Abstract
The great variety of genome organizations means that most plant positive strand viral RNAs differ from the standard 5′-cap/3′-poly(A) structure of eukaryotic mRNAs. The cap and poly(A) tail recruit initiation factors that support the formation of a closed loop mRNA conformation, the state in which translation initiation is most efficient. We review the diverse array of cis-acting sequences present in viral mRNAs that compensate for the absence of a cap, poly(A) tail, or both. We also discuss the cis-acting sequences that control translation strategies that both amplify the coding potential of a genome and regulate the accumulations of viral gene products. Such strategies include leaky scanning initiation of translation of overlapping open reading frames, stop codon readthrough, and ribosomal frameshifting. Finally, future directions for research on the translation of plant positive strand viruses are discussed.
Introduction
Our understanding of post-transcriptional gene regulation has changed vastly over the last sesquidecade and firmly established the downstream steps of the gene expression cascade as essential and varied contributors to gene expression control. In the realm of translation, conceptual changes include (i) the description of internal ribosome entry sites (IRES), (ii) the realization that the poly(A) tail participates directly in translation initiation, and (iii) a better understanding and prediction of unusual translation ‘‘recoding’’ events. Viral RNAs have been at the center of many of the groundbreaking studies.
Efficient translation of eukaryotic mRNAs is thought to occur in a closed loop format (Fig. 1A), in which the 5′- and 3′-termini are brought into close proximity through the mediation of interactions involving translation initiation factors (Hentze, 1997; Sachs et al., 1997). The key interactions involve poly(A) binding protein (PABP) bound to the 3′-poly(A) tail and initiation factor 4E (eIF4E) bound to the 5′cap, and the interaction of both proteins with eIF4G (the variants eIFiso4G and eIFiso4E found in plants participate in similar interactions). These simultaneous interactions mutually increase binding affinities (Le et al., 1997; Wei et al., 1998), stabilizing the closed loop. The 40S small ribosomal subunit associates with the 5′-end of the mRNA via its interaction with eIF3, which simultaneously binds eIF4G, and then initiates scanning towards the 3′-end (Pestova et al., 2001). Members of less than 20% of plant positive strand RNA viral genera have genomic and subgenomic mRNAs structured like host mRNAs, with a 5′-cap and poly(A) tail (van Regenmortel et al., 2000). The majority lacks one or both of these features. In understanding the translation of these RNAs, research has focused on how these non-canonical RNAs interact with the translation initiation factors. The evidence indicates that the closed loop model of the mRNA is applicable in these cases, with a variety of novel interactions substituting for the molecular bridging contacts that occur in normal cellular mRNAs (Fig. 1).
Fig. 1.
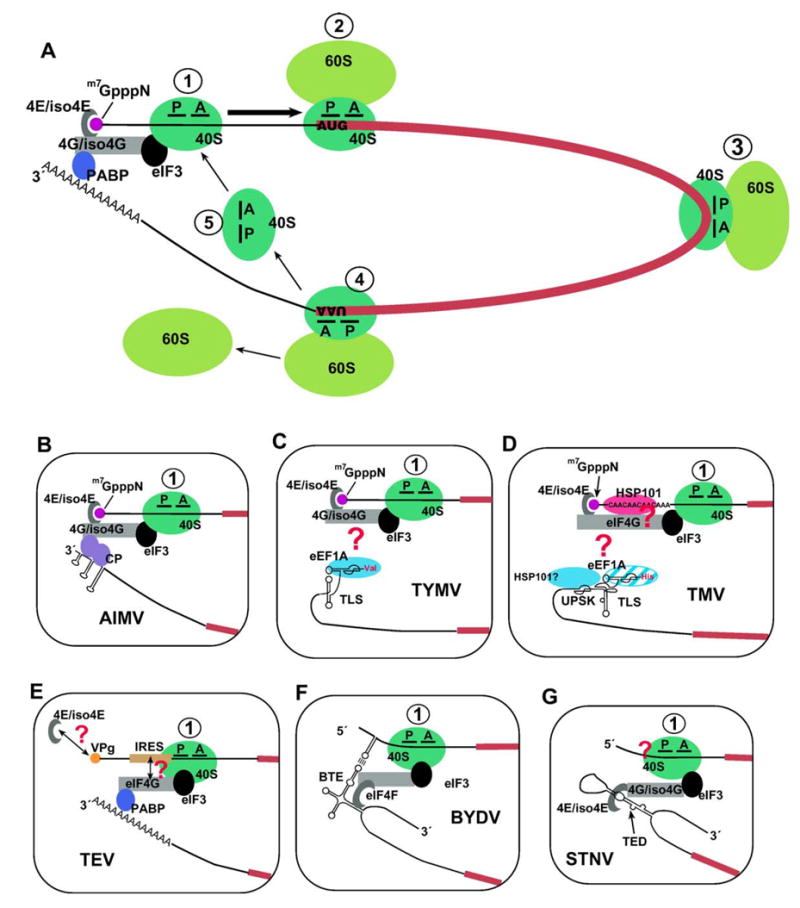
