Abstract
Purpose:
Guidelines for implantation of cardioverter defibrillators (ICD) are increasingly including indications for primary prevention of sudden cardiac death in high-risk groups, where ICDs were traditionally implanted for secondary prevention. We performed a single-centre audit to evaluate adherence to the recent Dutch guidelines.
Methods:
All 1886 patients visiting a large regional Dutch teaching hospital (attending 1.8 to 2.0% of the Dutch population) in November 2005 were screened using the recently updated Dutch guidelines. Patients fulfilling these criteria were categorised as having an ICD indication for primary or secondary prevention.
Results:
135 patients had an indication for ICD, 19 of whom had one or received one. Of the remaining 116 patients, 14 were ‘new’ to the department of cardiology. The 102 ‘known’ patients had 466 doctor-patient contacts in the previous year, which averages 4.57 cardiology contacts per patient per year. Patients were more likely to receive an ICD for the secondary prevention of SCD (10/11, 91%) than for primary prevention (9/124, 7%).
Conclusion:
In a large regional teaching hospital in the Netherlands, only a small proportion of patients eligible for ICD implantation actually receive one. Cardiologists tend to implant ICDs for secondary prevention of SCD. The low ICD implantation rate for primary prevention of SCD may relate to logistics (e.g. permission to implant ICDs, the presence of an electrophysiology lab) or the perceived low cost-benefit ratio. Our results indicate that once the substantial backlog (13,500 ICDs) has been addressed, the annual implantation of new ICDs should rise from the current 125 to at least 510 per million inhabitants per year in the Netherlands. (Neth Heart J 2007;15:129-32.)
Keywords: implantable cardioverter defibrillator, guidelines, audit
In recent years, insertion of an implantable cardioverter defibrillator (ICD) has become routine clinical practice to prevent death in patients with life-threatening arrhythmias (e.g. in survivors of sudden cardiac death). We call this secondary prevention. Increasingly, ICDs are now being implanted to prevent sudden cardiac death in high-risk patients, particularly patients with ischaemic heart disease and left ventricular systolic dysfunction (primary prevention).1 The MADIT I study demonstrated that patients with an ejection fraction (EF) <35% following myocardial infarction and with nonsustained ventricular tachycardia (nsVT) on Holter monitoring should be considered for an ICD if electrophysiological (EP) studies demonstrate sustained ventricular tachycardia (sVT), which can not be suppressed by procainamide.2 The MADIT II study revealed a benefit of ICD implantation in patients with an EF of ≤30% following myocardial infarction, regardless of Holter or EP studies.3 This led to an update of the ESC guidelines to include an EF ≤30% following myocardial infarction as a Class IIA indication.4
Recent studies have convincingly demonstrated striking differences in ICD implantation rates both between European countries and between regions (e.g. a fourfold difference in ICD implantation rates in the UK).5 It is unlikely that these differences can merely be explained by differences in patient characteristics. Financial resources, the presence of an electrophysiology lab and permission to implant ICDs may also play an important role. By adopting the MADIT II criteria the ICD implantation rate is bound to increase substantially, with serious financial consequences.6 In the Netherlands, the ESC guidelines are mirrored in the Dutch guidelines.7 We performed a one-month audit in a large teaching hospital in the Netherlands to study the consequences of rigorously applying the new guidelines. As ICDs are increasingly combined with cardiac resynchronisation therapy (CRT) the audit was extended to include CRT.
Methods
The study was carried out in the Department of Cardiology of the Meander Medical Centre in Amersfoort, a regional teaching hospital servicing an area with 330,000 inhabitants, reflecting 2.0% of the Dutch population. Routine cardiological evaluations, procedures and treatments (including CRT) are carried out by a staff of seven cardiologists. Patients are referred to the University Medical Centre Utrecht and St Antonius Hospital, Nieuwegein, for coronary revascularisation (both surgical and percutaneous), electrophysiological studies and ICD implantations.
