Abstract
Transcriptional suppression of 15-lipoxygenase-1 (15-LOX-1) helps enable human colorectal cancer cells escape apoptosis, a critical mechanism for colonic tumorigenesis. GATA-6 is strongly expressed in vitro in cancer cells; its downregulation by pharmaceuticals is associated with reversal of 15-LOX-1 transcriptional suppression. The mechanistic contribution of GATA-6 overexpression to colonic tumorigenesis, especially concerning 15-LOX-1 transcriptional suppression, remains unknown. We tested whether GATA-6 is differentially overexpressed in human colorectal cancers and whether reversing GATA-6 overexpression in colon cancer cells is sufficient to restore 15-LOX-1 expression and influence cell proliferation or apoptosis. The expression of GATA-6 RNA and protein was measured in paired human colorectal cancer and normal tissues from two separate patient groups. We used GATA-6 small interfering RNA transfection to downregulate GATA-6 expression and examine the effects of this downregulation on 15-LOX-1 expression, cell proliferation, and apoptosis in Caco-2 and HCT-116 colon cancer cells with and without the nonsteroidal anti-inflammatory drug NS-398 or the histone deacetylase inhibitor sodium butyrate. GATA-6 mRNA and protein expressions were higher in cancer than normal epithelia of the colon. GATA-6 knockdown was insufficient by itself but contributed significantly to restoring 15-LOX-1 expression and inducing apoptosis by NS-398 or sodium butyrate. Maintaining 15-LOX-1 transcriptional silencing in cancer cells is a multifactorial process involving GATA-6 overexpression and other regulatory events.
Keywords: transcriptional regulation, colon cancer, apoptosis
Introduction
GATA is a family of transcriptional regulatory proteins with a highly conserved DNA-binding domain containing two zinc fingers that target the DNA sequence (A/T)GATA(A/G) (1). GATA-4, -5, and -6 are expressed in overlapping patterns during the development of endoderm-derived organs such as the intestines (2).
15-Lipoxygenase-1 (15-LOX-1) is an enzyme that is involved in oxidative metabolism of linoleic acid, which is expressed during terminal differentiation of normal cells (3-5) but transcriptionally silenced in cancer cells (6). Expression of 15-LOX-1 is downregulated in colonic tumorigenesis (7-9); restoring 15-LOX-1 expression induces apoptosis and inhibits tumorigenesis in colon cancer cells (8, 10-12, 13). GATA-6 expression is inversely related to the induction of 15-LOX-1 expression during induced terminal differentiation in Caco-2 colon cancer cells by sodium butyrate (NaBT) treatment (14), and GATA-6 inhibits the ability of nonsteroidal anti-inflammatory drugs (NSAIDs) to induce 15-LOX-1 transcription and thus apoptosis in colon cancer cells (6). These data suggested that GATA-6 promotes tumorigenesis by acting as a transcriptional suppressor of 15-LOX-1 to inhibit apoptosis in cancer cells. Additionally, the following lines of evidence suggest that GATA-6 may promote tumorigenesis, especially in the intestines. First, GATA-6 expression is higher in the proliferating region of the intestinal crypts than in the tips of the intestinal villi, where cells undergo differentiation and apoptosis (15). Second, GATA-6 is highly expressed in gastric, colonic, pancreatic, pulmonary, and prostatic cancer cell lines (16-18). Third, in contrast with the GATA-4 and -5 promoters, which are methylated in human gastric, colorectal (17), and lung cancers (18), the GATA-6 promoter remains unmethylated, so GATA-6 expression may contribute to tumorigenesis in these sites.
The relative mechanistic contribution of GATA-6 overexpression to colonic tumorigenesis and 15-LOX-1 transcriptional suppression has, however, remained unknown: specifically, whether GATA-6 overexpression occurs in vivo in human cancers and the biologic effects of reversing GATA-6 overexpression on cancer cells. Therefore, we examined whether GATA-6 is overexpressed in human colorectal cancer in vivo and the effects of GATA-6 overexpression reversal on important molecular events in colorectal cancer such as 15-LOX-1 expression, cell proliferation, and apoptosis.
MATERIALS AND METHODS
Cells, antibodies, and reagents
We obtained Caco-2 human colon carcinoma and HCT-116 colon cancer cell lines from the American Type Culture Collection (Manassas, VA). We purchased antihuman GATA-6 antibodies from Santa Cruz Biotechnology (Santa Cruz, CA); siGENOME SMARTpool small interfering RNA (siRNA) for GATA-6 and a nonspecific control siRNA (siGLO RISC-Free siRNA) were obtained from Dharmacon (Lafayette, CO). Rabbit polyclonal antiserum to recombinant human 15-LOX-1 was obtained as described previously (11). Caffeic acid (CAF) was purchased from Cayman Chemical Co. (Ann Arbor, MI). Dibutyryl cAMP (dbcAMP) was purchased from Sigma-Aldrich (St. Louis, MO). Other reagents, molecular-grade solvents, and chemicals were obtained from commercial manufacturers or as specified.
Acquisition of clinical samples
We obtained surgically resected specimens of normal and malignant colorectal tissues from patients at The University of Texas M. D. Anderson Cancer Center through the Tissue Procurement and Banking Facility. For each patient, samples were procured from both the tumor area and the normal-appearing mucosa. Fresh-frozen paired colorectal normal and malignant mucosa samples were obtained from each of 33 patients. Tissue blocks were kept frozen at -70°C until processed. M. D. Anderson Cancer Center’s Institutional Review Board approved this study.
Cell cultures
Caco-2 cells were grown in Eagle’s Minimal Essential Medium containing 15% fetal bovine serum, and HCT-116 cells were grown in RPMI 1640 medium containing 10% fetal bovine serum in a humidified atmosphere containing 5% CO2 at 37°C. Cell culture media were supplemented with 1% penicillin/streptomycin.
Analysis of GATA-6 expression by immunohistochemical staining
Frozen (-20°C) 5-μm-thick sections from both the tumor area and the normal-appearing mucosa were cut, air dried, and fixed in acetone for 30 sec. At the time of staining, the sections were incubated with 3% hydrogen peroxidase in ethanol for 30 min to inactivate endogenous peroxides. Nonspecific antibody binding sites were blocked using 20% goat serum. Tissue sections were incubated overnight in 1:50 rabbit anti-human GATA-6 polyclonal antibody (H92) (Santa Cruz Biotechnology) at 4°C. The next day, the sections were washed and then incubated with biotinylated anti-rabbit antibody solution followed by avidin-biotinylated horseradish peroxidase complex (Vectastain Elite ABC; Vector Laboratories, Burlingame, CA). Slides were washed, reincubated in a solution of 0.1 M 3,3′-diaminobenzidine in 0.05 M tris-buffered saline with 0.5 ml of 3% hydrogen peroxide DAB solution enhanced with nickel cobalt (DAB-Ni kit, Zymed Laboratories, San Francisco, CA), and then counterstained with 1% methyl green for 2 min. For negative-control experiments, rabbit serum was substituted for the primary GATA-6 antibody solution.
GATA-6 expression analyses by cDNA arrays
The Cancer Profiling Array I (BD Biosciences, Clontech, Palo Alto, CA) consists of 241 paired cDNA samples representing 13 different tissue types. Each pair consists of a tumor sample and a corresponding normal tissue sample obtained from the same individual. Colon tissues represented on this array included 38 normal and tumor pairs from 34 patients. Clinical characteristics of samples are available on the Web site http://bioinfo.clontech.com/dparray.