The closed loop scheme for initiation of translation: thematic variations used by plant positive strand RNA viruses. Panel A shows in simplified terms (not all factors are shown; not to scale) the circularized format in which efficiently translated cellular mRNAs are believed to exist. The key responsible cis-acting features are the 5′m7GpppN cap (purple dot) and 3′-poly(A) tail, which synergistically enhance expression. Bridging interactions through eIF4E or eIF-iso4E (the cap-binding proteins), eIF4G or eIF-iso4G, and the poly(A) binding protein (PABP) bring the 5′- and 3′-termini into close proximity, and the interactions are mutually stabilizing. The first stage of translation initiation, the recruitment of the 40S small ribosome subunit to the 5′-end, depends on simultaneous interaction of eIF3 (a complex of multiple proteins) with eIF4G or eIF-iso4G and the 40S subunit. This step is depicted in step (1) and is followed by ribosome scanning (arrow) along the 5′-UTR (black line) in search of the AUG initiation codon. In response to base-pairing with the initiator tRNA (not shown) located in the ribosomal P site, the 60S large ribosome subunit joins (step 2) to initiate the elongation phase of translation. During peptide elongation (step 3), the codons in the ORF (thick red line) are read by tRNAs entering the ribosomal A site, until a termination codon, such as UAA, is reached (step 4), triggering termination of protein synthesis and subunit dissociation and release (step 5). Because of the closed loop format, ribosomes are near the 5′-end upon termination, facilitating new initiation. The boxed diagrams (B–G) illustrate variations of the initial 40S subunit recruitment step for viral mRNAs that lack a cap, poly(A) tail, or both, and which are discussed in the text. Red question marks indicate unknown or uncertain details. AMV, TYMV, and TMVare examples of viruses whose RNAs have a cap but no poly(A) tail. For AMV, a coat protein (CP) dimer binds to the 3′-terminal region and to eIF4G/iso4G. For TYMV RNA, aminoacylation (indicated by Val in the diagram) of the 3′-terminal tRNA-like structure (TLS) is needed for full 3′-translational enhancement, and it has been postulated that eEF1A binding is involved in closed loop formation. TMV RNA also has a 3′ TLS capable of aminoacylation (His) and eEF1A binding, but 3′-translational enhancement relies on an upstream pseudoknot (UPSK). Intriguingly, this feature can also bind eEF1A, which may be involved in closed loop formation, apparently in a way that predominates over a TYMV-like interaction involving the TLS. TEV RNA has a poly(A) tail, but no 5′-cap. The 5′-end is covalently linked to VPg, which is not needed for translation but does interact with eIF4E and eIF-iso4E; it is not known whether this interaction influences translation. 40S ribosome subunits are recruited to the 5′-UTR through an IRES element whose function requires eIF4G and that may involve direct base-pairing to 18S ribosomal RNA. BYDV and STNV RNAs lack both canonical terminal elements and possess translational enhancer elements (BTE, TED) in an internal position (not at the 3′-terminus) of the 3′-UTR. These elements recruit translation initiation factors that are normally recruited to the 5′-end by the cap. In BYDV RNA, ribosome delivery to the 5′-end is accomplished through direct RNA base-pairing between elements in the 5′- and 3′-UTRs, while in STNV RNA, base-pairing between the 5′-UTR and rRNA may be involved. See text for details.
