsmeyer S.J., Flavell R.A., Mak T.W., Lindsten T., Korsmeyer S.J., Mak T.W., Lindsten T., Korsmeyer S.J., Lindsten T., Korsmeyer S.J., Korsmeyer S.J. BCL-2, BCL-XL sequesters BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis. Mol. Cell. 2001;8:705–711. doi: 10.1016/s1097-2765(01)00320-3. [DOI] [PubMed] [Google Scholar]
- Chiou S.K., White E., White E. p300 binding by E1A cosegregates with p53 induction but is dispensable for apoptosis. J. Virol. 1997;71:3515–3525. doi: 10.1128/jvi.71.5.3515-3525.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coultas L., Bouillet P., Stanley E.G., Brodnicki T.C., Adams J.M., Strasser A., Bouillet P., Stanley E.G., Brodnicki T.C., Adams J.M., Strasser A., Stanley E.G., Brodnicki T.C., Adams J.M., Strasser A., Brodnicki T.C., Adams J.M., Strasser A., Adams J.M., Strasser A., Strasser A. Proapoptotic BH3-only Bcl-2 family member Bik/Blk/Nbk is expressed in hemopoietic and endothelial cells but is redundant for their programmed cell death. Mol. Cell. Biol. 2004;24:1570–1581. doi: 10.1128/MCB.24.4.1570-1581.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coultas L., Bouillet P., Loveland K.L., Meachem S., Perlman H., Adams J.M., Strasser A., Bouillet P., Loveland K.L., Meachem S., Perlman H., Adams J.M., Strasser A., Loveland K.L., Meachem S., Perlman H., Adams J.M., Strasser A., Meachem S., Perlman H., Adams J.M., Strasser A., Perlman H., Adams J.M., Strasser A., Adams J.M., Strasser A., Strasser A. Concomitant loss of proapoptotic BH3-only Bcl-2 antagonists Bik and Bim arrests spermatogenesis. EMBO J. 2005;24:3963–3973. doi: 10.1038/sj.emboj.7600857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuconati A., White E., White E. Viral homologs of BCL-2: Role of apoptosis in the regulation of virus infection. Genes & Dev. 2002;16:2465–2478. doi: 10.1101/gad.1012702. [DOI] [PubMed] [Google Scholar]
- Cuconati A., Degenhardt K., Sundararajan R., Anschel A., White E., Degenhardt K., Sundararajan R., Anschel A., White E., Sundararajan R., Anschel A., White E., Anschel A., White E., White E. Bak and Bax function to limit adenovirus replication through apoptosis induction. J. Virol. 2002;76:4547–4558. doi: 10.1128/JVI.76.9.4547-4558.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuconati A., Mukherjee C., Perez D., White E., Mukherjee C., Perez D., White E., Perez D., White E., White E. DNA damage response and MCL-1 destruction initiate apoptosis in adenovirus-infected cells. Genes & Dev. 2003;17:2922–2932. doi: 10.1101/gad.1156903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danial N.N., Korsmeyer S.J., Korsmeyer S.J. Cell death: Critical control points. Cell. 2004;116:205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- Datta S.R., Ranger A.M., Lin M.Z., Sturgill J.F., Ma Y.C., Cowan C.W., Dikkes P., Korsmeyer S.J., Greenberg M.E., Ranger A.M., Lin M.Z., Sturgill J.F., Ma Y.C., Cowan C.W., Dikkes P., Korsmeyer S.J., Greenberg M.E., Lin M.Z., Sturgill J.F., Ma Y.C., Cowan C.W., Dikkes