Abstract
This study investigated the binaural temporal window in adults and children 5–10.5 years of age. Detection thresholds were estimated for a brief, interaurally out-of-phase (Sπ) 500 Hz pure tone signal masked by bandpass, 100–2000 Hz Gaussian noise. In one set of conditions, the masker was consistently either in phase (No) or out of phase (Nπ). In another set of conditions, the masker changed abruptly in interaural phase (NoNπ or NπNo), and threshold was estimated at a range of delays with respect to the phase transition. Masked thresholds were also obtained in further conditions where the masker interaural phase was steady and the signal was of long duration. Age effects obtained with dynamic maskers could be accounted for by positing that children have a binaural temporal window with a relatively prolonged leading edge or that the children position the binaural temporal window relatively late with respect to the signal. Modeling of the reduced masking-level difference shown by children for a brief Sπ signal presented in a steady No or Nπ masker was more consistent with late placement of a symmetrical binaural temporal window than a binaural temporal window having a relatively prolonged leading edge.
I. INTRODUCTION
The present study investigated the binaural temporal window (Kollmeier and Gilkey, 1990; Culling and Summerfield, 1998; Holube et al., 1998; Bernstein et al., 2001) in adults and school-aged children. The binaural temporal window refers to the temporal epoch during which the auditory system integrates information related to binaural difference cues. Experiments on the binaural temporal window are often performed in the context of the masking-level difference (MLD) paradigm (Hirsh, 1948), where the signal and the masker are presented to the two ears with different interaural phase characteristics. In the paradigm used here Kollmeier and Gilkey, 1990; Holube et al., 1998), the binaural temporal window is measured using a brief, interaurally out-of-phase signal (Sπ) as a function of the temporal position of the signal with respect to an abrupt interaural phase transition of the noise masker. This transition is either from in phase to out of phase (NoNπ) or from out of phase to in phase (NπNo). The time constant of the binaural temporal window is estimated, in part, from the steepness with which the detection thresholds of the brief signals change with respect to the temporal occurrence of the signal relative to the interaural phase transition of the masking noises. Estimates of binaural time constants using this method are longer than typical estimates of monaural time constants under analogous stimulus conditions (Kollmeier and Gilkey, 1990; Holube et al., 1998), consistent with the proposal that the binaural system is “sluggish” (Grantham and Wightman, 1978; Grantham and Wightman, 1979).
Although we are not aware of any previous comparison of the binaural temporal windows in adults and school-aged children, several studies have examined monaural temporal processing in children. For example, studies have found that monaural temporal gap detection thresholds are often elevated in children (Irwin et al., 1985; Wightman et al., 1989). Children also have relatively high monaural thresholds for the detection of a pure tone signal in narrow bands of noise that are sinusoidally amplitude modulated (Grose et al., 1993). The temporal modulation transfer function (TMTF) paradigm (Viemeister, 1979) has also been used to study monaural temporal resolution in children (Hall and Grose, 1994). In this method, sensitivity to the presence of amplitude modulation is determined as a function of the modulation rate. The TMTF results indicated that although thresholds for the detection of modulation were higher in children than in adults, they were uniformly higher across low and high modulation rates, with the result that derived monaural time constants did not vary across the age range tested (four years to adult). The TMTF results therefore indicated that although children were less sensitive to the presence of modulation than adults, there was no difference in monaural temporal resolution, per se.
One motivation for the present work was to provide basic, new information on the development of temporal resolution for binaural hearing. A more specific motivation concerns previous results that have been obtained on MLDs in children. Whereas the MLD for a pure-tone signal in a wideband masker appears to be adult-like by age 5–6 years (Hall and Grose, 1990), studies have indicated that 5–10-year-old children have reduced MLDs when the masker is a narrow-band noise (Grose et al., 1995; Grose et al., 1997). Narrow-band noise maskers possess prominent envelope fluctuations (e.g., Bos and de Boer, 1966), and recent studies have indicated that the Sπ thresholds for tones presented in narrow-band noise are determined largely by information coincident with masker envelope minima (Grose and Hall, 1998; Hall et al., 1998; Buss et al., 2003), where the binaural difference cues are the largest (Buss et al., 2003). Although the monaural signal-to-noise ratio is also favorable in the envelope minima of narrowband noise maskers, listeners are not able to exploit these epochs in monaural detection, but instead apply equal weight across envelope maxima and minima (Buss et al., 1996). Listeners are able to take advantage of the favorable signal-to-noise ratios available in the masker envelope minima of monaural narrowband noise stimuli only when the narrowband noise is multiplied by a low-frequency modulator (e.g., Carlyon et al., 1989; Grose et al., 1993) or when comodulated flanking noise bands are present (e.g., Hall et al., 1984). Thus, for a single, narrowband Gaussian noise masker, listeners are able to take advantage of the good signal-to-noise ratios associated with masker envelope minima for binaural but not for monaural detection.
A developmental study by Hall et al. 2004 suggested that the relatively small MLDs shown by children in narrow-band masking noise may be due to a reduced ability to take advantage of the binaural information occurring in the relatively brief masker envelope minima. The ability to take advantage of the temporal epochs containing the most favorable binaural detection cues (masker envelope minima) may depend upon the binaural temporal window, both in terms of its duration (a shorter duration associated with better acuity), and in terms of the temporal relation between the center of the window and the optimal time to listen for the signal (optimal temporal alignment associated with better acuity). The present study examined the binaural temporal window in adults and in children aged 5–10.5 years.
II. METHODS
A. Listeners
All listeners had pure-tone detection thresholds of 20 dB Hearing Level (HL) or better at octave frequencies from 250 to 8000 Hz (ANSI, 1996). None had a history of chronic ear disease, and none had a known history of otitis media within a three-year period preceding testing. Thirteen children were recruited into the study. Two of these children were dropped from the study due to high test-retest variability (more than 15 dB variation among threshold estimates). The remaining 11 listeners (seven females and four males) ranged in age from 5 to 10.5 years, with a mean age of 8.0 years (standard deviation 1.5 years). There were 12 adult listeners (nine females and three males), ranging in age from 18 to 43 years, with a mean age of 26.6 years (standard deviation 9.2 years). All listeners were paid for participation and provided data in four sessions lasting no more than 1 h each.
B. Stimuli
The signal was a 500 Hz pure tone, ramped on and off with 5 ms cos2 ramps and 10 ms of steady state. All signals were presented in Sπ phase. Maskers were Gaussian noise samples, bandpass filtered 100–2000 Hz, with onset and offset ramps imposed by Finite Impulse Response (FIR) filters. The total duration of each masker sample was 950 ms. The masker was presented at a level of 40 dB/Hz sound pressure level (SPL). Stimulus conditions are illustrated schematically in Fig. 1. In the steady masker conditions, the masker was either No or Nπ throughout its presentation. In the dynamic masker conditions, the phase changed abruptly in the temporal center of the masker.
