Abstract
Background.
Left ventricular volumes, ejection fraction and regional wall motion are cardiac parameters which provide valuable information for patient management in a large variety of cardiac conditions. Differences in regional wall motion are of relevance in the field of cardiac resynchronisation therapy. We quantified three-dimensional echocardiographic measurements of left ventricular volumes, ejection and regional wall motion (e.g. expressed as systolic dyssynchrony index (SDI)) in two patient cohorts: patients with normal conduction and patients with complete left bundle branch block.
Methods.
Thirty-five patients scheduled for routine cardiac examination underwent three-dimensional echocardiography: 23 patients with normal conduction and 12 patients with a complete left bundle branch block. Full-volume datasets were analysed and end-systolic volume (ESV), end-diastolic volume (EDV) and ejection fraction (EF) were obtained. SDI was derived from the standard deviation of the measured times to reach minimal regional volume for each of the 16 segments of the left ventricle.
Results.
A significant difference was observed in left ventricular volumes, ejection fraction and SDI between the two groups. Patients with complete left bundle branch block showed higher EDV (p=0.025) and ESV (p<0.01) and a lower EF (p<0.01) than patients with normal conduction. SDI is significantly higher in patients with complete left bundle branch block (p=0.004) expressing a higher amount of ventricular dyssynchrony. Intraobserver variability showed excellent correlation coefficients: r=0.99 for EDV, ESV and SDI and r=0.98 for EF.
Conclusion.
Three-dimensional echocardiography is a feasible and reproducible method for the quantification of left ventricular volumes, left ventricular ejection fraction and regional wall motion. Differences can be assessed between normal patients and patients with left bundle branch block. (Neth Heart J 2007;15:89-94.)
Keywords: echocardiography (three-dimensional), bundle branch block (left), dyssynchrony (systolic)
Left ventricular volumes and left ventricular ejection fraction are considered important prognostic parameters in a large number of cardiac diseases.1-3 Therefore, accurate quantification plays an essential role in patient management. At the present time, these parameters are assessed using either conventional twodimensional (2D) echocardiography or nuclear techniques. Studies have indicated that three-dimensional (3D) echocardiography is superior to two-dimensional echocardiography when evaluating a variety of cardiac conditions and several cardiac parameters.4-9
The algorithms used to determine ventricular volumes and ejection fraction by 2D echocardiography are largely based on geometrical assumptions concerning cavity shape and position and orientation of imaging planes. This is an important limitation to conventional echocardiography and can result in significant errors and variability.10,11 With 3D echocardiography, these limitations can largely be overcome, mainly by avoiding the need for geometrical assumptions. Three-dimensional echocardiography uses algorithms that derive ventricular volumes and ejection fraction from the complete 3D dataset, and therefore makes geometrical assumptions unnecessary.
In ventricular dyssynchrony there is a decrease in symmetrical ventricular contraction resulting in decreased haemodynamics, which leads to increased ventricular volumes, decreased ejection fraction and a decrease in overall ventricular function and efficiency.
Studies have indicated that assessment and quantification of ventricular dyssynchrony is of great value in diagnostic and prognostic considerations of cardiac disease. Recently, assessment and quantification of dyssynchrony has become increasingly important as cardiac resynchronisation therapy has established itself as an effective treatment for chronic heart failure patients.12-14 Techniques used to quantify ventricular synchrony include M-mode echocardiography, visual estimation using 2D echocardiography, 2D echocardiographic measurements, tissue Doppler and strain. Widespread use of these techniques has been hindered by limitations in acquisition and analysis, such as the inability to compare multiple segments at once. Threedimensional echocardiography allows comparison of synchrony of all ventricular segments and can be considered an accurate method for the assessment and quantification of ventricular dyssynchrony.15-17
Complete left bundle branch block is a conduction disorder demonstrating abnormal sequence of activation and contraction of the heart. Left ventricular systole and diastole are delayed compared with right ventricular contraction and relaxation. Characteristically, the left ventricle shows abnormal septum activation and movement occurs from right to left, with early activation of the septal wall region and late activation of the posterolateral wall region.12,18 Left bundle branch block is associated with cardiac disease and has prognostic implications.
The purpose of this study is to assess the feasibility of three-dimensional echocardiography in daily practice in a nonacademic centre. We quantified 3D echocardiographic measurements of left ventricular volumes and ejection fraction in two patient groups: patients with normal conduction and patients with complete left bundle branch block. Regional wall motion was also quantified in these same patient groups.