We used polymerase chain reaction (PCR) to amplify a human GATA-6 cDNA fragment (486 bp) from the template of GATA-6 expression vector (pCMV6-XL6-GATA6 vector, OriGene Technologies, Inc., Rockville, MD) with the primers 5′-GAG GGA ATT CAA ACC AGG AA-3′ (forward) and 5′-CAA GCC TCT TGG GAA AAA CA-3′ (reverse). The cDNA probe was labeled with α-32P-deoxycytidine triphosphate by random primer labeling and hybridized to the Cancer Profiling Array I membrane according to the manufacturer’s protocol. Equal loading was confirmed by probing randomly selected arrays from the same lot of printed array with ubiquitin as part of the printing quality control. Data were acquired and quantified using the Storm PhosphoImager and ImageQuant version 5.2 software (Molecular Dynamics, Sunnyvale, CA).
siRNA transfection and treatment with NS-398 and NaBT
Cells were cultured to 40%-50% confluence and then transfected with 100 nmol of a pooled mixture of four SMART-selected siRNA duplexes (SMARTpool; Dharmacon) for GATA-6 or a nonspecific control siRNA (siGLO RISC-Free siRNA; Dharmacon) using Lipofectamine 2000 (Invitrogen, Carlsbad, CA). In some experiments, NS-398 (30 μM), or NaBT (0.25 mM), or an equal amount of solvent (control) was added 48 h after the siRNA transfection.
Quantitative real-time reverse-transcriptase PCR
Total RNA was extracted from cells using TRI reagent (Molecular Research Center, Cincinnati, OH). The isolated RNA was size fractionated by electrophoresis on a 1% agarose-formaldehyde gel, stained with ethidium bromide, and confirmed to be of adequate quality (clear RNA bands for 18S, 28S, and 5S; 28S:18S of 2:1). Extracted RNA was quantified using an RNA quantitation kit (RiboGreen; Molecular Probes, Eugene, OR). A 500-ng aliquot of each RNA sample was reverse transcribed in a 20-μl reaction volume using the iScript cDNA synthesis kit (Bio-Rad Laboratories, Hercules, CA). Real-time reverse-transcriptase (RT)-PCR was carried out in 25 μl of a reaction mixture containing 1 μl of cDNA (25 ng/μl), 12.5 μl of 2× TaqMan Universal PCR Master Mix (Applied Biosystems, Foster City, CA), 10.25 μL double distilled water, and 1.25 μl of primer and probe mixture (Applied Biosystems). Real-time PCR assays were performed in triplicate using a 7300 real-time PCR system (Applied Biosystems) with the following conditions: 50°C for 2 min, 95°C for 10 min, 40 cycles at 95°C for 15 sec, and 60°C for 1 min. A sequence-detection program calculated a threshold cycle number at which the probe cleavage-generated fluorescence exceeded the background signal (19).
Measurement of relative RNA expression level
We calculated the relative RNA expression level using a comparative threshold cycle method (19). The sets of gene primer and probe for the target genes (e.g., GATA-6 and 15-LOX-1) were confirmed to have amplification efficiency equal to that of the internal reference gene (HPRT1). The relative expression level of an individual target gene was normalized to the reference gene and to a calibrator sample that was run on the same plate. We calculated the normalized relative expression level of a target gene in an individual sample using the following formula:
in which the real-time RT-PCR efficiency of the target gene transcript is denoted by Etarget and that of the reference gene transcript by Ereference; CT = threshold cycle number (19). Thus, the relative RNA expression level of a gene is a unitless number relative to that of the calibrator sample (20). We calculated relative RNA expression with commercial software (SDS V1.2; Applied Biosystems).
Western blot analyses
Cells were homogenized in lysis buffer [0.5% NP40, 20 mM MOPS (pH 7.0), 2 mM EGTA, 5 mM EDTA, 30 mM sodium fluoride, 40 mM β-glycerophosphate, 2 mM sodium orthovanadate, 1 mM phenylmethysulfonylfluoride, and 1× complete protease inhibitor cocktail (Roche Applied Science, Indianapolis, IN)]. Equivalent amounts of protein (60 μg crude protein/sample) were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane. Blots were incubated with a rabbit antibody to human GATA-6 (1:500, Santa Cruz Biotechnology) overnight at 4°C and then analyzed by the enhanced chemiluminescence method. HCT-116 cells transfected with GATA-6 full-length cDNA expression vector (pCMV6-XL6-GATA6 vector) were used as a positive control, and histone H1 was used as an internal control. Cells were processed for 15-LOX-1 Western blot analyses as has been described previously (21).
Chromatin immunoprecipitation assays
Caco-2 and HCT-116 cells were transfected with GATA-6 SMARTpool siRNA and nonspecific control siRNA (siGLO RISC-Free siRNA) as described previously. Cross-linking was performed 48 h after transfection by adding formaldehyde to the culture medium to a final concentration of 1% and incubating for 10 min at 37°C. We performed chromatin immunoprecipitation (ChIP) assays using a commercial assay kit according to the manufacturer’s protocol (Upstate Cell Signaling Solutions, Waltham, MA). Chromatin was immunoprecipitated using an affinity purified goat anti-human GATA-6 polyclonal antibody (C-20) (Santa Cruz Biotechnology). To amplify a 355-bp fragment of the human 15-LOX-1 promoter, we used the primers 5′-TAC ACA CGT GCA TAA CTC C-3′ (sense) and 5′-CCC ATC TTG CTC AAA GAT G-3′ (antisense) using the following conditions: 94°C for 3 min and then 94°C for 20 sec; 60°C for 30 sec; and 71.5°C for 70 sec for 28 cycles. We optimized the PCR conditions for the primer set to ensure that the product yield was within the linear range (data not shown).
In vitro cell-survival assays
We measured cell survival for Caco-2 and HCT-116 cells in vitro using the sulforhodamine B (SRB) technique (22). Cells were plated in 96-well plates at equal cell densities and cells were transfected the next day with GATA-6 siRNA, nonspecific siRNA, or transfection medium (Lipofectamine) alone. At 72 and 96 h after transfection, the cells were fixed with trichloracetic acid and stained with SRB. Optical densities were measured at 490 nm with a microplate reader (Emax; Molecular Devices, Sunnyvale, CA).
Enzyme immunoassay measurements
13-S-HODE levels were measured in cell lysates and cell culture media by use of a commercially available enzyme immunoassay (EIA) kit (Assay Designs Inc., Ann Arbor, MI), as described previously (21).
Assessment of apoptosis
We measured apoptosis by caspase-3 activity and DNA fragmentation assays. Cells were plated to 50% confluence in 100 cm2 dishes and were then transfected with GATA-6 siRNA, nonspecific siRNA, or transfection medium alone. Cells were harvested at 72 and 96 h after transfection. Caspase-3 activity and DNA fragmentation assays were performed as described previously (13).