P., Korsmeyer S.J., Greenberg M.E., Sturgill J.F., Ma Y.C., Cowan C.W., Dikkes P., Korsmeyer S.J., Greenberg M.E., Ma Y.C., Cowan C.W., Dikkes P., Korsmeyer S.J., Greenberg M.E., Cowan C.W., Dikkes P., Korsmeyer S.J., Greenberg M.E., Dikkes P., Korsmeyer S.J., Greenberg M.E., Korsmeyer S.J., Greenberg M.E., Greenberg M.E. Survival factor-mediated BAD phosphorylation raises the mitochondrial threshold for apoptosis. Dev. Cell. 2002;3:631–643. doi: 10.1016/s1534-5807(02)00326-x. [DOI] [PubMed] [Google Scholar]
- Degenhardt K., White E., White E. A mouse model system to genetically dissect the molecular mechanisms regulating tumorigenesis. Clin. Cancer Res. 2006;12:5298–5304. doi: 10.1158/1078-0432.CCR-06-0439. [DOI] [PubMed] [Google Scholar]
- Degenhardt K., Chen G., Lindsten T., White E., Chen G., Lindsten T., White E., Lindsten T., White E., White E. BAX and BAK mediate p53-independent suppression of tumorigenesis. Cancer Cell. 2002a;2:193–203. doi: 10.1016/s1535-6108(02)00126-5. [DOI] [PubMed] [Google Scholar]
- Degenhardt K., Sundararajan R., Lindsten T., Thompson C., White E., Sundararajan R., Lindsten T., Thompson C., White E., Lindsten T., Thompson C., White E., Thompson C., White E., White E. Bax and Bak independently promote cytochrome c release from mitochondria. J. Biol. Chem. 2002b;277:14127–14134. doi: 10.1074/jbc.M109939200. [DOI] [PubMed] [Google Scholar]
- Degenhardt K., Mathew R., Beaudoin B., Bray K., Anderson D., Chen G., Mukherjee C., Shi Y., Gelinas C., Fan Y., Mathew R., Beaudoin B., Bray K., Anderson D., Chen G., Mukherjee C., Shi Y., Gelinas C., Fan Y., Beaudoin B., Bray K., Anderson D., Chen G., Mukherjee C., Shi Y., Gelinas C., Fan Y., Bray K., Anderson D., Chen G., Mukherjee C., Shi Y., Gelinas C., Fan Y., Anderson D., Chen G., Mukherjee C., Shi Y., Gelinas C., Fan Y., Chen G., Mukherjee C., Shi Y., Gelinas C., Fan Y., Mukherjee C., Shi Y., Gelinas C., Fan Y., Shi Y., Gelinas C., Fan Y., Gelinas C., Fan Y., Fan Y., et al. Autophagy promotes tumor cell survival and restricts necrosis, inflammation, and tumorigenesis. Cancer Cell. 2006;10:51–64. doi: 10.1016/j.ccr.2006.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong F., Pirbhai M., Xiao Y., Zhong Y., Wu Y., Zhong G., Pirbhai M., Xiao Y., Zhong Y., Wu Y., Zhong G., Xiao Y., Zhong Y., Wu Y., Zhong G., Zhong Y., Wu Y., Zhong G., Wu Y., Zhong G., Zhong G. Degradation of the proapoptotic proteins Bik, Puma, and Bim with Bcl-2 domain 3 homology in Chlamydia trachomatis-infected cells. Infect. Immun. 2005;73:1861–1864. doi: 10.1128/IAI.73.3.1861-1864.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elangovan B., Chinnadurai G., Chinnadurai G. Functional dissection of the pro-apoptotic protein Bik. Heterodimerization with anti-apoptosis proteins is insufficient for induction of cell death. J. Biol. Chem. 1997;272:24494–24498. doi: 10.1074/jbc.272.39.24494. [DOI] [PubMed] [Google Scholar]