Methods
Patient selection
Thirty-five consecutive patients who were referred to our echo department during a period of approximately eight weeks and who fulfilled the criteria were included in this study. Only patients with acceptable image quality were included, defined as a good four-chamber view window with conventional 2D echocardiography. Patients were divided into two groups, primarily based on the ECG. Group I comprised patients with sinus rhythm, normal ECG, no conduction abnormalities, no cardiac history and a normal 2D echocardiogram, while group II was made up of patients with a complete left bundle branch block.
When patients were included in group I or II, a 3D echocardiogram was carried out. There were 23 patients in group I (age 57±19) and 12 in group II (age 66±11). Four patients in group II had a history of cardiac disease (myocardial infarction: n=2, cardiomyopathy: n=2).
Three-dimensional data acquisition
Data acquisition was performed using an ultrasound scanner (Sonos 7500, Philips Medical Systems) that was equipped with specialised software for 3D data acquisition and a special transducer. The transducer is a matrix-array transducer containing 3000 piezoelectric elements (X4, Philips) which renders images in realtime. The 3D data acquisition is normally accomplished in a relatively narrow sector width of 30°x50° (azimuth and elevation direction, respectively). In order to acquire the entire left ventricular volume in the 3D dataset, full-volume acquisition is used. With full-volume acquisition, a pyramidal volume of approximately 90°x90° is scanned by acquiring four subvolumes in real-time of approximately 90°x22°. The subvolumes are acquired in eight successive cardiac cycles and acquisition is gated on every other R wave of the electrocardiogram. To prevent discordance between the subvolumes, patients are asked to breath in, breath out and then hold their breath, after which acquisition is started, with the transducer being placed in an apical four-chamber position. The breath-hold needs to last until acquisition is finished and since acquisition is completed in eight cardiac cycles, duration of the breathhold is dependent on heart rate. When acquisition is finished, the 3D dataset is transferred to a workstation for off-line data analysis.
Three-dimensional data analysis
The 3D echocardiographic data were analysed off-line using 4D LV-Analysis CRT software (version 1.0, TomTec, Unterschleissheim, Germany). After allocation of three landmarks (mitral valve, aortic valve and apex) the volume dataset is automatically sliced into eight equally rotated imaging planes and end-systolic frame and end-diastolic frame have to be defined. In each of the eight imaging planes, the apex and two annulus points have to be determined in both an end-systolic and end-diastolic position. Next, endocardial borders are traced using an automated contour detection system and they are manually adjusted if automatic detection is not accurate and satisfactory. Finally, a fullvolume 3D model of the left ventricle (‘bag’) is reconstructed, which is divided into 16 segments (figure 1). End-systolic and end-diastolic volume as well as ejection fraction are displayed. Furthermore, both global and segmental volume and ejection fraction are presented in a curve.
Figure 1.
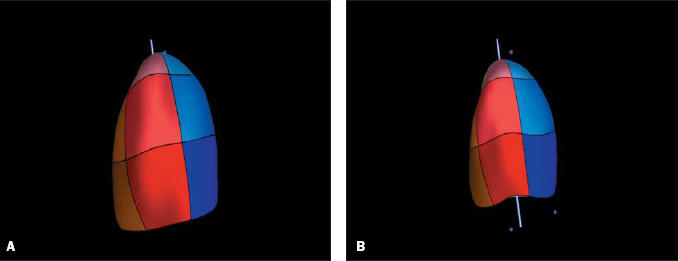
Three-dimensional reconstruction of the left ventricle, divided into 16 segments, in end-diastole (1A) and end-systole (1B).
Regional wall motion
Regional wall motion was assessed using a systolic dyssynchrony index (SDI) as defined by Kapetanakis et al.16 For each of the 16 segments of the left ventricle, the time taken to reach its minimum volume as a percentage of the cardiac cycle is calculated. The systolic dyssynchrony index is defined as the standard deviation of these percentages. A higher SDI value denotes a higher degree of ventricular dyssynchrony. The SDI is calculated from a percentage of the heart cycle rather than in milliseconds in order to be able to make comparisons between patients with significantly different heart rates.
Statistical analysis
All variables are presented as mean ± SD. Independent samples T tests were performed for comparison of parameters between patient groups. Intraobserver variability was assessed twice, first in the non-experienced observer and then later on when the observer had gained experience in data analysis, expressing the learning curve of the observer. To assess intraobserver variability, data of 13 randomly selected patients were analysed twice by the same observer within a period of time between the two observations of approximately two weeks. Intraobserver variability is expressed as mean difference ± SD between the two observations and Pearson’s correlation coefficients are calculated as well.