Statistical analyses
We used the sign test for nonparametric data analyses for GATA-6 expression (immunohistochemistry and cDNA arrays). We used one-way analysis of variance (ANOVA) to compare various quantifiable outcome measures (e.g., 15-LOX-1 and GATA-6 expression levels) in different experimental conditions (e.g., GATA-6 siRNA, nonspecific siRNA, or transfection medium alone) after log transformation of data. Data were analyzed with SAS software (SAS Institute, Cary, NC). Reported P values were two-sided, were considered statistically significant at the 0.05 level, and reflected the Bonferroni adjustment for multiple comparisons.
RESULTS
GATA-6 overexpression in human colorectal cancers
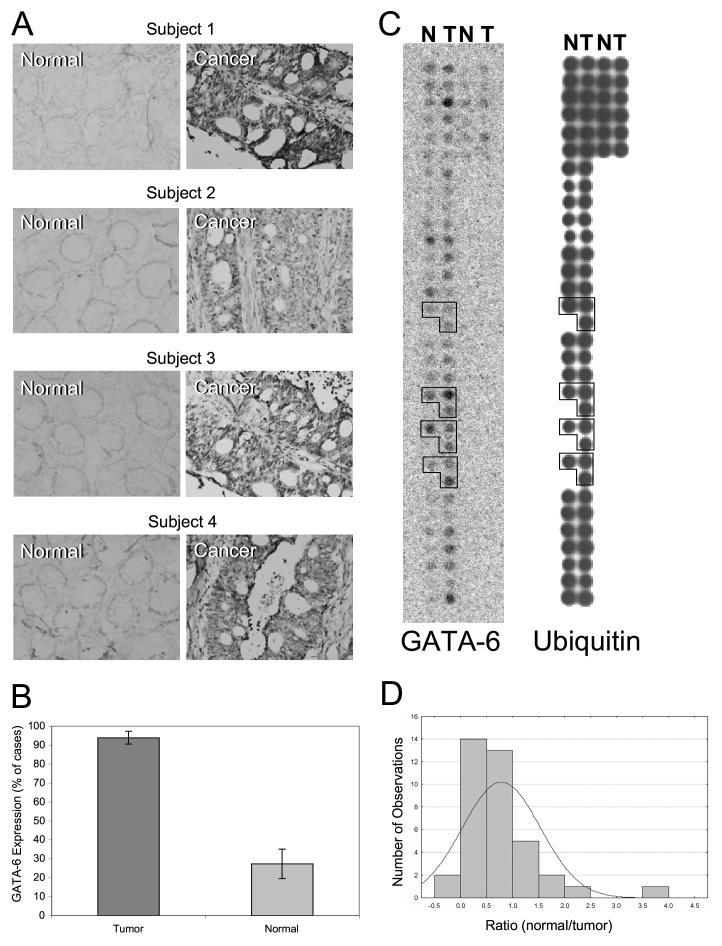
GATA-6 protein expression, analyzed by immunohistochemical staining, was markedly higher in colon cancer epithelial cells than in their paired normal colorectal epithelia in 31 (94%) of 33 stained pairs (P < 0.01) (Fig. 1 A). GATA-6 immunohistochemical staining (regardless of intensity) was detected in 94% of cancer mucosa compared with only 23% of normal mucosa (P < 0.01) (Fig. 1 B). The median intensity of staining was +2 in the tumors and 0 in the normal tissues. GATA-6 mRNA expression in the colon cancer part of the Cancer Profiling Array I that consisted of cDNA prepared from paired normal and cancerous colon tissues showed that GATA-6 mRNA was overexpressed in the majority of tumors (29 of 38 pairs, 76%; P < 0.01) (Fig. 1 C) with a median ratio of normal-to-tumor expression of 0.69 (Fig. 1 D). The normal-to-tumor GATA-6 expression ratio was higher in patients with stages I-III disease [0.92 ± 0.15 (mean ± SE)] than in patients with stage IV disease (0.466 ± 0.12) (P = 0.058).
Figure 1.
GATA-6 expression in normal and colorectal cancer tissues. A) Paired normal and colorectal cancer tissues that were immunohistochemically stained for expression of GATA-6. GATA-6 nuclear staining is shown in brown. Sections were counterstained with methyl green (greenish blue color). B) Proportion of cases with positive immunohistochemical staining for GATA-6 in paired colorectal cancer and normal tissues shown in A. C) Expression profile of GATA-6 mRNA in normal and tumor colon tissue samples in the colon cancer part of the Cancer Profiling Array I hybridized with a human GATA-6 cDNA probe labeled with α-32P-deoxycytidine triphosphate. N = normal samples; T = tumor samples. For some patients, samples were obtained for both early and late tumor-development stages (marked with open polygons). A randomly selected array from the same lot of printed arrays was probed with ubiquitin to confirm equal loading. D) The ratio of GATA-6 expression in paired normal and cancer tissues shown in C.
GATA-6 knockdown effects on 15-LOX-1 expression
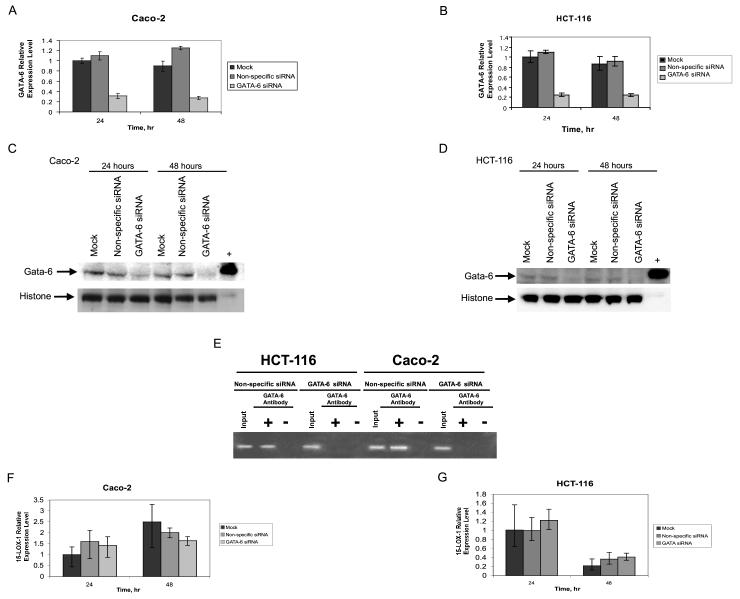
GATA-6 siRNA decreased GATA-6 mRNA expression by approximately 72% at 24 h and by 78% at 48 h in Caco-2 cells (P < 0.0001), as measured by real-time RT-PCR assays (Fig. 2 A). Similarly, GATA-6 siRNA reduced GATA-6 mRNA expression by 75% in HCT-116 cells at 24 and 48 h (P < 0.0001) (Fig. 2 B). Transfection with nonspecific siRNA failed to reduce GATA-6 expression [compared with transfection with medium alone (mock)] at either 24 or 48 h in either Caco-2 or HCT-116 cells (Fig. 2 A and B). GATA-6 siRNA transfection also downregulated GATA-6 protein expression in Caco-2 and HCT-116 cells (Fig. 2 C and D). GATA-6 binding to the 15-LOX-1 promoter was detected in both Caco-2 and HCT-116 cells with ChIP assays using GATA-6 antibodies in cells transfected with the control (nonspecific) siRNA but not in cells transfected with GATA-6 siRNA (Fig. 2 E). Expression levels for 15-LOX-1 mRNA were similar in cells transfected with nonspecific siRNA or GATA-6 siRNA in either the Caco-2 or HCT-116 cell lines at 24 h (P = 0.12 for Caco-2 and P = 0.29 for HCT-116) or 48 h (P = 0.13 for Caco-2 and P = 0.54 for HCT-116) (Fig. 2 F and G). As in Caco-2 and HCT-116, GATA-6 downregulation by GATA-6 siRNA failed to increase 15-LOX-1 mRNA expression levels in RKO or DLD-1 colorectal cancer cells (data not shown). GATA-6 siRNA transfections significantly reduced the expression of human hepatocyte nuclear factor 4-alpha (HNF4A), a GATA-6 target gene, compared with this expression in mock- or nonspecific siRNA-transfected cells (data not shown) in Caco-2 cells (P < 0.0001) and HCT-116 cells (P < 0.0001).