The records of all patients (both inpatients and outpatients) visiting the Department of Cardiology in November 2005 were screened using the established criteria for ICD implantation and cardiac resynchronisation therapy (CRT).7,8
For primary prevention by ICD implantation in patients with ischaemic cardiomyopathy the following criteria were used: (i) EF ≤30% or (ii) EF ≤40% with spontaneous nsVT on Holter ECG (>3 weeks post myocardial infarction). For dilated (nonischaemic) cardiomyopathy, patients had to be NYHA III or IV with an EF of ≤35% to be eligible for ICD implantation. Additional criteria for ICD implantation are listed in table 1. CRT criteria were similar to those used in the CARE HF study: EF ≤35% as obtained by an objective imaging technique, NYHA III or IV and either QRS duration ≥150 ms or QRS >120 ms with asynchrony shown on echocardiography.8
Table 1.
Specification of the ICD-indicated group and ICD implantations.
| Primary prevention | Secondary prevention | |
|---|---|---|
| N (ICD)* | N (ICD)* | |
| Ischaemic cardiomyopathy | ||
| - EF ≤30% | 116 (7) | |
| - EF ≤40% + spontaneous nsVT | 7 (2) | |
| - Resuscitated VT/VF | - | 7 (7) |
| - Spontaneous haemodynamic non- tolerated sVT | - | 1 (1) |
| Dilated cardiomyopathy | ||
| - EF ≤35% + NYHA III or IV | 1 (0) | 1 (0) |
| Hypertrophic cardiomyopathy** | - | - |
| Right ventricular cardiomyopathy** | - | 1 (1) |
| Long QT syndrome** | - | 1 (1) |
| Brugada syndrome** | - | - |
| Total | 124 (9) | 11 (10) |
EF=ejection fraction, nsVT=non-sustained VT, sVT=sustained VT, PES=programmed electrical stimulation,*N=total number of patients with ICD indication, ICD=patients with ICD, **Selected patients.
Patients who had visited the Department of Cardiology during the previous 12 months were considered ‘prevalent’ or old cases, those who had not ‘incident’ or new cases. A three-month follow-up (December 2005 to February 2006) was performed of patients with an indication for device therapy who did not receive a device during the audit period (November 2005).
Patients in whom insufficient information was available for a complete assessment were considered to have no indication for ICD implantation or CRT; for example, a patient with an EF of 35% following myocardial infarction who had not undergone Holter monitoring or electrophysiological testing. Similarly, patients without information on LV function could not be indicated for ICD implantation or CRT.
The chart review was carried out by one person (CJWB). A sample of 17% of the patient records was double checked by another investigator (AM), indicating that no ICD or CRT indications had been missed.
Results
A total of 1886 patients were included in the study, 276 of whom were admitted to hospital a d 1610 were visiting the outpatient’s clinic. The inpatient group was older (68±13 vs. 65±14 years), comprised slightly more me (64 vs. 60%) a d had a higher proportion with a indication for ICD implantation (12 vs. 6.5%). Twenty-six patients already had a device (17 ICD only, 7 CRT only a d 2 combined ICD and CRT). Of the 1877 patients without CRT, 12 had a indication for CRT. Of the 1867 patients without a ICD, 116 were found to have a indication for a ICD (table 2), 11 of whom had a indication for both ICD a d CRT. Fourteen of all the patients indicated for a ICD who had ot had o e during the audit ( =116) were ‘new’ (i.e. not having visited the cardiology department during the previous 12 months), resulting in a yearly ICD implantation rate of 168 (14 ‘new’ patients per month x 12 months) in our population, upon rigorous application of the Dutch guidelines.7
Table 2.
ICD indications and ICD implantations.
| Indication for ICD | No indication for ICD | Total | |
|---|---|---|---|
| ICD | 19 | 0 | 19 |
| No ICD | 116 | 1751 | 1867 |
| Total | 135 | 1751 | 1886 |
ICD=implantable cardioverter defibrillator.
The remaining 102 ‘old’ patients had 466 cardiologistpatient contacts (admissions or outpatient clinic visits) in the previous year, averaging 4.57 contacts per patient per year. This translates into a backlog of 270 ICD implantations (102 x 12 months / 4.57). These patients have had contact with a cardiologist in the previous year, are indicated for an ICD but did not receive the device.
Table 1 provides an overview of patients eligible for ICD implantation according to the current guidelines. Only a small proportion of patients with an indication for device implantation receive an ICD (19/135, 14%) or CRT (9/21, 43%). Patients with a secondary indication for ICD implantation are more likely to have the device implanted than those with a primary indication (91 vs. 7%).