- Fischer S.F., Vier J., Kirschnec S., Klos A., Hess S., Ying S., Hacker G., Vier J., Kirschnec S., Klos A., Hess S., Ying S., Hacker G., Kirschnec S., Klos A., Hess S., Ying S., Hacker G., Klos A., Hess S., Ying S., Hacker G., Hess S., Ying S., Hacker G., Ying S., Hacker G., Hacker G. Chlamydia inhibits host cell apoptosis by degradation of proapoptotic BH3-only proteins. J. Exp. Med. 2004;200:905–916. doi: 10.1084/jem.20040402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelinas C., White E., White E. BH3-only proteins in control: Specificity regulates MCL-1 and BAK-mediated apoptosis. Genes & Dev. 2005;19:1263–1268. doi: 10.1101/gad.1326205. [DOI] [PubMed] [Google Scholar]
- Gerdes K., Christensen S.K., Lobner-Oleson A., Christensen S.K., Lobner-Oleson A., Lobner-Oleson A. Prokaryotic toxin antitoxin stress response loci. Nat. Rev. Microbiol. 2005;3:371–382. doi: 10.1038/nrmicro1147. [DOI] [PubMed] [Google Scholar]
- Germain M., Mathai J.P., Shore G.C., Mathai J.P., Shore G.C., Shore G.C. BH3-only BIK functions at the endoplasmic reticulum to stimulate cytochrome c release from mitochondria. J. Biol. Chem. 2002;277:18053–18060. doi: 10.1074/jbc.M201235200. [DOI] [PubMed] [Google Scholar]
- Gil J., Esteban M., Esteban M. Induction of apoptosis by the dsRNA-dependent protein kinase (PKR): Mechanism of action. Apoptosis. 2000;5:107–114. doi: 10.1023/a:1009664109241. [DOI] [PubMed] [Google Scholar]
- Goldmacher V.S. Cell death suppression by cytomegaloviruses. Apoptosis. 2005;10:251–265. doi: 10.1007/s10495-005-0800-z. [DOI] [PubMed] [Google Scholar]
- Han J., Sabbatini P., White E., Sabbatini P., White E., White E. Induction of apoptosis by human Nbk/Bik, a BH3-containing protein that interacts with E1B 19K. Mol. Cell. Biol. 1996;16:5857–5864. doi: 10.1128/mcb.16.10.5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazan R., Engelberg-Kulka H., Engelberg-Kulka H. Escherichia coli mazEF-mediated cell death as a defense mechanism that inhibits the spread of phage P1. Mol. Genet. Genomics. 2004;272:227–234. doi: 10.1007/s00438-004-1048-y. [DOI] [PubMed] [Google Scholar]
- Hedge R., Srinivasula S.M., Ahmad M., Fernandes-Alnemri T., Alnemri E.S., Srinivasula S.M., Ahmad M., Fernandes-Alnemri T., Alnemri E.S., Ahmad M., Fernandes-Alnemri T., Alnemri E.S., Fernandes-Alnemri T., Alnemri E.S., Alnemri E.S. Blk, a BH3-containing mouse protein that interacts with Bcl-2 and Bcl-XL, is a potent death agonist. J. Biol. Chem. 1998;273:7783–7786. doi: 10.1074/jbc.273.14.7783. [DOI] [PubMed] [Google Scholar]
- Hur J., Chesnes J., Coser K.R., Lee R.S., Geck P., Isselbacher K.J., Shioda T., Chesnes J., Coser K.R., Lee R.S., Geck P., Isselbacher K.J., Shioda T., Coser K.R., Lee R.S., Geck P., Isselbacher K.J., Shioda T., Lee R.S., Geck P., Isselbacher K.J., Shioda T., Geck P., Isselbacher K.J., Shioda T., Isselbacher K.J., Shioda T., Shioda T. The Bik BH3-only protein is induced in estrogen-starved and antiestrogen-exposed breast cancer cells and provokes apoptosis. Proc. Natl. Acad. Sci. 2004;101:2351–2356. doi: 10.1073/pnas.0307337101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioffe M.L., White E., Nelson D.A., Dvorzhinski D., DiPaola R.S., White E., Nelson D.A., Dvorzhinski D., DiPaola R.S., Nelson D.A., Dvorzhinski D., DiPaola R.S., Dvorzhinski D., DiPaola R.S., DiPaola R.S. Epothilone induced cytotoxicity is dependent on p53 status in prostate cells. Prostate. 2004;61:243–247. doi: 10.1002/pros.20108. [DOI] [PubMed] [Google Scholar]