Figure 2.
GATA-6 knockdown effects on 15-lipoxygenase-1 (15-LOX-1) expression in colorectal cancer cells. A and B) Effects of GATA-6 small interfering RNA (siRNA) transfection on GATA-6 RNA expression. A) Caco-2 and B) HCT-116 cells were transfected with a pool of four SMART-selected siRNA duplexes for GATA-6 (GATA-6 siRNA), a nonspecific siRNA sequence (nonspecific siRNA), or transfection media only (mock), and GATA mRNA expression was measured by real-time reverse-transcriptase polymerase chain reaction (RT-PCR) for the indicated times. The relative expression levels were calculated as the values relative to that of the calibrator sample (mock at 24 h). Values shown are the means ± SDs of triplicate measurements (repeated experiments showed similar results). C and D) Effects of GATA-6 siRNA on GATA-6 protein expression, measured by Western blotting. C) Caco-2 and D) HCT-116 cells were transfected with GATA-6 siRNA, nonspecific siRNA, or mock, as described in A and B. Cells were harvested at the indicated time points following transfection. The “+” indicates positive control (HCT-116 cells transfected with a GATA-6 full-length cDNA expression vector). E) HCT-116 and Caco-2 cells were transfected with GATA-6 siRNA or nonspecific siRNA. Cells were formaldehyde cross-linked 48 h after transfection and subjected to chromatin immunoprecipitation (ChIP) assays using GATA-6 antibody. A 355-bp fragment of the 15-LOX-1 promoter was amplified by PCR as described in the “Materials and Methods” section. GATA-6 antibody “+” or “-” indicates immunoprecipitations with or without GATA-6 antibody, respectively, and “Input” indicates total DNA before immunoprecipitation. F and G) Effects of GATA-6 downregulation on 15-LOX-1 RNA expression. F) Caco-2 and G) HCT-116 cells were transfected with GATA-6 siRNA or nonspecific siRNA, as described in A and B. 15-LOX-1 mRNA expression was measured by real-time RT-PCR at the indicated times. The relative expression levels were calculated as the values relative to that of the calibrator sample (mock at 24 h). Values shown are the means ± SDs of triplicate measurements (repeated experiments showed similar results).
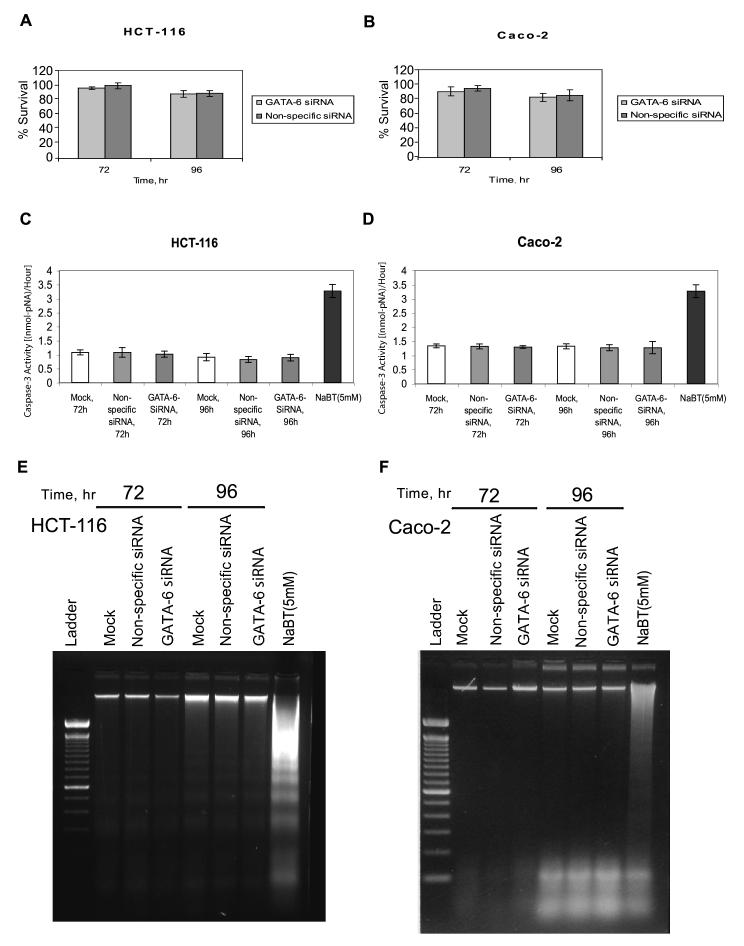
GATA-6 knockdown effects on colon cancer cell proliferation and apoptosis
Survival rates after GATA-6 siRNA transfection were identical to those of Caco-2 and HCT-116 cells transfected with nonspecific siRNA or mock transfection at 72 h (P = 0.2 for Caco-2 and P = 0.09 for HCT-116) and 96 h (P = 0.23 for Caco-2 and P = 0.55 for HCT-116) (Fig. 3 A and B). Apoptosis induction rates as measured by caspase-3 activity at 72 and 96 h were similar in Caco-2 and HCT-116 cells transfected with GATA-6 siRNA, nonspecific siRNA or mock (P = 0.95 for Caco-2 and P = 0.1 for HCT-116) (Fig. 3 C and D). Treatment of both Caco-2 and HCT-116 cells with NaBT at 5 mM (as positive control) significantly increased caspase-3 activity (P < 0.0001 for both). Apoptosis measurement by DNA laddering at 72 and 96 h also showed no differences between Caco-2 or HCT-116 cells transfected with either GATA-6, nonspecific siRNA or mock, whereas treatment with NaBT(5 mM) induced the typical DNA laddering indicative of apoptosis (Fig. 3 E and F).
Figure 3.
Effects of GATA-6 on cell proliferation and apoptosis of colon cancer cells. A and B) Effects of GATA-6 knockdown on colon cancer cell proliferation. A) HCT-116 and B) Caco-2 cells were transfected with a pool of four SMART-selected small interfering RNA (siRNA) duplexes for GATA-6 (GATA-6 siRNA), a nonspecific siRNA sequence (nonspecific siRNA), or transfection media only (mock [no siRNA]). GATA-6 effects on cell survival were determined by sulforhodamine B assays for the indicated times. The relative survival percentages were calculated as the values relative to that of the mock experiment. Values shown are the means ± SDs of six experiments. C-F) GATA-6 effects on apoptosis. HCT-116 cells (C and E) and Caco-2 cells (D and F) transfected with GATA-6 siRNA, nonspecific siRNA, or transfection media only (mock) were harvested at the indicated times, and apoptosis was measured by caspase-3 enzymatic activity assays (C and D) or by DNA ladder assays (E and F). NaBT 5 mM represents experiments in which HCT-116 cells were treated with 5 mM sodium butyrate (positive control) and harvested at 48 h.