Discussion
During a one-month audit, 135 patients visiting the Department of Cardiology of a regional teaching hospital (without EP lab) were found to fulfil the criteria of the recently updated Dutch guidelines for device implantation (either ICD implantation, CRT, or a combination of both). Nineteen of 135 patients (14%) fulfilling ICD criteria already had an ICD or received one during the audit or three-month followup. For CRT the corresponding figures were 9/21 (43%). ICDs were predominantly implanted for secondary prevention of cardiac death.
Several factors may be responsible for the observed low implantation rate of devices: patient characteristics (age, comorbidity, life-expectancy), the treating physician’s perception of cost/harm to benefit ratio, logistics (the presence of an EP lab and the permission to implant devices), and the fact that the implementation of guidelines takes time.
The high number of ICD candidates is mainly driven by the results of the MADIT II study, which included 742 patients (mean age 64±10 years, 84% men) with an EF <30% following myocardial infarction in the ICD arm, compared with an average age of 71±10 years (68% men) in our ICD-indicated group. A 31% reduction in mortality at 20-month follow-up (19.8 vs. 14.2%) was demonstrated in MADIT II. This survival benefit only became apparent after nine months and a lower EF identified patients at increased risk of death. The increasingly aggressive management of acute coronary syndrome (e.g. virtual disappearance of thrombolysis in patients with an acute ST-segment elevation coronary syndrome in favour of primary percutaneous intervention in the Netherlands) may result in a different group of patients than those studied in MADIT II (1997 to 2001). Patients with an EF <30% following myocardial infarction are more likely to shy away from device therapy the longer they have been stable following the incident.
To put this into perspective: the results of the SCD-HeFT study (ICD implantation for primary prevention of sudden cardiac death in patients with heart failure) were highly significant: 7.2% mortality reduction (36.1 vs. 28.9%). This translates into the implantation of 14 ICDs to prevent one death over the course of five years; of those 14 patients receiving an ICD, four will die regardless of ICD implantation, three to five persons will experience inappropriate shocks, and device-related complications (infections, thrombosis, etc) are always lurking.9
The threshold to implant devices is probably higher for centres without an EP lab or permission to implant ICDs. The higher percentage of patients receiving CRT in our study (43% of those with an indication) compared with ICD (14%) may well reflect this phenomenon, as CRT implantations in the Netherlands are not restricted to a limited number of hospitals. Lastly, ICD implantation should only be considered in patients without reversible cardiac dysfunction, who receive optimal pharmacological management.1,10
Limitations of our study relate to the short period of the audit, the fact that this was a single-centre study and the absence of a complete work-up, which should have included assessment of left ventricular systolic function and Holter monitoring in all patients, and detailed echocardiographic studies for the detection of asynchrony and /or electrophysiological studies in selected patients. This does not detract from the fact that the study reflects current day-to-day practice in a large regional teaching hospital in the Netherlands without an electrophysiological lab. If a full work-up of all patients had been carried out the number of patients eligible for device therapy would have even been higher.
The potential implications of rigidly applying current guidelines for ICD implantations in the Netherlands are considerable. Extrapolating the data from a hospital addressing 2.0% of the population to the total Dutch population results in 13,500 [(270) x (100 / 2.0)] ‘old’ patients deserving an ICD and 8400 [(168) x (100 / 2.0)] ‘new’ patients eligible for ICD implantation on a yearly basis. These figures lead to an incidence of 510 ICDs per million inhabitants per year in the Netherlands. Currently, 2100 ICDs are implanted yearly in the Netherlands (125 ICDs per million per year). If indications are extended to patients with nonischaemic cardiomyopathies, implantation rates are bound to increase even more.9
Even if the substantial financial consequences of increasing numbers of ICD implantations are not taken into consideration,6 the consequences of the concomitant increase in device replacements should not be taken lightly. Figure 1 shows the (anticipated) yearly number of primo ICD implantations and ICD replacements if a clinic starts implanting ICDs at a rate of 100 per year. In the ninth year of the programme the number of reimplantations exceeds the number of primo implantations, as 79% of implanted ICDs need replacement after four years (based on the year 1999 ICD technology) and 55% after eight years.11
Figure 1.