- Jeffers J.R., Parganas E., Lee Y., Yang C., Wang J., Brennan J., MacLean K.H., Han J., Chittenden T., Ihle J.N., Parganas E., Lee Y., Yang C., Wang J., Brennan J., MacLean K.H., Han J., Chittenden T., Ihle J.N., Lee Y., Yang C., Wang J., Brennan J., MacLean K.H., Han J., Chittenden T., Ihle J.N., Yang C., Wang J., Brennan J., MacLean K.H., Han J., Chittenden T., Ihle J.N., Wang J., Brennan J., MacLean K.H., Han J., Chittenden T., Ihle J.N., Brennan J., MacLean K.H., Han J., Chittenden T., Ihle J.N., MacLean K.H., Han J., Chittenden T., Ihle J.N., Han J., Chittenden T., Ihle J.N., Chittenden T., Ihle J.N., Ihle J.N., et al. Puma is an essential mediator of p53-dependent and -independent apoptotic pathways. Cancer Cell. 2003;4:321–328. doi: 10.1016/s1535-6108(03)00244-7. [DOI] [PubMed] [Google Scholar]
- Jiang A., Clark E.A., Clark E.A. Involvement of Bik, a proapoptotic member of the Bcl-2 family, in surface IgM-mediated B cell apoptosis. J. Immunol. 2001;166:6025–6033. doi: 10.4049/jimmunol.166.10.6025. [DOI] [PubMed] [Google Scholar]
- Letai A., Bassik M.C., Walensky L.D., Sorcinelli M.D., Weiler S., Korsmeyer S.J., Bassik M.C., Walensky L.D., Sorcinelli M.D., Weiler S., Korsmeyer S.J., Walensky L.D., Sorcinelli M.D., Weiler S., Korsmeyer S.J., Sorcinelli M.D., Weiler S., Korsmeyer S.J., Weiler S., Korsmeyer S.J., Korsmeyer S.J. Distinct BH3 domains ether sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell. 2002;2:183–192. doi: 10.1016/s1535-6108(02)00127-7. [DOI] [PubMed] [Google Scholar]
- Lo H.W., Day C.P., Hung M.C., Day C.P., Hung M.C., Hung M.C. Cancer-specific gene therapy. Adv. Genet. 2005;54:235–255. doi: 10.1016/S0065-2660(05)54010-0. [DOI] [PubMed] [Google Scholar]
- Luo X., Budihardjo I., Zou H., Slaughter C., Wang X., Budihardjo I., Zou H., Slaughter C., Wang X., Zou H., Slaughter C., Wang X., Slaughter C., Wang X., Wang X. Bid, a Bcl-2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell death receptors. Cell. 1998;94:481–490. doi: 10.1016/s0092-8674(00)81589-5. [DOI] [PubMed] [Google Scholar]
- Mathai J.P., Germain M., Shore G.C., Germain M., Shore G.C., Shore G.C. BH3-only BIK regulates BAX, BAK-dependent release of Ca2+ from endoplasmic reticulum stores and mitochondrial apoptosis during stress-induced cell death. J. Biol. Chem. 2005;280:23829–23836. doi: 10.1074/jbc.M500800200. [DOI] [PubMed] [Google Scholar]