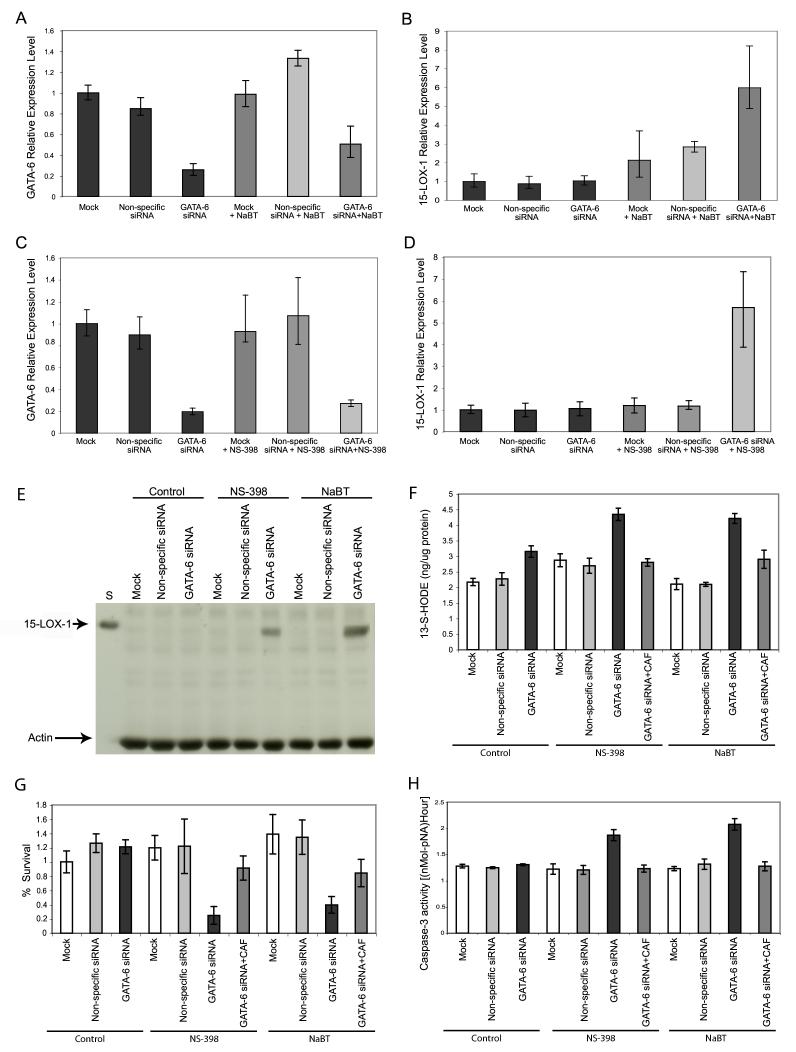
GATA-6 knockdown effects on 15-LOX-1 expression and apoptosis induction by NS-398 and NaBT
NaBT at 0.25 mM [markedly lower than the previously reported concentration (5 mM) used to downregulate GATA-6 and induce apoptosis and 15-LOX-1 expression (14, 23)] failed to downregulate GATA-6 in Caco-2 cells (P = 0.079) (Fig. 4 A). GATA-6 siRNA significantly reduced GATA-6 expression in Caco-2 cells with and without NaBT treatment [compared with cells transfected with either nonspecific siRNA or transfection media alone (mock)] (P < 0.0001) (Fig. 4 A). GATA-6 siRNA transfection alone failed to increase 15-LOX-1 expression, when compared with mock or nonspecific siRNA transfection (P = 0.96) (Fig. 4 B). Caco-2 cells transfected with GATA-6 siRNA and treated with NaBT, however, had significantly higher 15-LOX-1 expression than did cells either transfected with nonspecific siRNA and treated with NaBT or transfected with GATA-6 siRNA but not treated with NaBT (P < 0.0001) (Fig. 4 B).
Figure 4.
Effects of GATA-6 knockdown on NS-398 and sodium butyrate (NaBT) induction of 15-lipoxygenase-1 (15-LOX-1) expression and apoptosis in colon cancer cells. A) Effects of GATA-6 siRNA transfection and NaBT (0.25 mM) treatment on GATA-6 RNA expression, measured by real-time reverse-transcriptase polymerase chain reaction (RT-PCR). Caco-2 cells were transfected with a pool of four SMART-selected small interfering RNA (siRNA) duplexes for GATA-6 (GATA-6 siRNA), a nonspecific siRNA sequence (nonspecific siRNA), or transfection media only (mock). Cells were treated 48 h post-transfection with 0.25 mM NaBT for 6 h and then harvested for RNA measurements. GATA-6 mRNA expression was measured by real-time RT-PCR. Values shown are the means ± SDs of triplicate measurements (repeated experiments showed similar results). B) Effects of GATA-6 siRNA transfection and NaBT (0.25 mM) treatment on 15-LOX-1 RNA expression, measured by real-time RT-PCR, as described in A. C) Effects of GATA-6 siRNA transfection and NS-398 (30 μM) treatment on GATA-6 RNA expression, measured by real-time RT-PCR. Caco-2 cells were transfected with GATA-6 siRNA, nonspecific siRNA or mock, as for Fig. 4A, and then treated with 30 μM NS-398 for 6 h. Values shown are the means ± SDs of triplicate measurements (repeated experiments showed similar results). D) Effects of GATA-6 siRNA transfection and NS-398 (30 μM) treatment on 15-LOX-1 RNA expression, measured by real-time RT-PCR, as described in C. E-H) Effects of GATA-6 knockdown by GATA-6 siRNA combined with either NS-398 or NaBT on 15-LOX-1 protein expression, 15-LOX-1 enzymatic activity, cell survival, and apoptosis. Caco-2 cells were transfected with GATA-6 siRNA, nonspecific siRNA, or transfection media only (mock) for 48 h and then treated with 30 μM NS-398 or 0.25 mM NaBT for another 48 h. Caffeic acid (CAF) (2.2 μM) was added in experiments in which 15-LOX-1 enzymatic activity was inhibited (E). Cells were harvested, and 15-LOX-1 expression was assessed by Western blotting; S, standard positive control of HCT-116 cells stably transfected with a vector expressing 15-LOX-1 (repeated experiments showed similar results) (F). 15-LOX-1 enzymatic activity was assessed by measuring 13-S-HODE levels (enzyme immunoassay [EIA]) (G). Cell survival was evaluated by sulforhodamine B assays. The relative survival percentages were calculated as the values relative to that of the control-mock experiment. Apoptosis was assessed by measuring caspase-3 activity level (H); values shown are the means ± SDs of triplicate measurements (repeated experiments showed similar results).