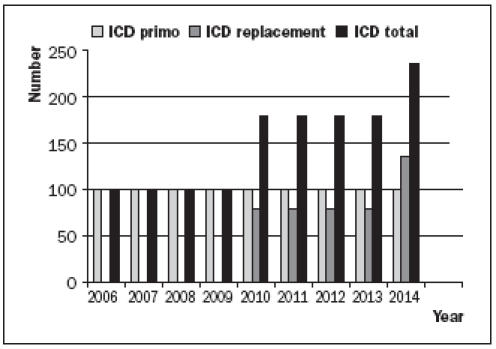
Anticipated yearly number of ICD implantations, when started in 2006 at a rate of 100/year.
Conclusion
The guidelines for ICD implantation, particularly for the primary prevention of sudden cardiac death, are not reflected in day-to-day clinical practice. The logistic and financial consequences of rigidly applying current guidelines would be considerable. A balanced, thoughtful approach and optimisation regarding risk assessment are mandatory to implant devices in those patients who are most likely to benefit.
References
- 1.Priori SG, Aliot E, Blomstrom-Lundqvist C, Bossaert L, Breithardt G, Brugada P, et al. Task Force on Sudden Cardiac Death of the European Society of Cardiology. Eur Heart J 2001;22:1374-1450. [DOI] [PubMed] [Google Scholar]
- 2.Moss AJ, Hall WJ, Cannom DS, Daubert JP, Higgins SL, Klein H, et al. Improved survival with an implanted defibrillator in patients with coronary disease at risk for ventricular arrhythmia. Multicenter Automatic Defibrillator Implantation Trial Investigators. New Engl J Med 1996;335:1933-940. [DOI] [PubMed] [Google Scholar]
- 3.Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, et al. Multicenter Automatic Defibrillator Implantation Trial II Investigators. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. New Engl J Med 2002;346:877-83. [DOI] [PubMed] [Google Scholar]
- 4.Priori SG, Aliot E, Blomstrom-Lundqvist C, Bossaert L, Breithardt G, Brugada P, et al. Update of the guidelines on sudden cardiac death of the European Society of Cardiology. Eur Heart J 2003; 24:13-5. [DOI] [PubMed] [Google Scholar]
- 5.Cunningham AD, Plummer CJ, McComb JM, Lord SW, Cunningham MW, Toussiant JM, et al. The implantable cardioverter-defibrillator: postcode prescribing in the UK 1998-2002. Heart 2005;91:1280-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Khatib SM, Anstrom KJ, Eisenstein EL, Peterson ED, Jollis JG, Mark DB, et al. Clinical and economic implications of the Multicenter Automatic Defibrillator Implantation Trial-II. Ann Intern Med 2005;142:593-600. [DOI] [PubMed] [Google Scholar]
- 7.van Erven L, van Dessel PFHM, Simmers TA, van Gelder IC, Hauer RNW, Wever EFD, et al. Guidelines ICD implantation 2005 – an update. http://www.cardiologie.nl/2/pagecontent/main_richtlijnen/20060821/icd2005.pdf .
- 8.Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, et al. Cardiac Resynchronization-Heart Failure (CARE-HF) Study Investigators. New Engl J Med 2005;352: 1539-49. [DOI] [PubMed] [Google Scholar]
- 9.Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, et al. Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) Investigators. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. New Engl J Med 2005;352:225-37. [DOI] [PubMed] [Google Scholar]
- 10.Swedberg K, Cleland J, Dargie H, Drexler H, Follath F, Komajda M, et al.; Task Force for the Diagnosis and Treatment of Chronic Heart Failure of the European Society of Cardiology. Guidelines for the diagnosis and treatment of chronic heart failure: executive summery (update 2005): The Task Force for the Diagnosis and Treatment of Chronic Heart Failure of the European Society of Cardiology. Eur Heart J 2005;26:1115-40. [DOI] [PubMed] [Google Scholar]
- 11.Hauser RG. The growing mismatch between patient longevity and the service life of implantable cardioverter-defibrillators. J Am Coll Cardiol 2005;45:2022-5. [DOI] [PubMed] [Google Scholar]