FIG. 1.
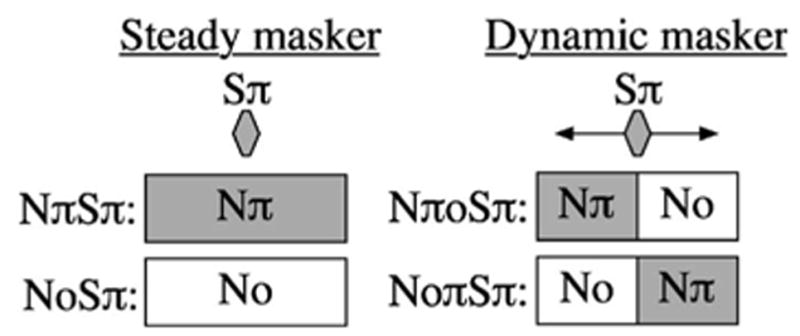
Schematic (not to scale) of the stimuli used in the steady masker and dynamic masker conditions of the main experiment. In the steady masker conditions, the signal was presented in the temporal center of the masker. In the dynamic masker conditions, the signal was presented at a range of delays relative to the abrupt interaural phase transition occurring at the temporal center of the masker.
Listeners completed the steady masker conditions first. In these conditions, the signal was coincident with the temporal center of the masker. In the dynamic masker conditions, all signal delays were defined in terms of the relation between the masker transition point and the temporal center of the signal. Listeners were randomly assigned to two groups: one group completed the NoNπ conditions before the NπNo conditions, and the other group completed these conditions in the reverse order.
Because testing time was limited with the children, procedures for estimating thresholds in the dynamic masker conditions were designed to maximize efficiency. To this end, the function associated with each set of conditions was first broadly characterized by estimating thresholds at four signal delays: one at each estimated asymptote (upper and lower), one near the masker phase transition, and one well into the region of improved thresholds. Based on the pattern of results obtained for these four delays, two additional delays were identified and tested, with the goal of increasing the number of thresholds obtained in the most steeply sloping portion of the function.
For the NoNπ condition, the four initial delays were −320, −120, −20, and 280 ms. The two additional delays were based on comparison of (1) the midpoint between the thresholds for the −320 and −20 ms delay conditions and (2) the threshold for the −120 ms delay condition. If the threshold for the −120 ms delay condition was lower than the midpoint (indicating that appreciable changes in threshold were confined to delays near the abrupt transition), then the two additional delays were −70 and 30 ms (relatively short delays, placing the signal near the abrupt transition). This was the case for all of the adults and for 10 of the 11 children. For the remaining child (age=10.5 years), the threshold for the −120 ms delay condition was higher than the midpoint between the −320 and −20 ms delay conditions (indicating an appreciable change in threshold over a longer time period), and the two additional delays were −220 and 180 ms (relatively long temporal separation relative to the abrupt transition).
The same general procedure was followed for the NπNo conditions. The four initial delays were −320, −20, 80, and 280 ms. If the threshold for the 80 ms delay condition was lower than the midpoint between the 280 and −20 ms delay conditions, then the two additional thresholds were obtained for the relatively short delays of −70 and 30 ms. As with the NoNπ conditions, this occurred for all of the adults and for 10 of the 11 children. For the remaining child (age =6.5 years), the threshold for the 80 ms delay condition was higher than the midpoint between the 280 and −20 ms delay conditions, and the two additional delays were −220 and 180 ms.
C. Threshold estimation procedure
The task used a three-alternative forced-choice procedure with the signal level adjusted in a three-down one-up track estimating the 79% correct point on the psychometric function. The signal level was adjusted in steps of 4 dB for the first two reversals and then in steps of 2 dB for the remaining six reversals. Thresholds were computed as the average level at the last six reversals. All thresholds were obtained in blocks, by condition. Two estimates were obtained in each condition, with a third estimate obtained only in cases where the first two varied by 3 dB or more. Listening intervals were marked visually using animation on a video monitor. Over the course of a threshold run, a cartoon picture was unmasked, in the style of a jigsaw puzzle, with one piece revealed following each correct response. This cartoon was completely unmasked and performed a 2 s animation at the end of the threshold run. All listeners used this interface.
D. Modeling the binaural temporal window
Thresholds were fitted with a double-sided exponential window, using the fmins function in MATLAB (MathWorks). This window acts upon and integrates interaural correlation associated with the masking stimulus. The double-sided exponential window was described by Kollmeier and Gilkey (1990) and takes the form of
| (1) |
where t is time and τ1 and τ2 are the time constants associated with the lagging and leading edges of the window, respectively. Following Kollmeier and Gilkey (1990), masking at the output of this window was estimated based on the equalization and cancellation model (Durlach, 1963), with
| (2) |
where LM is the monaural threshold, K is internal noise, and r(t) is the time-varying interaural correlation of the windowed input. The parameter K was estimated based on the MLD in steady masker condition, while the parameter LM was allowed to vary freely. This reflects an assumption that the maximum MLD in the dynamic masker conditions is equal to the MLD obtained in the steady masker conditions, but that all thresholds may suffer from the presence of the masker phase transition, those based on binaural cues and those based on monaural cues alike. As in previous studies using this fitting technique (Kollmeier and Gilkey, 1990; Holube et al., 1998), three parameters were allowed to vary: time constants for the lagging and leading edges of the window (τ1 and τ2) and an estimate of overall processing efficiency (LM). As in Kollmeier and Gilkey (1990), it was assumed that the binaural temporal window was centered on the onset of the Sπ signal in the NoNπ masker and on the offset of the Sπ signal in the NπNo masker. This assumption was based on the idea that “off-time” listening would allow improvement of the effective signal-to-noise ratio at the output of the binaural temporal window. The data for the NoNπ conditions and the NπNo conditions were fitted simultaneously.