NS-398 at 30 μM [below the concentration (120 μM) previously reported to downregulate GATA-6, induce 15-LOX-1 expression, and induce apoptosis (6)] did not reduce GATA-6 expression in Caco-2 cells (Fig. 4 C). GATA-6 siRNA reduced GATA-6 to similar levels in cells whether treated or not with NS-398 (Fig. 4 C); the reduction was significantly different, however, from mock- or nonspecific siRNA-transfected cells (P < 0.0001). Although GATA-6 siRNA transfection alone failed to increase 15-LOX-1 expression (P = 0.15) (Fig. 4 D), GATA-6 siRNA transfection plus NS-398 (30 μM) increased 15-LOX-1 expression significantly, compared with cells transfected with nonspecific siRNA plus NS-398 treatment and compared with cells transfected with GATA-6 siRNA but not treated with NS-398 (P < 0.0001) (Fig. 4 D). GATA-6 downregulation alone or combined with either NS-398 or NaBT did not significantly change 15-LOX-2 or 5-LOX RNA expression; COX-2 expression increased modestly in association with GATA-6 siRNA transfection alone or combined with NS-398 (< one fold) (P < 0.0001) but not with NaBT (data not shown). As with GATA-6 siRNA, dbcAMP (2 mM) reduced GATA-6 expression in Caco-2 cells (data not shown) but failed (by itself) to induce 15-LOX-1 expression (P = 0.09). dbcAMP (2 mM) combined with NaBT (0.25 mM) increased 15-LOX-1 mRNA expression 3.5-fold compared with control or dbcAMP alone (P = 0.0008) (data not shown). GATA-6 siRNA alone failed to induce 15-LOX-1 protein expression, but GATA-6 siRNA combined with a low concentration of either NS-398 or NaBT did induce 15-LOX-1 protein expression (Fig. 4 E) and significantly increased 13-S-HODE, the main product of 15-LOX-1 (versus GATA-6 siRNA, NS-398, or NaBT alone) (P < 0.0001) (Fig. 4 F). The increased 13-S-HODE level was reversed by CAF at 2.2 μM, a concentration that specifically inhibits 15-LOX-1 enzymatic activity (10) (P < 0.0001) (Fig. 4 F). GATA-6 siRNA transfection combined with either NS-398 or NaBT also significantly reduced cell survival (as measured by the number of attached cells and SRB assay) compared with effects of GATA-6 siRNA transfection alone or either agent alone (P < 0.0001) (Fig. 4 G for SRB assays; data not shown for cell count), and CAF (2.2 μM) reversed this inhibition of cell survival (P < 0.0001) (Fig. 4 G for SRB assays; data not shown for cell count). GATA-6 siRNA transfection without treatment (either NS-398 or NaBT) failed to induce apoptosis, as measured by caspase-3 activation levels (Fig. 4 H) (P = 0.52). NS-398 (30 μM) plus GATA-6 siRNA transfection significantly increased caspase-3 activation levels, compared with NS-398 plus mock or nonspecific siRNA transfections (P < 0.0001). Similarly, NaBT (0.25 mM) plus GATA-6 siRNA transfection significantly increased caspase-3 activation levels, compared with NaBT plus mock or nonspecific siRNA transfection (P < 0.0001) (Fig. 4 H). CAF, at 2.2 μM concentration, reduced caspase-3 activation levels by GATA-6 siRNA combined with either NS-398 or NaBT to levels that were similar to those produced by GATA-6 siRNA transfection, NS-398, or NaBT alone or by other controls (nonspecific siRNA or mock transfection) (P = 0.52) (Fig. 4 H).
DISCUSSION
We found that 1) GATA-6 was overexpressed in human colorectal cancer tissue at the RNA and protein levels compared with GATA-6 expression in paired normal tissue, 2) GATA-6 knockdown was insufficient by itself to reverse 15-LOX-1 transcriptional silencing, and 3) GATA-6 enhanced the ability of an otherwise inactive low dose of an NSAID or histone deacetylase (HDAC) inhibitor to reverse 15-LOX-1 silencing in colon cancer cells.
We confirmed our findings of GATA-6 overexpression by assessing two different patient groups and using two different methods of measuring expression (RNA and protein). To our knowledge, this is the first report showing differential GATA-6 expression between malignant and normal clinical colorectal mucosa. The ratios of GATA-6 RNA expression in tumors to that in paired normal tissues were approximately twofold higher in patients with distant metastases than in patients with earlier stage disease. The statistical significance of this finding was only borderline, possibly because the sample size was small. If confirmed in larger studies, this finding would indicate an association between the degree of GATA-6 overexpression and the potential for distant metastases in human colorectal tumors.
Although not studied directly, a protumorigenic role (possibly via inhibiting the expression of 15-LOX-1) for GATA-6 has been suggested by prior data. For example, restoring terminal cell differentiation in Caco-2 colon cancer cells is associated with downregulation of GATA-6 expression (14). GATA-6 overexpression inhibits NSAIDs from inducing both 15-LOX-1 expression and apoptosis (6). Since the direct role of GATA-6 in tumorigenesis has not been examined previously, we evaluated the effects of reversing GATA-6 overexpression on 15-LOX-1 expression, cell proliferation, and apoptosis in Caco-2 and HCT-116 colon cancer cells. We selected Caco-2 because it has higher GATA-6 expression than do various other colorectal cancer cell lines (data not shown) and because it has the special ability to undergo terminal differentiation with NaBT treatment, thus resembling normal colonic cells (24). Caco-2 terminal differentiation has been associated with GATA-6 downregulation (14) and mechanistically linked to 15-LOX-1 expression (21). For comparison with effects in Caco-2, we used HCT-116 cells, which have one of the lowest levels of GATA-6 expression (data not shown). The siRNA approach allowed us to adequately reduce GATA-6 expression and to block GATA-6 binding to the 15-LOX-1 promoter, where GATA-6 acts as a transcriptional suppressor (14). We also measured the expression of HNF4, which is a target gene for the transcriptional activity of GATA-6 (25), to further confirm that GATA-6 downregulation modulated GATA-6 transcriptional activity. GATA-6 knockdown by GATA-6 siRNA significantly reduced HNF4, when compared with mock or nonspecific siRNA transfection, thus confirming that knocking down GATA-6 effectively modulated its transcriptional activity. Contrary to our expectations, however, downregulating GATA-6 failed to induce 15-LOX-1 expression or induce apoptosis in HCT-116 or Caco-2 colon cancer cells.