- Meijerman I., Blom W.M., de Bont H.J., Mulder G.J., Nagelkerke J.F., Blom W.M., de Bont H.J., Mulder G.J., Nagelkerke J.F., de Bont H.J., Mulder G.J., Nagelkerke J.F., Mulder G.J., Nagelkerke J.F., Nagelkerke J.F. Induction of apoptosis and changes in nuclear G-actin are mediated by different pathway: The effect of inhibitors of protein and RNA synthesis in isolated rat hepatocytes. Toxicol. Appl. Pharmacol. 1999;156:46–55. doi: 10.1006/taap.1998.8616. [DOI] [PubMed] [Google Scholar]
- Nelson D.A., Tan T.T., Rabson A.B., Anderson D., Degenhardt K., White E., Tan T.T., Rabson A.B., Anderson D., Degenhardt K., White E., Rabson A.B., Anderson D., Degenhardt K., White E., Anderson D., Degenhardt K., White E., Degenhardt K., White E., White E. Hypoxia and defective apoptosis drive genomic instability and tumorigenesis. Genes & Dev. 2004;18:2095–2107. doi: 10.1101/gad.1204904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijhawan D., Fang M., Traer E., Zhong Q., Gao W., Due F., Wang X., Fang M., Traer E., Zhong Q., Gao W., Due F., Wang X., Traer E., Zhong Q., Gao W., Due F., Wang X., Zhong Q., Gao W., Due F., Wang X., Gao W., Due F., Wang X., Due F., Wang X., Wang X. Elimination of MCL-1 is required for the initiation of apoptosis following ultraviolet irradiation. Genes & Dev. 2003;17:1475–1486. doi: 10.1101/gad.1093903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez D., White E., White E. TNF-α signals apoptosis through a bid-dependent conformational change in Bax that is inhibited by E1B 19K. Mol. Cell. 2000;6:53–63. [PubMed] [Google Scholar]
- Radetzki S., Kohne C.H., von Haefen C., Gillissen B., Sturm I., Dorken B., Daniel P.T., Kohne C.H., von Haefen C., Gillissen B., Sturm I., Dorken B., Daniel P.T., von Haefen C., Gillissen B., Sturm I., Dorken B., Daniel P.T., Gillissen B., Sturm I., Dorken B., Daniel P.T., Sturm I., Dorken B., Daniel P.T., Dorken B., Daniel P.T., Daniel P.T. The apoptosis promoting Bcl-2 homologues Bak and Nbk/Bik overcome drug resistance in Mdr-1-negative and Mdr-1-overexpressing breast cancer cell lines. Oncogene. 2002;21:227–238. doi: 10.1038/sj.onc.1205010. [DOI] [PubMed] [Google Scholar]
- Roulston A., Marcellus R.C., Branton P.E., Marcellus R.C., Branton P.E., Branton P.E. Viruses and apoptosis. Annu. Rev. Microbiol. 1999;53:577–628. doi: 10.1146/annurev.micro.53.1.577. [DOI] [PubMed] [Google Scholar]
- Samuelson A.V., Lowe S.W., Lowe S.W. Selective induction of p53 and chemosensitivity in RB-deficient cells by E1A mutants unable to bind the RB-related proteins. Proc. Natl. Acad. Sci. 1997;94:12094–12099. doi: 10.1073/pnas.94.22.12094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibue T., Takeda K., Oda E., Tanaka H., Murasawa H., Takaoka A., Morishita Y., Akira S., Taniguchi T., Tanaka N., Takeda K., Oda E., Tanaka H., Murasawa H., Takaoka A., Morishita Y., Akira S., Taniguchi T., Tanaka N., Oda E., Tanaka H., Murasawa H., Takaoka A., Morishita Y., Akira S., Taniguchi T., Tanaka N., Tanaka H., Murasawa H., Takaoka A., Morishita Y., Akira S., Taniguchi T., Tanaka N., Murasawa H., Takaoka A., Morishita Y., Akira S., Taniguchi T., Tanaka N., Takaoka A., Morishita Y., Akira S., Taniguchi T., Tanaka N., Morishita Y., Akira S., Taniguchi T., Tanaka N., Akira S., Taniguchi T., Tanaka N., Taniguchi T., Tanaka N., Tanaka N. Integral role of Noxa in p53-mediated apoptotic response. Genes & Dev. 2003;17:2233–2238. doi: 10.1101/gad.1103603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman R.H. Implications for RNase L in prostate cancer biology. Biochemistry. 2003;42:1805–1812. doi: 10.1021/bi027147i. [DOI] [PubMed] [Google Scholar]