III. RESULTS AND DISCUSSION
A. Developmental differences for the steady masker
Although the main aim of the present study was to investigate binaural temporal resolution for maskers with dynamically varying interaural phase, MLDs were also obtained for No and Nπ steady maskers in order to provide a basis for estimating K (see Methods Sec. II). Table I summarizes findings for the steady masker conditions. As indicated in Table I, the children had higher thresholds than the adults in the NπSπ and the NoSπ conditions, and also had smaller MLDs. A repeated measures analysis of variance showed that the NπSπ thresholds were higher than the NoSπ thresholds (F1,21=650; p < 0.001), that the children had higher thresholds than the adults 9F1,21=65.5; p < 0.001), and also that there was a significant interaction between threshold and group (F1,21=10.6; p < 0.005). This interaction reflects the fact that the developmental effect (higher thresholds for the children) was greater for the NoSπ threshold than for the NπSπ threshold, resulting in a smaller MLD for the children. The smaller MLD of the children in the relatively wideband masking noise used here might appear to be at odds with results reported in the studies of Hall and Grose (1990) and Grose et al. (1997), where it was found that children 5–6 years of age and older had adult-like MLDs for masking noise bandwidths wider than approximately 300 Hz. However, both Hall and Grose (1990) and Grose et al. 1997 used a long duration (400 ms) signal, so it is possible that the discrepancy between the present and past findings is related to signal duration. This possibility will be considered further below.
TABLE I.
Mean NπSπ and NoSπ thresholds (dB SPL) and derived MLDs for the steady masker conditions, with results shown separately for the two age groups. Standard deviations are in parentheses.
| NπSπ | NoSπ | MLD | |
|---|---|---|---|
| Adult | 67.5 (0.7) | 51.0 (1.6) | 16.5 (1.7) |
| Child | 70.4 (1.4) | 57.6 (3.1) | 12.8 (3.5) |
B. Developmental differences for the dynamic maskers
1. Time constants and off-time listening
Individual results for the adults and children are shown in Figs. 2 and 3, respectively, with the Sπ signal threshold plotted as a function of delay of the temporal center of the signal with respect to the masker transition point. The unfilled, downward pointing triangles represent data for the NoNπ conditions and the filled, upward pointing triangles represent data for the NπNo conditions. The columns of Table II under the “onset/offset fit” heading show the individual and median values for τ1 and τ2 and the percent variance accounted for by the data fits (the “fitted shift fit” results in the table will be considered in the following section). As can be seen in Table II, the percent of variance accounted for by the data fits varied considerably among listeners, ranging from 52% to 98% in the adults and from 43% to 94% in the children. In the statistics below that are related to parameter estimates, the median rather than the mean is used to characterize central tendency and nonparametric tests are used to test for parameter differences. This approach was adopted because evaluations of kurtosis and skewness associated with the parameters of interest were often not consistent with a normal distribution, particularly for the children. An alpha criterion of p<0.05 was adopted for statistical significance.
FIG. 2.
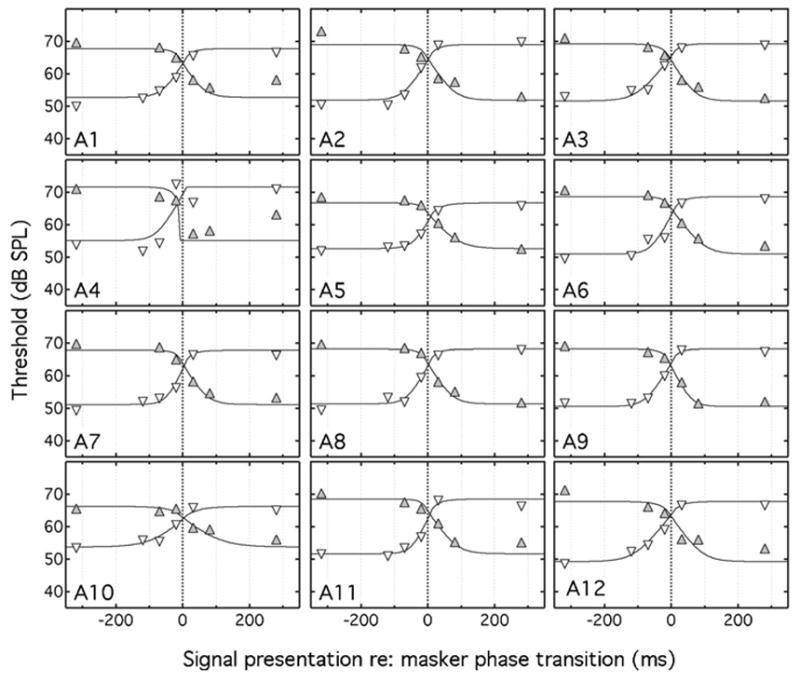
Mean Sπ thresholds for each adult (A1–A12) listener are shown by the unfilled, downward pointing triangles for the NoNπ conditions, and by the filled, upward pointing triangles for the NπNo conditions. Thresholds are plotted as a function of the delay between the signal and the interaural phase transition of the masker. The solid lines show modeled fits based on a double-sided, exponential temporal window. The dotted vertical line represents the timing of the masker phase transition.
FIG. 3.
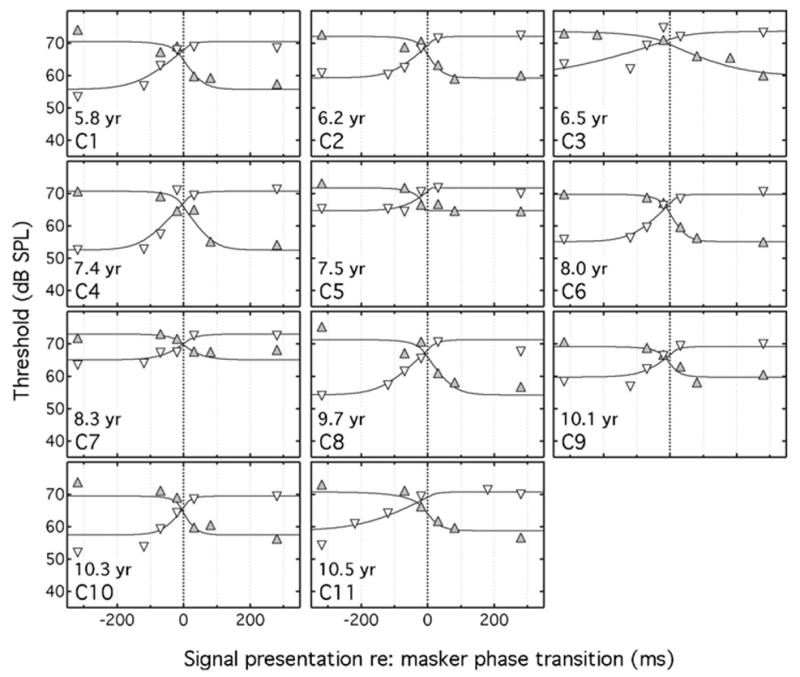
Mean Sπ thresholds for each child (C1–C11) listener are shown, following the plotting conventions of Fig. 2. Also shown is the child’s age in years.
TABLE II.