Results
Patient characteristics
Characteristics of the patients in groups I and II are presented in table 1.
Table 1.
Demographic and clinical characteristics of patients in groups I and II.
| Normal conduction(group I) | Left bundle branch block(group II) | p value | |
|---|---|---|---|
| n | 23 | 12 | |
| Age (years) | 57±19 | 66±11 | NS |
| Male gender, n (%) | 16 (73) | 4 (33) | p<0.05 |
| QRS duration (ms) | 95±8 | 147±15 | p<0.05 |
| BSA (m2) | 1.83±0.19 | 1.79±0.19 | NS |
| Cardiovascular history | |||
| - MI, n (%) | 2 (16) | ||
| - Cardiomyopathy, n (%) | 2 (16) |
BSA=body surface area, MI=myocardial infarction, NS=not significant.
Quantification of left ventricular volumes and ejection fraction
The results of the quantification of left ventricular volumes and ejection fraction in the two patient groups are summarised in table 2. For all three parameters, results differ significantly between group I and group II (p=0.025, p=0.000 and p=0.000 for EDV, ESV and EF, respectively).
Quantification of regional wall motion
The results of the quantification of regional wall motion in group I and group II are summarised in table 2. Results show a significant difference between SDI measured in patients in groups I and II (p=0.004). See also figures 2 and 3.
Table 2.
Quantification of left ventricular volumes, ejection fraction and regional wall motion in patients in groups I and II.
| Group I (Mean±SD) | Group II (Mean±SD) | Mean difference±SD | p value | 95% CI | |
|---|---|---|---|---|---|
| EDV (ml) | 104.2±34.1 | 139.5±54.6 | -35.3±15.0 | 0.025 | -65.7 to -4.8 |
| ESV (ml) | 40.0±17.6 | 78.8±41.5 | -38.7±10.0 | 0.000 | -58.9 to -18.4 |
| EF (%) | 62.7±6.7 | 45.4±11.7 | 17.3±3.1 | 0.000 | 11.0 to 23.6 |
| SDI (%) | 2.5±1.3 | 7.9±5.1 | 17.3±3.1 | 0.004 | -8.7 to -2.1 |
CI=confidence interval, EDV=end-diastolic volume, ESV=end-systolic volume, EF=ejection fraction, SDI=systolic dyssynchrony index.
Figure 2.
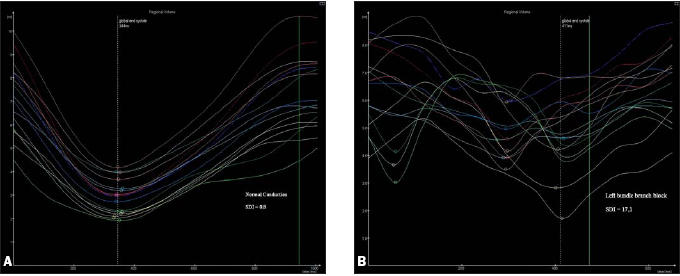
Regional volume curves representing all 16 segments in a patient with normal conduction (2A) and a patient with left bundle branch block (2B).
Figure 3.
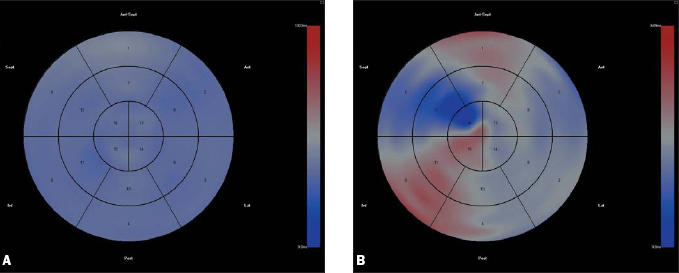
Parametric representation of left ventricular activation in a patient with normal conduction (3A) and a patient with left bundle branch block (3B). Areas that are coloured red represent areas with late activation compared with global activation. Blue areas represent areas with early contraction. A homogenous colour means that left ventricular contraction is synchronous.
Intraobserver variability
The intraobserver variability for measurements of left ventricular volumes, ejection fraction and SDI is summarised in table 3. Intraobserver variability in the experienced observer shows high correlation coefficients and low variability (r=0.99 for EDV, ESV and SDI, r=0.98 for EF).
Table 3.