- Sturm I., Stephan C., Gillissen B., Siebert R., Janz M., Radetzki S., Jung K., Loening S., Dorken B., Daniel P.T., Stephan C., Gillissen B., Siebert R., Janz M., Radetzki S., Jung K., Loening S., Dorken B., Daniel P.T., Gillissen B., Siebert R., Janz M., Radetzki S., Jung K., Loening S., Dorken B., Daniel P.T., Siebert R., Janz M., Radetzki S., Jung K., Loening S., Dorken B., Daniel P.T., Janz M., Radetzki S., Jung K., Loening S., Dorken B., Daniel P.T., Radetzki S., Jung K., Loening S., Dorken B., Daniel P.T., Jung K., Loening S., Dorken B., Daniel P.T., Loening S., Dorken B., Daniel P.T., Dorken B., Daniel P.T., Daniel P.T. Loss of the tissue-specific proapoptotic BH3-only protein Nbk/Bik is a unifying feature of renal cell carcinoma. Cell Death Differ. 2006;13:619–627. doi: 10.1038/sj.cdd.4401782. [DOI] [PubMed] [Google Scholar]
- Suzuki M., Zhang J., Liu M., Woychik N.A., Inouye M., Zhang J., Liu M., Woychik N.A., Inouye M., Liu M., Woychik N.A., Inouye M., Woychik N.A., Inouye M., Inouye M. Single protein production in living cells facilitated by an mRNA interferase. Mol. Cell. 2005;18:253–261. doi: 10.1016/j.molcel.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Tan T.T., Degenhardt K., Nelson D.A., Beaudoin B., Nieves-Neira W., Bouillet P., Villunger A., Adams J.M., White E., Degenhardt K., Nelson D.A., Beaudoin B., Nieves-Neira W., Bouillet P., Villunger A., Adams J.M., White E., Nelson D.A., Beaudoin B., Nieves-Neira W., Bouillet P., Villunger A., Adams J.M., White E., Beaudoin B., Nieves-Neira W., Bouillet P., Villunger A., Adams J.M., White E., Nieves-Neira W., Bouillet P., Villunger A., Adams J.M., White E., Bouillet P., Villunger A., Adams J.M., White E., Villunger A., Adams J.M., White E., Adams J.M., White E., White E. Key roles of BIM-driven apoptosis in epithelial tumors and rational chemotherapy. Cancer Cell. 2005;7:227–238. doi: 10.1016/j.ccr.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Teodoro J.G., Shore G.C., Branton P.E., Shore G.C., Branton P.E., Branton P.E. Adenovirus E1A proteins induce apoptosis by both p53-dependent and p53-independent mechanisms. Oncogene. 1995;11:467–474. [PubMed] [Google Scholar]
- Verma S., Zhao L.J., Chinnadurai G., Zhao L.J., Chinnadurai G., Chinnadurai G. Phosphorylation of pro-apoptotic protein BIK. J. Biol. Chem. 2001;276:4671–4676. doi: 10.1074/jbc.M008983200. [DOI] [PubMed] [Google Scholar]