Values of τ1, τ2(ms) and percent of variance accounted for the onset/offset approach are shown on the left part of the table, and values of τ (ms), SHIFT (ms) and percent of variance accounted for in the fitted shift approach are shown on the right side of the table. Adults (A1–A12) are shown at the top of the table and children (C1–C11) are shown at the bottom of the table. AX refers to adult median values for τ and mean value for percent of variance accounted for. CX refers to like values for children. Numbers in parentheses are the lower and upper quartiles around the median.
| Onset/offset fit
|
Fitted shift fit
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Listener | τ1 | τ2 | %Var | τ | NπNo SHIFT | % var | τ | NπNo SHIFT | %var |
| A1 | 24 | 24 | 77 | 32 | 60 | 98 | 28 | −4 | 99 |
| A2 | 26 | 25 | 93 | 40 | 60 | 99 | 16 | 0 | 99 |
| A3 | 24 | 31 | 90 | 34 | 42 | 98 | 36 | −28 | 99 |
| A4 | −1 | 24 | 52 | 140 | −78 | 68 | 6 | 52 | 81 |
| A5 | 33 | 20 | 98 | 32 | 12 | 99 | 40 | −18 | 100 |
| A6 | 29 | 21 | 87 | 34 | 18 | 99 | 28 | −42 | 99 |
| A7 | 23 | 18 | 95 | 32 | 44 | 100 | 24 | −24 | 99 |
| A8 | 24 | 21 | 94 | 26 | 18 | 99 | 32 | −30 | 97 |
| A9 | 20 | 24 | 97 | 22 | 14 | 99 | 20 | −12 | 99 |
| A10 | 47 | 42 | 91 | 52 | 16 | 99 | 36 | −18 | 98 |
| A11 | 27 | 18 | 88 | 40 | 58 | 99 | 20 | −10 | 98 |
| A12 | 29 | 32 | 86 | 26 | 14 | 99 | 28 | −38 | 100 |
|
| |||||||||
| AX | 25 (24/29) | 24 (21/26) | 87 | 33 (30/40) | 18 (14/47) | 96 | 28 (20/33) | −18 (−28/−8) | 97 |
| C1 | 24 | 47 | 91 | 16 | 26 | 91 | 26 | 50 | 100 |
| C2 | 22 | 41 | 75 | 38 | 8 | 98 | 30 | 0 | 99 |
| C3 | 83 | 121 | 43 | 70 | −4 | 100 | 38 | 16 | 99 |
| C4 | 28 | 36 | 58 | 36 | 54 | 96 | 6 | 56 | 99 |
| C5 | 0 | 42 | 80 | 32 | 60 | 100 | 28 | 24 | 95 |
| C6 | 21 | 39 | 94 | 34 | 40 | 100 | 34 | 8 | 100 |
| C7 | 35 | 40 | 74 | 140 | 74 | 74 | 40 | −2 | 97 |
| C8 | 27 | 42 | 77 | 20 | 26 | 97 | 46 | 30 | 99 |
| C9 | 20 | 40 | 78 | 38 | 34 | 97 | 12 | 46 | 93 |
| C10 | 19 | 27 | 69 | 116 | 78 | 90 | 22 | 56 | 99 |
| C11 | 23 | 75 | 81 | 46 | 22 | 98 | 64 | 48 | 98 |
| CX | 23 (20/28) | 41 (39/45) | 75 | 38 (35/58) | 34 (24/57) | 95 | 30 (24/39) | 30 (12/49) | 98 |
The estimated time constants associated with the lagging and leading edges of the binaural temporal window (τ1 and τ2) were similar for the adults, with a median value of 25 ms for τ1 and 24 ms for τ2 (see Table II). These results are consistent with those of Kollmeier and Gilkey (1990), where τ1 and τ2 were relatively symmetrical, both ranging between approximately 17 and 40 ms. A Wilcoxon signed ranks test indicated no significant difference between τ1 and τ2 for the adults tested here (Z=0.76; p=0.45). For children, the median values of τ1 and τ2 were 23 and 41 ms, respectively. The signed ranks test indicated that the difference between the τ1and τ2 values of the children was significant (Z =2.9;p=0.003). A Mann-Whitney U test indicated that τ1 did not differ between adults and children (U=53.0;p =0.45), but that τ2 was longer for children than for adults (U=9.0;p<0.001).
2. Evaluation of the possibility that children place the binaural temporal window late
Although the above analysis indicates that τ2 differed between adults and children, it is not clear that the duration of the time constant is the best way to characterize the difference in data patterns between the adults and children. As noted above, Kollmeier and Gilkey (1990) assumed that their adult listeners were able to improve the effective signal-to-noise ratio by listening off time and attempted to take this into account by centering the binaural temporal window at the beginning of the Sπ signal in the NoNπ conditions and the end of the signal in the NπNo conditions. It is possible that children might not be as adept as adults in approaching optimal temporal placement of the binaural temporal window. One simple possibility that is qualitatively consistent with the data pattern observed here is that, whereas adults tend to optimize the position of the binaural temporal window (as suggested by Kollmeier and Gilkey), children have a general tendency to listen late. With respect to optimal off-time listening, the penalty associated with listening late might be relatively minor for an Sπ signal in an NπNo masker because listening late would tend to improve the signal-to-noise ratio for a signal occurring near the masker phase transition. This interpretation is consistent with the finding that τ1 did not differ significantly for adults and children in the present study. A more significant penalty to listening late would be expected for an Sπ signal in an NoNπ masker because listening late in this case would tend to worsen the effective signal-to-noise ratio, particularly for a signal near the masker phase transition. This would create a difference between adults and children, as adults are assumed to listen “early” in order to improve the effective signal-to-noise ratio in this condition.