Intraobserver variability
| Non-experienced | Experienced | |||
|---|---|---|---|---|
| Mean difference± SD | R | Mean difference± SD | R | |
| EDV | -5.6 ±13.9 | 0.48 | -0.1±17.8 | 0.99 |
| ESV | 1.1±7.0 | 0.31 | -0.2±12.4 | 0.99 |
| EF (%) | 0.59±6.7 | 0.86 | -0.2±3.4 | 0.98 |
| SDI (%) | -0.9±2.8 | 0.26 | -0.1±0.2 | 0.99 |
EDV=end-diastolic volume, ESV=end-systolic volume, EF=ejection fraction, SDI=systolic dyssynchrony index, R=Pearson’s correlation coefficient.
Discussion
This study compared left ventricular volumes, ejection fraction and regional wall motion in patients with normal conduction and without apparent cardiac abnormalities and patients with complete left bundle branch block. The results show that: 1) three-dimensional echocardiographic measurements of left ventricular volumes and ejection fraction differ significantly between the two investigated groups with higher endsystolic and end-diastolic volumes and a lower ejection fraction in patients with left bundle branch block; 2) the systolic dyssynchrony index differs significantly between patients with normal conduction and patients with complete left bundle branch block, being higher in patients with left bundle branch block indicating a higher degree of ventricular dyssynchrony; 3) there is low intraobserver variability for 3D echocardiographic measurements of left ventricular volumes and left ventricular ejection fraction.
Results show a clear difference between the two investigated groups of patients, especially in ejection fraction (group I: 62.7±6.7%, group II: 45.4±11.7%). Left bundle branch block is often associated with other cardiac conditions, particularly with cardiomyopathy. These associated cardiac diseases could solely (i.e. independent from the presence of left bundle branch block) account for a lower left ventricular function and therefore it is very important to consider these diseases when evaluating study results in order to prevent bias. Four out of the 12 investigated patients in group II had a history of cardiac disease (cardiomyopathy: n=2, myocardial infarction: n=2). Ejection fractions of the patients who had a myocardial infarction are 46.04 and 23.39%. When excluding both these variables from the group mean, the group mean increases to 47.51±10.43%, which is still significantly different from patients in group I (p<0.05). Ejection fractions of the patients with cardiomyopathy are both higher than the group mean (46.4 and 55.68%) and when excluding both variables mean ejection fraction decreases to 44.2±12.4%. Therefore, it is unlikely that presence of cardiomyopathy accounts for the significantly lower ejection fraction in group II compared with group I in this study.
Results show that 3D echocardiography is a feasible method for the quantification of left ventricular volumes, ejection fraction and regional wall motion in a non-academic centre. Considering the clinical value of these parameters, routine use of 3D echocardiography would be helpful in clinical practice (e.g. in the follow-up of patients with valve pathologies, cardiomyopathy or postinfarction). A new generation of left ventricular analysis software will soon be commercially available. This software uses new algorithms and can reduce postprocessing time back to two or three minutes, therefore offering important potential for routine clinical application of 3D echocardiography in the near future.
Comparison of intraobserver variability in the experienced observer and in the non-experienced observer demonstrates the learning curve of the observer (table 3).
Only patients with good image quality were included in this study, which means that patients who fulfil the criteria but who have a poor echo window are not considered. We found that acquisition of a good 3D echocardiogram and accurate 3D data analysis could be obtained in 80 to 85% of all patients.
Study limitations
In order to encompass the entire left ventricle in a fullvolume 3D dataset acquisition of four subvolumes is required. These subvolumes are subsequently as- sembled to create the complete 3D echocardiographic dataset. Acquisition is gated on every other R wave during a breath-hold. Therefore, acquisition of a 3D dataset in patients with severe arrhythmias or patients who cannot hold their breath (i.e. patients with severe dyspnoea) is difficult, because it causes discordance between the four subvolumes which makes it impossible to perform accurate data analysis.
A 3D echocardiographic dataset is acquired in a relatively large pyramidal volume of 93°x84°. Nevertheless, acquisition of a full-volume dataset in patients with severely dilated ventricles is problematic. In patients with severe heart failure, for example, often the complete left ventricle cannot be encompassed in the 3D dataset and parts of the left ventricle are excluded from the dataset. Excluded parts are interpolated during analysis which can cause imprecise analysis outcomes due to over- or underestimation of ventricular volumes.
Conclusion
Three-dimensional echocardiography is a feasible and reproducible method of quantification and comparison of left ventricular volumes, ejection fraction and regional wall motion. Results show a significant difference between patients with normal conduction and patients with complete left bundle branch block. The investigated patients with complete left bundle branch block display higher end-systolic and end-diastolic volumes, a lower ejection fraction and a higher SDI expressing a higher degree of ventricular dyssynchrony.