Another active area of research has been the deciphering of translational expression of viral genomes. Because of genome size constraints and the essentially monocistronic nature of eukaryotic translation, positive strand RNA plant viruses exhibit a range of devices that expand the expressible gene content. Alteration of ribosomal behavior by ‘‘recoding’’ permits readthrough and frameshifting, and leaky scanning allows use of alternative translation start sites. These phenomena are outlined in Fig. 2 and discussed in the second part of this review. We lack space in this review for comprehensive coverage of all translational control mechanisms in all positive strand RNA plant viruses. Instead, we provide examples that illustrate concepts and translational regulation strategies that we anticipate are widely applicable to these viruses.
Fig. 2.

Expanded expression repertoire resulting from leaky scanning and translational recoding. Panel A depicts in simple terms the coding content of a standard mRNA, which directs protein synthesis between an initiation codon, typically the 5′-most AUG (black diamond), and a termination codon (red hexagon). The encoded protein is indicated by the thick line below the RNA. (B) In many viral RNAs, more than one initiation site can be used. This can occur by leaky scanning when the 5′-most initiation codon is weakly recognized by ribosomes because it is in a weak context, as occurs with pyrimidines (Y) in the −3 and/or +4 positions (as indicated) or if initiation occurs at a non-AUG codon (not shown). When the initiation sites are in different reading frames, two proteins of unrelated sequence are made (as shown); when the initiation sites are in-frame (not shown), the encoded proteins are identical except for the presence of an N-terminal extension on one of the proteins. The product of the downstream ORF is generally less efficiently expressed, indicated by the thinner line. (C) In viral RNAs such as TMV RNA, a suppressible termination codon (cross-hatched hexagon) is embedded in an ORF. In conjunction with the downstream recoding signal CARYYA (R = purine; Y = pyrimidine), a small proportion of ribosomes avoids termination, permitting the synthesis of an elongated version of the upstream protein. (D) Frameshifting (typically −1) occurs in mRNAs, which have a pair of recoding signals: a ‘‘slippery’’ heptanucleotide (XXxNNNZ, where X and N can be any base and Z is any base except G) and a feature such as a pseudoknot that is thought to induce ribosome pausing. The encoded products represent translation of the entire upstream ORF and a longer chimeric protein derived from the different ORFs upstream and downstream of the frameshift point.
Closed loop formation for viral RNAs lacking a cap or poly(A) tail
Viral mRNAs with capped 5′-ends and non-polyadenylated 3′-termini
Viruses in more than a third of the genera of positive strand RNA plant viruses have genomes with a 5′-cap but no poly(A) tail. In transfection experiments delivering reporter mRNAs into plant protoplasts, the poly(A) tail has been shown to serve as a potent translational enhancer that acts in synergy with the 5′-cap (Gallie, 1991). Its major effect on protein expression is to influence translational efficiency, with a smaller role in stabilizing the RNA (Gallie, 1991). Similar functions are provided by the 3′-UTRs of Tobacco mosaic virus (TMV) (Gallie and Walbot, 1990), Alfalfa mosaic virus (AMV) (Krab et al., 2005), and Turnip yellow mosaic virus (TYMV) (Matsuda and Dreher, 2004) RNAs, and it appears reasonable to expect that such a function is an important role of the 3′-UTRs of all positive strand viral genomic RNAs. These cisacting elements can be detected in reporter assays, in which they enhance protein expression when added to mRNAs with appropriate negative control 3′-UTRs. Translation enhancing activity may be evident only under appropriate circumstances, however, such as in the presence of coat protein (for AMV) or upon aminoacylation (for TYMV) (Fig. 1).