In order to evaluate the idea that adults and children have similar binaural time constants but that children place the binaural temporal window relatively late in time, two additional data fitting approaches were pursued. The first employed a fitting procedure very similar to that of Kollmeier and Gilkey (1990), but with separate fits to the NoNπ and NπNo data and different free parameters. The parameter K was estimated based on the MLD in steady masker condition, while the parameters LM, the time constant (τ) and placement of the temporal window (SHIFT) were allowed to vary freely. A single value of τ and single value of SHIFT were fitted to the NπNo data set, and a single value of τ and a single value of SHIFT were fitted to the NoNπ data set. With the single value of τ, the leading and lagging edges of the temporal window were forced to be the same. The SHIFT parameter allowed the temporal center of the binaural temporal window to vary, instead of being fixed, at values assumed to be near optimal. SHIFT represents the time difference between the center of the window and the center of the signal, with positive SHIFTs associated with a temporal window position after the center of the signal, and negative shifts associated with a temporal window position before the center of the signal. This “fitted shift” approach will be contrasted with the onset/offset shift approach used above, where the temporal position of the binaural temporal window was assumed to be either at the onset or at the offset of the signal (depending on the dynamic masker phase condition). If adults and children have similar binaural temporal window time constants, but differ in terms of the temporal placement of the binaural temporal window (adults place the window to enhance the effective signal-to-noise ratio but children place the window relatively late), the following outcomes were expected from the fitted shift approach: (1) Neither the NoNπ value of τ nor the NoNπ value of τ would differ between adults and children; (2) Adults would show SHIFT values that were negative for the NoNπ condition and positive for the NπNo condition; Children would show SHIFT values that were positive for both NoNπ and NπNo. The results of the data fits were consistent with these expectations (see fitted shift fit columns in Table II). The variance accounted for in the fitted shift approach was considerably greater than found in the onset/offset fit approach (see Table II). The primary reason for this is that, whereas the NoNπ and NπNo data functions were fitted simultaneously in the onset/offset fit approach, each function was fitted separately in the fitted shift approach. The variance accounted for in the fitted shift approach was better than 90% for all listeners except for one adult (A4) and one child (C7) (see Table II) for whom the variance accounted for was considerably less. These two listeners were excluded from the statistical tests that follow (although the pattern of statistical significance was the same regardless of this exclusion). A Mann-Whitney test indicated that the children and adults did not differ for τ based on the NπNo data (U=42.5;p=0.39) or τ based on the NoNπ data (U=50.5;p=0.76); furthermore, Wilcoxon signed ranks tests indicated that the two values of τ did not differ from each other significantly either for the adults (Z =1.4;p=0.15) or for the children (Z=1.2;p=0.24). For the adults, estimates of SHIFT differed significantly for the two masker conditions (Z=2.9;p=0.003), with a positive SHIFT derived from the NoNπ conditions and a negative SHIFT derived from the NπNo conditions (see Table II). The SHIFT values for the children did not differ significantly (Z =0.05;p=0.96), with a positive median value for both the NoNπ conditions and the NπNo conditions (see Table II). The fact that the fitted SHIFT differed in sign for the adults is consistent with Kollmeier and Gilkey’s assumption that adults can shift the binaural temporal window in a direction that achieves a better effective signal-to-noise ratio. The fact that the fitted values of SHIFT were both positive for the children is consistent with an interpretation that children do not shift the binaural temporal window in a way that improves the effective signal-to-noise ratio, but instead listen late in all conditions.
There are at least two caveats that should be considered with respect to the fitted shift approach. One concerns the assumption that the listener can position the temporal window to enhance the effective signal-to-noise ratio. Although this assumption appears to be reasonable, at least for adult listeners, it is likely that listeners would not use a constant SHIFT across all signal delays in either the NoNπ or the NπNo conditions. It would seem more likely that the SHIFT would be greater relatively near the masker phase transition and, perhaps, negligible for longer delays, where the signal is well removed from the masker phase transition. This is a consideration not only for the case where SHIFT is a free parameter, but also in the onset/offset shift approach used here and by Kollmeier and Gilkey (1990). The second caveat concerns a penalty to be paid in terms of reduced effective signal level when the center of the binaural temporal window is shifted away from the temporal center of the signal. In the Kollmeier and Gilkey fitting procedure, K is derived from the steady masker condition where it can be assumed (at least for adults) that the binaural temporal window is centered on the signal and, therefore, the signal is minimally attenuated by the window. For the dynamic masker phase conditions, where a shift in the position of the binaural temporal window may improve the effective signal-to-noise ratio, it is important to consider the effective attenuation of signal level that may result from such a shift. Consideration of this issue may be particularly important in children, where an assumption of optimal off-time listening would appear to be very questionable. For example, in the NoNπ conditions, the median SHIFT for the children using the fitted shift fitting procedure was 34 ms, and this SHIFT was in a temporal direction opposite to that of the SHIFT thought to be optimal in these conditions. These observations raise the question of whether the modeling of a free SHIFT parameter should take into account a reduction in the effective signal level due to the difference between the temporal position of the signal and the temporal position of the binaural temporal window. This question is difficult to answer with certainty, but it could be argued that the value of K used in modeling the NoNπ and NπNo data of the children already takes into account the difference between the temporal position of the signal and the temporal position of the binaural temporal window. If the premise is correct—that children place the binaural temporal window late in all conditions—then the effective reduction in signal amplitude resulting from late placement of the binaural temporal window is reflected in the steady masker condition from which the value of K was derived. Indeed, placing the binaural temporal window late with respect to the signal temporal position in the steady masker condition could be one explanation for the finding that children showed smaller MLDs than adults in this condition. Recall that previous studies had shown comparable MLDs for adults and children for long-duration signals presented in relatively broadband noise, and, in these cases, placement of the binaural temporal window would have less of an impact on thresholds because the long duration signal would introduce multiple opportunities distributed in time in which to detect the signal. This possibility will be considered in more detail, below. In summary, the validity of the parameter estimates from fitted shift analysis is questionable due to additional effects of signal attenuation due to misalignment of the signal and the binaural temporal window. However, the results of the fitted shift analysis are broadly consistent with the assumption of Kollmeier and Gilkey (1990) that adults place the temporal window in a temporal direction consistent with optimal placement, and with an interpretation that children place the temporal window late.