We previously reported that GATA-6 ectopic expression in RKO and DLD-1 colorectal cancer cells inhibits the induction of 15-LOX-1 expression by NSAIDs (6). As in Caco-2 and HCT-116, however, our present study showed that GATA-6 knockdown failed to reverse 15-LOX-1 transcriptional suppression in RKO or DLD-1 cells. These findings suggested that although GATA-6 is sufficient to initiate 15-LOX-1 transcriptional suppression (6), GATA-6 is not the sole transcriptional factor maintaining 15-LOX-1 silencing in cancer cells. Therefore, we examined the relative contribution of GATA-6 toward maintaining 15-LOX-1 transcriptional silencing by evaluating whether specific GATA-6 knockdown by GATA-6 siRNA acts with other transcriptional modulatory events to reverse 15-LOX-1 transcriptional suppression. The HDAC inhibitor NaBT and the NSAID NS-398 (which is a selective cyclooxygenase-2 inhibitor) downregulate GATA-6 and induce 15-LOX-1 expression and apoptosis at high concentrations (5 mM for NaBT and 120 μM for NS-398) (6, 14). We found that a much lower concentration of NaBT (0.25 mM) or NS-398 (30 μM) failed to downregulate GATA-6. We examined the effects of combining each drug at the low concentration (that failed to induce GATA-6 downregulation) with specific GATA-6 downregulation (via GATA-6 siRNA). These combinations induced 15-LOX-1 RNA and protein expression, 13-S-HODE production, and apoptosis and inhibited cell survival. Downregulation of GATA-6 was not increased by the combinations over that by siRNA alone, indicating that modulation of transcriptional events other than GATA-6 downregulation was required to restore 15-LOX-1 transcription. We also used the experimental approach of cAMP treatment to test the effects of downregulating GATA-6 on 15-LOX-1 transcription. cAMP alone at a concentration reported to induce GATA-6 proteolysis (26) failed to induce 15-LOX-1 expression. The same concentration of cAMP combined with NaBT (0.25 mM), however, increased 15-LOX-1 expression by approximately 3.5-fold (compared with cAMP treatment alone). These results indicate 1) that GATA-6 downregulation alone is insufficient to induce 15-LOX-1 expression and apoptosis but significantly enhances the reversal of 15-LOX-1 suppression by an HDAC inhibitor or NSAID, 2) that 15-LOX-1 transcriptional silencing in cancer cells is multifactorial because reversal of 15-LOX-1 silencing required GATA-6 downregulation combined with the modulation of other transcriptional and/or epigenetic factors by NS-398 or NaBT, and 3) that induction of apoptosis was related to the induction of 15-LOX-1 expression since the reversal of 15-LOX-1 enzymatic activity by CAF [at a concentration that specifically inhibits 15-LOX-1 enzymatic activity (10)] blocked apoptosis induction. This last conclusion is consistent with our prior findings that 15-LOX-1 expression significantly contributes to apoptosis induction by either NSAIDs or HDAC inhibitors in colorectal cancer cells (11, 21). The specific link between apoptosis and 15-LOX-1 expression induction also was supported by our findings that GATA-6 siRNA transfection combined with either NS-398 or NaBT induced 15-LOX-1 expression but failed to alter the expression of 5-LOX or 15-LOX-2 or to downregulate COX-2 expression. HDAC inhibitors such as NaBT and NSAIDs such as NS-398 are known to influence various transcriptional/epigenetic factors such as histone acetylation and protein kinase G (12, 26). These and other factors, such as signal transducers and activators of transcription-6 (27, 28) and methylation (29), have been reported to individually influence 15-LOX-1 transcriptional regulation in cancer cells.
Our current findings demonstrate that the transcriptional regulation of 15-LOX-1 is combinatorial and that the modulation of more than one individual factor is required to reverse 15-LOX-1 suppression in cancer cells. These results provide a rationale for evaluating therapeutic strategies using GATA-6-targeting interventions combined with NSAIDs and HDAC inhibitors. Both classes of agents have shown promising antitumorigenic activity in the clinic. They also appear to have limited efficacy, however, and certainly have important potential adverse effects (e.g., cardiovascular toxicity of NSAIDs) that would limit their use at high doses (30-32). Both limitations may be improved by adding GATA-6-inhibiting approaches. Future mechanistic studies of the other transcriptional and/or epigenetic factors that significantly contribute to maintaining 15-LOX-1 transcriptional silencing will further elucidate the mechanisms of this silencing and thus may lead to improved approaches for treating colonic tumorigenesis.
Acknowledgments
Grant support:This research was supported by the National Cancer Institute, National Institutes of Health, Department of Health and Human Services R01 grant CA106577 (I.S.); the American Cancer Society Scholar Award RSG-04-020-01-CNE (I.S.); The University of Texas M. D. Anderson Cancer Center physician scientist grant (I.S.); Sense Corp Texas 4000 For Cancer; and the National Colorectal Cancer Research Alliance.
We thank Dr. Susan M. Fischer for her helpful feedback and Karen Phillips, from the Department of Scientific Publications, for editing the manuscript.
References
- 1.Molkentin JD. The Zinc Finger-containing Transcription Factors GATA-4, -5, and -6. UBIQUITOUSLY EXPRESSED REGULATORS OF TISSUE-SPECIFIC GENE EXPRESSION. J. Biol. Chem. 2000;275:38994–38952. doi: 10.1074/jbc.R000029200. [DOI] [PubMed] [Google Scholar]
- 2.Laverriere AC, MacNeill C, Mueller C, Poelmann RE, Burch JB, Evans T. GATA-4/5/6, a subfamily of three transcription factors transcribed in developing heart and gut. J Biol Chem. 1994;269:23177–23184. [PubMed] [Google Scholar]
- 3.Grullich C, Duvoisin RM, Wiedmann M, van Leyen K. Inhibition of 15-lipoxygenase leads to delayed organelle degradation in the reticulocyte. FEBS Lett. 2001;489:51–54. doi: 10.1016/s0014-5793(01)02080-4. [DOI] [PubMed] [Google Scholar]
- 4.van Leyen K, Duvoisin RM, Engelhardt H, Wiedmann M. A function for lipoxygenase in programmed organelle degradation. Nature. 1998;395:392–395. doi: 10.1038/26500. [DOI] [PubMed] [Google Scholar]
- 5.Hill EM, Eling T, Nettesheim P. Changes in expression of 15-lipoxygenase and prostaglandin-H synthase during differentiation of human tracheobronchial epithelial cells. Am J Respir Cell Mol Biol. 1998;18:662–669. doi: 10.1165/ajrcmb.18.5.2985. [DOI] [PubMed] [Google Scholar]
- 6.Shureiqi I, Jiang W, Fischer SM, Xu X, Chen D, Lee JJ, Lotan R, Lippman SM. GATA-6 transcriptional regulation of 15-lipoxygenase-1 during NSAID-induced apoptosis in colorectal cancer cells. Cancer Res. 2002;62:1178–1183. [PubMed] [Google Scholar]