References
- 1.Cohn PF, Gorlin R, Cohn L, Colins JJ. Left ventricular ejection fraction as a prognostic guide in surgical management of coronary and valvular heart disease. Am J Cardiol 1974;34:136-41. [DOI] [PubMed] [Google Scholar]
- 2.Cintron G, Johnson G, Francis G, et al. Prognostic significance of serial changes in left ventricular ejection fraction in patients with congestive heart failure. Circulation 1993;87(suppl VI):vi17-23. [PubMed] [Google Scholar]
- 3.McGhie AI, Willerson JT, Corbett JR. Radionuclide assessment of ventricular function and risk stratification after myocardial infarction. Circulation 1991;84(suppl I):i167-76. [PubMed] [Google Scholar]
- 4.Sugeng L, Weinert L, Lang RM. Left ventricular assessment using real time three dimensional echocardiography. Heart 2003;89: iii29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schroder KM, Sapin PM, King DL, et al. Three-dimensional echocardiographic volume computation: in vitro comparison to standard two-dimensional echocardiography. J Am Soc Echocardiogr 1993;6:467-75. [DOI] [PubMed] [Google Scholar]
- 6.Keller AM, Gopal AS, King DL. Left and right atrial volume by freehand three-dimensional echocardiography: in vivo validation using magnetic resonance imaging. Eur J Echocardiogr 000;1:55-65. [DOI] [PubMed] [Google Scholar]
- 7.Gopal AS, Keller AM, Rigling R, et al. Left ventricular volume and endocardial surface area by three-dimensional echocardiography: comparison with two-dimensional echocardiography and nuclear magnetic resonance imaging in normal subject. J Am Coll Cardiol 1993;22:258-70. [DOI] [PubMed] [Google Scholar]
- 8.Chuang ML, Beaudin RA, Riley MF, et al. Three-dimensional echocardiographic measurement of left ventricular mass: comparison with magnetic resonance imaging and two-dimensional echocardiographic determinations in man. Int J Card Imaging 2000;16:347-57. [DOI] [PubMed] [Google Scholar]
- 9.Gopal AS, Keller AM, Shen Z, Sapin PM, et al. Three-dimensional echocardiography: in vitro and in vivo validation of left ventricular mass and comparison with conventional echocardiographic methods. J Am Coll Cardiol 1994;24:504-13. [DOI] [PubMed] [Google Scholar]
- 10.King DL, Harrison MR, King DL Jr, Gopal AS, Kwan OL, DeMaria AN. Ultrasound beam orientation during standard twodimensional imaging: assessment by three-dimensional chocardiography. J Am Soc Echocardiogr 1992;5:569-76. [DOI] [PubMed] [Google Scholar]
- 11.King DL, Harrison MR, King DL Jr, Gopal AS, Martin RP, DeMaria AN. Improved reproducibility of left atrial and left ventricular measurements by guided three-dimensional echocardiography. J Am Coll Cardiol 1992;20:1238-45. [DOI] [PubMed] [Google Scholar]
- 12.Abraham WT, Hayes DL. Cardiac resynchronization therapy for heart failure. Circulation 2003;108:2596-603. [DOI] [PubMed] [Google Scholar]
- 13.Lane RE, Chow AWC, Chin D, Mayet J. Selection and optimisation of biventricular pacing: the role of echocardiography. Heart 2004;90(suppl VI):vi10-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu CM, Bax JJ, Monaghan M, Nihoyannopoulos P. Echocardiographic evaluation of cardiac dissynchrony for predicting a favourable response to cardiac resynchronisation therapy. Heart 2004;90(suppl VI):vi17-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liodakis E, Al Shareef O, Dawson D, Pearson P, Nihoyannopoulos P. Role of real-time transthoracic 3D echocardiography in the assessment of mechanical asynchrony. Heart 2005;91(suppl I):A31 [Google Scholar]
- 16.Kapetanakis S, Kearney MT, Siva A, et al. Real-time three-dimensional echocardiography: A novel technique to quantify global left ventricular mechanical dissynchrony. Circulation 2005;112:992-1000. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Q, Yu CM, Fung JW, et al. Assessment of the effect of cardiac resynchronization therapy on intraventricular mechanical synchronicity by regional volumetric changes. Am J Cardiol 2005; 95:126-9. [DOI] [PubMed] [Google Scholar]
- 18.Vassallo JA, Cassidy DM, Marchlinski FE, Buston AE, et al. Endocardial activation of left bundle branch block. Circulation 1984; 96:912-23. [DOI] [PubMed] [Google Scholar]