An additional data fitting approach for the steady masker condition further explored the question of whether the results of the children in the dynamic masker conditions might be better accounted for in terms of a relatively long τ2 or late placement of a symmetrical (adult-like) binaural temporal window. This approach examined whether the reduced MLD of the children found in the steady masker condition was more consistent with the assumption of a prolonged τ2 or late placement of the binaural temporal window. This approach assumed that the MLDs of adults and children are similar in conditions where the parameters of the binaural temporal window have minimal influence on the MLD (in agreement with finding that adults and children have similar MLDs for long-duration signals in wideband noise), and that the reduced MLDs of children for brief signals result from developmental differences in the binaural temporal window. Note that the assumption of similar MLDs in adults and children does not require similar binaural detection thresholds, but rather similar differences between the monaural and binaural thresholds. The crux of the data fitting approach was to pass an Sπ signal and No noise like those used in the steady masker condition, above, through a binaural temporal window representative of those fitted to the data of the adults using the onset/offset shift approach. The parameters of τ1 and τ2 were set to 25 and 24 ms, respectively, the median values fitted for the adult listeners. Effects were then examined of either prolonging the τ2 of the temporal window or increasing the relative delay between the center of the binaural temporal window and the temporal center of the signal, in order to gain insight into the question of whether the reduced MLD of the children in the steady masker could be better accounted for by a prolonged τ2 or late temporal placement of an adult-like temporal window. This approach assumed that, in the steady masker case, the optimal binaural temporal window placement is at the temporal center of the signal, as there are no transient masker features to corrupt base line (No) correlation. The first step was to model the correlation at the output of the binaural temporal window with a signal-plus-noise stimulus that corresponded to the median NoSπ threshold achieved by the adults in the steady masker condition (40 dB/Hz SPL Gaussian masker filtered from 100 to 2000 Hz; 500 Hz Sπ signal with 5 ms ramps and 10 ms steady state, set to the level of 51 dB SPL). A total of 10 000 masker samples, each of 1000 ms duration were generated. The stimuli were FIR filtered to a bandwidth of 78 Hz, the approximate width of the monaural auditory filter at 500 Hz (Moore and Glasberg, 1983). Each masker sample was copied and the signal was added at the center of one copy and subtracted from the other, resulting in a pair of arrays characterizing the steady masker, NoSπ condition. The interaural correlation at the output of the binaural temporal window centered on the signal was then computed as the integral of the instantaneous correlation weighted by the shape of the binaural temporal window, using integration limits of −200 to +200 ms. The mean correlation so determined was 0.981. Repeating this procedure using values of τ1 and τ2 estimated from child data (23 and 41 ms, respectively), the signal level required to produce a correlation of 0.981 was elevated by approximately 1.5 dB. Whereas this analysis suggests that the relatively longer τ2 estimated for the children would produce a reduction in the MLD of 1.5 dB, the observed reduction of the MLD shown by the children in the steady masker condition was approximately 3.5 dB. Thus the agreement between the modeled reduction of the steady masker MLD and the actual reduction was not particularly close. Further modeling indicated that the value of τ2 required to result in a 3.5 dB reduction of the MLD was approximately 75–80 ms.
A similar analysis was then performed to determine what delay between the center of the signal and the center of the binaural temporal window would be needed in order to account for a 3.5 dB reduction in the MLD in the steady masker condition. To examine this question, the adult estimates of τ1 and τ2 were maintained (25 and 24 ms, respectively) and the center of the binaural temporal window was progressively advanced with respect to the center of the signal. The analysis indicated that the advance associated with a Sπ threshold increase of 3.5 dB was approximately 25–30 ms. This value agrees well with the values suggested for the children in the fitted shift approach used above (see Table II). Thus, whereas the children’s prolonged τ2 of 41 ms estimated in the onset/offset shift approach would not appear to account for the reduced MLD of the children in the steady masker condition, the effect can be accounted for by late (25–30 ms) placement of the binaural temporal window. This interpretation will be considered further in Sec. III, below.
3. Consideration of possible confusion effects
Kollmeier and Gilkey (1990) noted that there appeared to be a general deleterious effect in the NπNoSπ condition such that even when the Sπ signal was presented well before the masker phase transition, the obtained threshold was approximately 2 dB higher (poorer) than that obtained in a steady Nπ masker. Although the source of this effect was not clear, Kollmeier and Gilkey speculated that the masker interaural phase transition might result in a sensation that could be confused with the signal. It was of interest to determine whether the magnitude of this effect was different between adults and children in the present study. We therefore examined the difference between the Sπ threshold in the steady masker to the Sπ threshold in the NπNo masker at the −320 ms delay. For our adult listeners, the threshold for the −320 ms delay condition was higher than that in the steady masker condition by an average of 2.2 dB (sd=1.6 dB), and for the children this effect averaged 1.6 dB (sd=1.8 dB). The difference between the adults and children was not significant (t21=0.89; p =0.38). We also examined the difference between the Sπ threshold in the steady masker and the Sπ threshold in the NπNo masker at the 280 ms delay. For our adult listeners, the threshold in the 280 ms delay condition was higher than that in the steady masker condition by an average of 2.1 dB (sd=1.7 dB), and for the children this effect averaged 0.9 dB (sd=1.6 dB). The difference between the adults and children was again not significant (t21 =1.8;p=0.09). These results suggest that it is reasonable to conclude that any general, deleterious effect associated with the masker transition was no worse for children than for adults.
IV. SUPPLEMENTARY CONDITIONS EXAMINING THE MLD FOR BRIEF AND LONG-DURATION SIGNALS
As discussed above, one way to account for the reduced MLD in wideband noise obtained by the children in the present study is by late placement of the binaural temporal window relative to the signal. If signal energy is present for only a brief time, even a relatively small error in the placement of the binaural temporal window could have a material negative consequence for binaural signal detection. However, if signal energy is present over several hundred milliseconds, as was the case in the studies of Hall and Grose (1990) and Grose et al. 1997, a relatively small (e.g., 25–30 ms) delay in the placement of the window would be of little consequence. This interpretation is consistent with the fact that the previous studies using long-duration signals found no developmental difference for the MLD, but the present study using a short-duration signal found a smaller MLD in children. This interpretation is undermined to some extent by the fact that it is based upon results that were obtained in different studies using different sets of listeners. Furthermore, the previous studies compared NoSo and NoSπ thresholds to compute the MLD, whereas the present study compared NπSπ and NoSπ thresholds to compute the MLD. Given the theoretical importance of the finding that the MLDs of children in relatively wideband noise are adult-like for long duration signals but are reduced for brief signals, we examined supplementary conditions to determine the effect of signal duration within a single set of listeners.
A. Listeners
All listeners had pure-tone detection thresholds of 20 dB HL or better at octave frequencies from 250 to 8000 Hz (ANSI, 1996). None had a history of chronic ear disease, and none had a known history of otitis media within a 3-year period preceding testing. Nine children were recruited (four females and five males), ranging in age from 5 to 10.5 years, with a mean age of 7.9 years (standard deviation 1.9 years). There were ten adult listeners (six females and four males), ranging in age from 21 to 44 years, with a mean of 31.5 years (standard deviation 8.5 years). All listeners were paid for participation and provided data in one session lasting approximately 1 h each. None of the children and only one of the adults had participated in the main experiment.
B. Stimuli and threshold estimation
The masker was a continuous Gaussian noise, bandpass filtered from 100 to 2000 Hz, and presented at a level of 40 dB/Hz SPL. The signal was a 500 Hz pure tone, ramped on and off with 5 ms cos2 ramps. The steady-state duration of the signal was either 10 or 400 ms. The signal was Sπ and the masker was either No or Nπ. The threshold estimation procedure and visual interface providing interval and feedback information were the same as for the main experiment (see above).