- Wei M.C., Lindsten T., Mootha V.K., Weiler S., Gross A., Ashiya M., Thompson C.B., Korsmeyer S.J., Lindsten T., Mootha V.K., Weiler S., Gross A., Ashiya M., Thompson C.B., Korsmeyer S.J., Mootha V.K., Weiler S., Gross A., Ashiya M., Thompson C.B., Korsmeyer S.J., Weiler S., Gross A., Ashiya M., Thompson C.B., Korsmeyer S.J., Gross A., Ashiya M., Thompson C.B., Korsmeyer S.J., Ashiya M., Thompson C.B., Korsmeyer S.J., Thompson C.B., Korsmeyer S.J., Korsmeyer S.J. tBID, a membrane-targeted death ligand, oligomerizes BAK to release cytochrome c. Genes & Dev. 2000;14:2060–2071. [PMC free article] [PubMed] [Google Scholar]
- White E. Mechanisms of apoptosis regulation by viral oncogenes in infection and tumorigenesis. Cell Death Differ. 2006;13:1–7. doi: 10.1038/sj.cdd.4401941. [DOI] [PubMed] [Google Scholar]
- Willis S.N., Adams J.M., Adams J.M. Life in the balance: How BH3-only proteins induce apoptosis. Curr. Opin. Cell Biol. 2005;17:1–9. doi: 10.1016/j.ceb.2005.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis S.N., Chen L., Dewson G., Wei A., Naik E., Fletcher J.I., Adams J.M., Hung D.C.S., Chen L., Dewson G., Wei A., Naik E., Fletcher J.I., Adams J.M., Hung D.C.S., Dewson G., Wei A., Naik E., Fletcher J.I., Adams J.M., Hung D.C.S., Wei A., Naik E., Fletcher J.I., Adams J.M., Hung D.C.S., Naik E., Fletcher J.I., Adams J.M., Hung D.C.S., Fletcher J.I., Adams J.M., Hung D.C.S., Adams J.M., Hung D.C.S., Hung D.C.S. Proapoptotic Bak is sequestered by Mcl-1 and Bcl-XL, but not Bcl-2, until displaced by BH3-only proteins. Genes & Dev. 2005;19:1294–1305. doi: 10.1101/gad.1304105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Feigenblum D., Schneider R.J., Feigenblum D., Schneider R.J., Schneider R.J. A late adenovirus factor induces eIF-4E dephosphorylation and inhibition of cell protein synthesis. J. Virol. 1994;68:7040–7050. doi: 10.1128/jvi.68.11.7040-7050.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Zhang J., Hoeflich K.P., Ikura M., Qing G., Inouye M., Zhang J., Hoeflich K.P., Ikura M., Qing G., Inouye M., Hoeflich K.P., Ikura M., Qing G., Inouye M., Ikura M., Qing G., Inouye M., Qing G., Inouye M., Inouye M. MazF cleaves cellular mRNAs specifically at ACA to block protein synthesis in Escherichia coli. Mol. Cell. 2003;12:913–923. doi: 10.1016/s1097-2765(03)00402-7. [DOI] [PubMed] [Google Scholar]
- Zong W.X., Lindsten T., Ross A.J., MacGregor G.R., Thompson C.B., Lindsten T., Ross A.J., MacGregor G.R., Thompson C.B., Ross A.J., MacGregor G.R., Thompson C.B., MacGregor G.R., Thompson C.B., Thompson C.B. BH3-only proteins that bind pro-survival Bcl-2 family members fail to induce apoptosis in the absence of Bax and Bak. Genes & Dev. 2001;15:1481–1486. doi: 10.1101/gad.897601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Y., Peng H., Zhou B., Wen Y., Wang S.C., Tsai E.M., Hung M.C., Peng H., Zhou B., Wen Y., Wang S.C., Tsai E.M., Hung M.C., Zhou B., Wen Y., Wang S.C., Tsai E.M., Hung M.C., Wen Y., Wang S.C., Tsai E.M., Hung M.C., Wang S.C., Tsai E.M., Hung M.C., Tsai E.M., Hung M.C., Hung M.C. Systemic tumor suppression by the proapoptotic gene bik. Cancer Res. 2002;62:8–12. [PubMed] [Google Scholar]