- 7.Shureiqi I, Wojno KJ, Poore JA, Reddy RG, Moussalli MJ, Spindler SA, Greenson JK, Normolle D, Hasan AA, Lawrence TS, Brenner DE. Decreased 13-S-hydroxyoctadecadienoic acid levels and 15-lipoxygenase-1 expression in human colon cancers. Carcinogenesis. 1999;20:1985–1995. doi: 10.1093/carcin/20.10.1985. [DOI] [PubMed] [Google Scholar]
- 8.Nixon JB, Kim KS, Lamb PW, Bottone FG, Eling TE. 15-Lipoxygenase-1 has anti-tumorigenic effects in colorectal cancer. Prostaglandins Leukot Essent Fatty Acids. 2004;70:7–15. doi: 10.1016/j.plefa.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 9.Heslin MJ, Hawkins A, Boedefeld W, Arnoletti JP, Frolov A, Soong R, Urist MM, Bland KI. Tumor-associated down-regulation of 15-lipoxygenase-1 is reversed by celecoxib in colorectal cancer. Ann Surg. 2005;241:941–946. doi: 10.1097/01.sla.0000164177.95620.c1. discussion 946-947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shureiqi I, Chen D, Lee JJ, Yang P, Newman RA, Brenner DE, Lotan R, Fischer SM, Lippman SM. 15-LOX-1: a novel molecular target of nonsteroidal anti-inflammatory drug-induced apoptosis in colorectal cancer cells. J Natl Cancer Inst. 2000;92:1136–1142. doi: 10.1093/jnci/92.14.1136. [DOI] [PubMed] [Google Scholar]
- 11.Shureiqi I, Jiang W, Zuo X, Wu Y, Stimmel JB, Leesnitzer LM, Morris JS, Fan HZ, Fischer SM, Lippman SM. The 15-lipoxygenase-1 product 13-S-hydroxyoctadecadienoic acid down-regulates PPAR-delta to induce apoptosis in colorectal cancer cells. Proc Natl Acad Sci U S A. 2003;100:9968–9973. doi: 10.1073/pnas.1631086100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deguchi A, Xing SW, Shureiqi I, Yang P, Newman RA, Lippman SM, Feinmark SJ, Oehlen B, Weinstein IB. Activation of protein kinase G up-regulates expression of 15-lipoxygenase-1 in human colon cancer cells. Cancer Res. 2005;65:8442–8447. doi: 10.1158/0008-5472.CAN-05-1109. [DOI] [PubMed] [Google Scholar]
- 13.Zuo X, Wu Y, Morris JS, Stimmel JB, Leesnitzer LM, Fischer SM, Lippman SM, Shureiqi I. Oxidative metabolism of linoleic acid modulates PPAR-beta/delta suppression of PPAR-gamma activity. Oncogene. 2006;25:1225–1241. doi: 10.1038/sj.onc.1209160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kamitani H, Kameda H, Kelavkar UP, Eling TE. A GATA binding site is involved in the regulation of 15-lipoxygenase-1 expression in human colorectal carcinoma cell line, caco-2. FEBS Lett. 2000;467:341–347. doi: 10.1016/s0014-5793(00)01155-8. [DOI] [PubMed] [Google Scholar]
- 15.Gao X, Sedgwick T, Shi YB, Evans T. Distinct functions are implicated for the GATA-4, -5, and -6 transcription factors in the regulation of intestine epithelial cell differentiation. Mol Cell Biol. 1998;18:2901–2911. doi: 10.1128/mcb.18.5.2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al-azzeh ED, Fegert P, Blin N, Gott P. Transcription factor GATA-6 activates expression of gastroprotective trefoil genes TFF1 and TFF2. Biochim Biophys Acta. 2000;1490:324–332. doi: 10.1016/s0167-4781(00)00013-0. [DOI] [PubMed] [Google Scholar]
- 17.Akiyama Y, Watkins N, Suzuki H, Jair KW, van Engeland M, Esteller M, Sakai H, Ren CY, Yuasa Y, Herman JG, Baylin SB. GATA-4 and GATA-5 transcription factor genes and potential downstream antitumor target genes are epigenetically silenced in colorectal and gastric cancer. Mol Cell Biol. 2003;23:8429–8439. doi: 10.1128/MCB.23.23.8429-8439.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo M, Akiyama Y, House MG, Hooker CM, Heath E, Gabrielson E, Yang SC, Han Y, Baylin SB, Herman JG, Brock MV. Hypermethylation of the GATA Genes in Lung Cancer. Clin Cancer Res. 2004;10:7917–7924. doi: 10.1158/1078-0432.CCR-04-1140. [DOI] [PubMed] [Google Scholar]
- 19.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu J, Marsh S, Ahluwalia R, McLeod HL. Ferredoxin reductase: pharmacogenomic assessment in colorectal cancer. Cancer Res. 2003;63:6170–6173. [PubMed] [Google Scholar]
- 21.Shureiqi I, Wu Y, Chen D, Yang XL, Guan B, Morris JS, Yang P, Newman RA, Broaddus R, Hamilton SR, Lynch P, Levin B, Fischer SM, Lippman SM. The Critical Role of 15-Lipoxygenase-1 in Colorectal Epithelial Cell Terminal Differentiation and Tumorigenesis. Cancer Res. 2005;65:11486–11492. doi: 10.1158/0008-5472.CAN-05-2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, Warren JT, Bokesch H, Kenney S, Boyd MR. New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst. 1990;82:1107–1112. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]
- 23.Kamitani H, Geller M, Eling T. Expression of 15-Lipoxygenase by human colorectal carcinoma Caco-2 cell lines during apoptosis and cell differentiation. J Bio Chem. 1998;273:21569–21577. doi: 10.1074/jbc.273.34.21569. [DOI] [PubMed] [Google Scholar]
- 24.Pinto M, Robine-Leon S, Appay M, Kedinger M, Triadou N, Dussaulx E, Lacroix B, Simon-Assmann P, Haffen K, Fogh J, Zweibaum A. Enterocyte-like differentiation and polarization of the human colon carcinoma cell line Caco-2 in culture. Biol Cell. 1983;47:323–330. [Google Scholar]
- 25.Morrisey EE, Tang Z, Sigrist K, Lu MM, Jiang F, Ip HS, Parmacek MS. GATA6 regulates HNF4 and is required for differentiation of visceral endoderm in the mouse embryo. Genes Dev. 1998;12:3579–3590. doi: 10.1101/gad.12.22.3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kamitani H, Taniura S, Ikawa H, Watanabe T, Kelavkar UP, Eling TE. Expression of 15-lipoxygenase-1 is regulated by histone acetylation in human colorectal carcinoma. Carcinogenesis. 2001;22:187–191. doi: 10.1093/carcin/22.1.187. [DOI] [PubMed] [Google Scholar]
- 27.Shankaranarayanan P, Chaitidis P, Kuhn H, Nigam S. Acetylation by histone acetyltransferase CREB-binding protein/p300 of STAT6 is required for transcriptional activation of the 15-lipoxygenase-1 gene. J Biol Chem. 2001;276:42753–42760. doi: 10.1074/jbc.M102626200. [DOI] [PubMed] [Google Scholar]
- 28.Conrad DJ, Lu M. Regulation of human 12/15-lipoxygenase by Stat6-dependent transcription. Am J Respir Cell Mol Biol. 2000;22:226–234. doi: 10.1165/ajrcmb.22.2.3786. [DOI] [PubMed] [Google Scholar]
- 29.Hsi LC, Xi X, Wu Y, Lippman SM. The methyltransferase inhibitor 5-aza-2-deoxycytidine induces apoptosis via induction of 15-lipoxygenase-1 in colorectal cancer cells. Mol Cancer Ther. 2005;4:1740–1746. doi: 10.1158/1535-7163.MCT-05-0218. [DOI] [PubMed] [Google Scholar]
- 30.Steinbach G, Lynch PM, Phillips RK, Wallace MH, Hawk E, Gordon GB, Wakabayashi N, Saunders B, Shen Y, Fujimura T, Su LK, Levin B. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med. 2000;342:1946–1952. doi: 10.1056/NEJM200006293422603. [DOI] [PubMed] [Google Scholar]
- 31.Hawk ET, Levin B. Colorectal cancer prevention. J Clin Oncol. 2005;23:378–391. doi: 10.1200/JCO.2005.08.097. [DOI] [PubMed] [Google Scholar]
- 32.Kelly WK, Marks PA. Drug insight: Histone deacetylase inhibitors--development of the new targeted anticancer agent suberoylanilide hydroxamic acid. Nat Clin Pract Oncol. 2005;2:150–157. doi: 10.1038/ncponc0106. [DOI] [PubMed] [Google Scholar]