C. Results and discussion
Table III summarizes findings for both the brief and long-duration signal conditions. The long-duration signal results will be considered first. A repeated measures analysis of variance showed that the NπSπ thresholds were higher than the NoSπ thresholds (F1,17=688.7; p <0.001), that the children had higher thresholds than the adults (F1,17=15.3; p =0.001), and there was no significant interaction between threshold and group (F1,17=1.3; p =0.27). The lack of a significant interaction indicates that the MLD magnitude did not differ significantly between adults and children. This uniformity in the MLD magnitude across the age range tested here is consistent with the previous results obtained by Hall and Grose (1990) and Grose et al. 1997 for NoSo and NoSπ stimuli and masking noise bandwidths of 300 Hz or wider. A repeated measures analysis of variance on the data for the brief signal showed that the NπSπ thresholds were higher than the NoSπ thresholds (F1,17=327; p <0.001), that the children had higher thresholds than the adults (F1,17 =14.6;p=0.001), and that there was a significant interaction between threshold and group (F1,17=7.6; p =0.01). This interaction reflects the fact that the MLD for a brief signal was smaller in children than in adults. Thus, in contrast to the results for the long-duration signal, the adults showed a larger MLD than the children for the brief signal (see Table III), a result that replicates the significant developmental difference obtained in the main experiment.
TABLE III.
Mean NπSπ and NoSπ thresholds (dB SPL) and derived MLDs for the two age groups. Data are shown for the long- and brief-duration signals of the supplementary conditions. Standard deviations are in parentheses.
| Long signal
|
Brief signal
|
|||||
|---|---|---|---|---|---|---|
| NπSπ | NoSπ | MLD | NπSπ | NoSπ | MLD | |
| Adult | 56.1 (1.0) | 43.0 (1.8) | 13.1 (1.6) | 68.1 (1.2) | 53.3 (1.6) | 14.8 (2.1) |
| Child | 58.7 (2.2) | 46.7 (2.8) | 12.0 (2.5) | 70.4 (2.3) | 59.6 (4.0) | 10.8 (3.2) |
Overall, the results of the supplementary conditions confirm that whereas children and adults have similar MLDs for a relatively wideband masker when the signal is of long duration, children have smaller MLDs than adults when the signal duration is brief. One interpretation that is consistent with this finding is that shifts in the binaural temporal window, such as those derived in the fitted shift procedure described above, are not restricted to dynamic masker phase conditions.
V. GENERAL DISCUSSION
The developmental findings of the main experiment are consistent with an interpretation that children have either an asymmetrical binaural temporal window with a relatively long τ2 or late placement of a symmetrical binaural temporal window. Although the analyses performed here do not rule out the possibility of an asymmetrical binaural temporal window in children, an interpretation based upon late placement of a symmetrical temporal window was favored on the basis of two data fitting/modeling approaches. The first approach indicated that if the temporal position of the binaural temporal window is allowed to vary, the shift is in opposite directions for the NoNπ and NπNo conditions (consistent with the optimal off-time listening suggested by Kollmeier and Gilkey (1990)) for adults, but it is in the same (late) direction for children. This approach indicated no significant difference between the NoNπ or NπNo time constants for either adults or children. The second approach indicated that the reduced MLDs of the children in the steady masker condition are not well accounted for by the prolonged τ2 derived in the onset/offset fitting approach, but are well accounted for by a 25–30 ms lag in the placement of the binaural temporal window. Late placement of the binaural temporal window should result in a reduced MLD for a brief signal, where the signal-to-noise ratio is good for only a short time, but should not result in a reduced MLD for a long-duration signal, where the signal-to-noise ratio is good over an extended time. The data of the supplementary conditions reported above were consistent with this interpretation, with children showing adult-like MLDs for a long-duration signal but MLDs that were smaller than those of adults for a brief signal.
The present interpretation that children are inefficient in the placement of the binaural temporal window is also relevant to previous findings indicating that children show reduced MLDs for long-duration signals presented in narrow-band masking noise (Grose et al., 1995; Grose et al., 1997). The results of a previous study (Hall et al., 2004) suggested that the reduced MLDs of children for narrowband noise maskers were related to a poor ability to take advantage of the binaural information occurring in the masker envelope minima, where the signal-to-noise ratio is most favorable. One interpretation of the present results is that children are not as adept as adults in weighting the temporal epochs associated with the most favorable binaural detection cues. This interpretation is also consistent with the reduced MLDs of children for narrowband masking noise, where optimal performance hinges upon the weighting of the good binaural cues that are present during the relatively brief masker envelope minima.
There are interesting parallels between the developmental results that have been obtained on monaural and binaural temporal processing. For example, the finding by Grose et al. 1995 that children were relatively poor in listening in the envelope minima of a monaural, amplitude-modulated narrowband noise is analogous to the finding that children have a reduced ability to exploit binaural information in the envelope minima of a narrowband noise (Hall et al., 2004). A further correspondence between these monaural and binaural findings is that neither of the effects may be driven by an essential deficit in temporal acuity: monaural TMTF results indicated no developmental difference in the monaural time constant (Hall and Grose, 1994), and some aspects of the present results are consistent with an interpretation that there is no developmental difference in the time constant of the binaural temporal window. We plan to investigate possible developmental parallels between monaural and binaural temporal resolution further by examining the monaural temporal window using a method that is analogous to the one used here to investigate the binaural temporal window. One possible approach would be that used by Kollmeier and Gilkey (1990), where the detection of a brief monaural signal is obtained as a function of its temporal relation to an abrupt 15 dB level transition in the monaural masker.
Deficits in auditory perception that occur despite apparent acuity in basic auditory functions, such as temporal and frequency resolution, are sometimes “accounted for” in terms of processing efficiency. The concept of poor processing efficiency has some utility in that it can guide the search for explanation away from the peripheral encoding of sound and toward the central analysis of that encoding. However, a significant limitation to the utility of the concept of processing efficiency is that the nature of any apparent inefficiency is often unspecified. The results of the present study may represent a step forward in this regard, as they suggest that an important component of inefficiency in the binaural hearing of children may be related to a reduced ability to optimize temporal weighting in the analysis of binaural sound sequences where the signal-to-noise ratio changes dynamically. Such dynamic changes can occur in narrowband noise maskers due to the fact that the inherent, pronounced fluctuations of the masking stimulus cause the signal-to-noise ratio to vary markedly over time. In the present study, such dynamic changes occurred in a wideband noise due to the imposition of an abrupt transition in the masker interaural phase. The ability to weight dynamic sequences of binaural information in an optimal way may be associated with a relatively protracted auditory development.
VI. CONCLUSIONS
The developmental differences found here for the dynamic masker conditions are consistent either with an interpretation that children have a binaural temporal window with a relatively long leading edge (τ2) or with an interpretation that children place the binaural temporal window relatively late with respect to the timing of the signal. Fitting procedures that allowed the temporal position of the binaural temporal window to vary and analyses that related the pattern of data for the dynamic maskers to the pattern of data for the steady masker were more consistent with the interpretation that children place the binaural temporal window late.
The interpretation that children are relatively poor in optimizing the temporal weighting of dynamically changing binaural information is also consistent with (a) previous findings suggesting that children have a reduced ability to exploit the robust binaural cues available in the masker envelope minima when a long-duration signal is presented in a narrowband noise; and (b) the present finding that children show adult-like MLDs for long-duration signals in wideband noise but reduced MLDs for brief signals in wideband noise.
Acknowledgments
This work was supported by NIH, RO1 DC00397. We thank Madhu B. Dev and Heidi Reklis for assistance in running subjects and technical support. We thank Michael Akeroyd and an anonymous reviewer for many helpful suggestions on a previous version of this manuscript. We also thank the associate editor, Armin Kohlrausch, for thoughtful suggestions that led to improvements in our approach to data analysis and interpretation.
References
- ANSI. ANSI S3.6-1996, “Specification for audiometers. American National Standards Institute; New York: 1996. [Google Scholar]
- Bernstein LR, Trahiotis C, Akeroyd MA, Hartung K. Sensitivity to brief changes of interaural time and interaural intensity. J Acoust Soc Am. 2001;109:1604–1615. doi: 10.1121/1.1354203. [DOI] [PubMed] [Google Scholar]
- Bos CE, de Boer E. Masking and discrimination. J Acoust Soc Am. 1966;39:708–715. [Google Scholar]
- Buss E, Hall JW, Grose JH. The masking level difference for signals placed in masker envelope minima and maxima. J Acoust Soc Am. 2003;114:1557–1564. doi: 10.1121/1.1598199. [DOI] [PubMed] [Google Scholar]
- Buus S, Zhang L, Florentine M. Stimulus-driven, time-varying weights for Comodulation Masking Release. J Acoust Soc Am. 1996;99:2288–2297. doi: 10.1121/1.415416. [DOI] [PubMed] [Google Scholar]
- Carlyon RP, Buss S, Florentine M. Comodulation masking release for three types of modulators as a function of modulation rate. Hear Res. 1989;42:37–46. doi: 10.1016/0378-5955(89)90116-0. [DOI] [PubMed] [Google Scholar]
- Culling JF, Summerfield AQ. Measurement of the binaural temporal window using a detection task. J Acoust Soc Am. 1998;103:3540–3553. [Google Scholar]
- Durlach NI. Equalization and cancellation theory of binaural masking-level differences. J Acoust Soc Am. 1963;35:1206–1218. [Google Scholar]
- Grantham DW, Wightman FL. Detectability of a pulsed tone in the presence of a masker with time-varying interaural correlation. J Acoust Soc Am. 1979;65:1509–1517. doi: 10.1121/1.382915. [DOI] [PubMed] [Google Scholar]
- Grantham W, Wightman FL. Detectability of varying interaural temporal differences. J Acoust Soc Am. 1978;63:511–523. doi: 10.1121/1.381751. [DOI] [PubMed] [Google Scholar]
- Grose J, Hall J, Dev M. MLD in children: Effects of signal and masker bandwidths. J Speech Lang Hear Res. 1997;40:955–959. doi: 10.1044/jslhr.4004.955. [DOI] [PubMed] [Google Scholar]
- Grose JH, Hall JW, Gibbs C. Temporal analysis in children. J Speech Hear Res. 1993;36:351–356. doi: 10.1044/jshr.3602.351. [DOI] [PubMed] [Google Scholar]
- Grose JH, Hall JW., III Masker fluctuation and the masking-level difference. J Acoust Soc Am. 1998;103:2590–2594. doi: 10.1121/1.422779. [DOI] [PubMed] [Google Scholar]
- Grose JH, Hall JW, Mendoza L. Developmental effects in complex sound processing. In: Manley GA, Klump GM, Koppl C, Fastl H, Oekinghaus H, editors. 10th International Symposium on Hearing. World Scientific; Singapore: 1995. pp. 97–104. [Google Scholar]
- Hall JW, Buss E, Grose JH, Dev MB. Developmental effects in the masking-level difference. J Speech Lang Hear Res. 2004;47:13–20. doi: 10.1044/1092-4388(2004/002). [DOI] [PubMed] [Google Scholar]
- Hall JW, Grose JH. The masking-level difference in children. J Am Acad Audiol. 1990;1:81–88. [PubMed] [Google Scholar]
- Hall JW, Grose JH. Development of temporal resolution in children as measured by the temporal modulation transfer function. J Acoust Soc Am. 1994;96:150–154. doi: 10.1121/1.410474. [DOI] [PubMed] [Google Scholar]
- Hall JW, Grose JH, Hartmann WM. The masking-level difference in low-noise noise. J Acoust Soc Am. 1998;103:2573–2577. doi: 10.1121/1.422778. [DOI] [PubMed] [Google Scholar]
- Hall JW, Haggard MP, Fernandes MA. Detection in noise by spectro-temporal pattern analysis. J Acoust Soc Am. 1984;76:50–56. doi: 10.1121/1.391005. [DOI] [PubMed] [Google Scholar]
- Hirsh IJ. Influence of interaural phase on interaural summation and inhibition. J Acoust Soc Am. 1948;20:536–544. [Google Scholar]
- Holube I, Kinkel M, Kollmeier B. Binaural and monaural auditory filter bandwidths and time constants in probe tone detection experiments. J Acoust Soc Am. 1998;104:2412–2425. doi: 10.1121/1.423773. [DOI] [PubMed] [Google Scholar]
- Irwin RJ, Ball AK, Kay N, Stillman JA, Bosser J. The development of auditory temporal acuity in children. Child Dev. 1985;56:614–620. [PubMed] [Google Scholar]
- Kollmeier B, Gilkey R. Binaural forward and backward masking: Evidence for sluggishness in binaural detection. J Acoust Soc Am. 1990;87:1709–1719. doi: 10.1121/1.399419. [DOI] [PubMed] [Google Scholar]
- Moore BCJ, Glasberg BR. Suggested formulae for calculating auditory filter bandwidths and excitation patterns. J Acoust Soc Am. 1983;74:750–753. doi: 10.1121/1.389861. [DOI] [PubMed] [Google Scholar]
- Viemeister NF. Temporal modulation transfer functions based upon modulation thresholds. J Acoust Soc Am. 1979;66:1364–1380. doi: 10.1121/1.383531. [DOI] [PubMed] [Google Scholar]
- Wightman F, Allen P, Dolan T, Kistler D, Jamieson D. Temporal resolution in children. Child Dev. 1989;60:611–624. [PubMed] [Google Scholar]