The observation of synergy between the 5′-cap and the 3′-UTR for TMV (Gallie and Walbot, 1990) and TYMV (Matsuda and Dreher, 2004) RNAs supports the notion that these viral RNAs are translated in the closed loop format, although the expected 5′–3′ molecular bridges have not yet been identified (Figs. 1C and D). In the case of AMV RNA, the viral coat protein (CP) has recently been shown to serve a bridging role analogous to that of PABP in normal translation. AMV CP binds with high affinity near the 3′-terminus to AUGC repeats separated by hairpins (Bol, 2005; Guogas et al., 2004) and is also able to interact with eIF4G or eIFiso4G (Krab et al., 2005) (Fig. 1B). This is analogous to the situation with rotavirus mRNAs (Varani and Allain, 2002). AMV RNA has the distinctive property of requiring the presence of a few molecules of coat protein to successfully launch an infection (the phenomenon has been termed ‘‘coat protein activation’’). This long-standing puzzle has now been explained by the realization that the 3′-terminal region of each of the AMV RNAs serves as a translational enhancer when bound by CP (Neeleman et al., 2004). This CP-RNA interaction replaces the PABP-poly(A) interaction as indicated by the observation that presence of an artificial poly(A) tail on AMV RNA obviates the need for CP for translation (Neeleman et al., 2001). The translation of ilarvirus RNAs is likely to be similarly controlled by CP binding.
Translational enhancement has also emerged as a major role of the aminoacylatable tRNA-like structure (TLS) found at the 3′-end of TYMV RNA. Full enhancing activity relies on the remarkable tRNA mimicry of the TLS, particularly its ability to be aminoacylated, and therefore requires a precise –CCA 3′-terminus (Matsuda and Dreher, 2004). This explains the inability of an earlier study to detect translational enhancement associated with the TYMV 3′-UTR (Gallie and Kobayashi, 1994). The TYMV 3′-translational enhancer (TE) is active when aminoacylatable with the natural valine or when discrete mutations have switched the specificity to methionine (Matsuda and Dreher, 2004). The common property of these aminoacylated variants is the ability to tightly bind eEF1A·GTP (Dreher et al., 1999), implicating this factor in TE function (Fig. 1C). Mutations that interfere with eEF1A interaction support this conclusion (Matsuda and Dreher, 2004), but direct evidence is needed to implicate eEF1A in a molecular bridge between the 5′- and 3′-ends. If eEF1A were indeed involved, this would be an unprecedented link between the elongation and initiation components of the translation machinery. Translational enhancement in vivo by the TYMV TLS does not depend on 5′ TYMV sequences and occurs by a mechanism that involves synergy with the cap (Matsuda et al., 2004a; Matsuda and Dreher, 2004). These studies refute a mechanism in which the TLS was proposed to direct cap-independent initiation from one of the tandem initiation sites at the 5′-end of TYMV RNA (Barends et al., 2003). That provocative scheme appears to have been based entirely on cell-free experiments using conditions in which viral TLSs support anomalous labeling of existing proteins with tritiated amino acid esterified at the –CCA terminus (compare with Barends et al., 2004).
Although TMV RNA terminates in a TLS that is capable of aminoacylation (with histidine) and interaction with eEF1A (Mans et al., 1991), its 3′-translational enhancer that acts as a poly(A) tail substitute (PAS) has been localized to a series of pseudoknots just upstream of the TLS (Leathers et al., 1993). This coincides with sequences essential for viral replication (Takamatsu et al., 1990) and sequences that could be cross-linked to eEF1A in an aminoacylation-independent manner (Zeenko et al., 2002). Heat shock chaperone HSP101 is another protein that is capable of interacting with the pseudoknots (Wells et al., 1998), but a role for this protein in 3′-PAS function has not been established. It is intriguing to speculate that eEF1A may participate in the translational enhancement of both TMV and TYMV RNAs, although via different RNA interactions.
It is also intriguing to consider why the TMV TLS is apparently not involved in translational enhancement, despite sharing similar tRNA-like properties with the TYMV TLS. Perhaps TMV RNA is an example of an mRNA that has acquired a second 3′-PAS upstream of its usual position at the 3′-terminus, supplanting the original 3′-terminal PAS element (the TLS). Evidently, 3′-PAS elements are relatively free of evolutionary constraints, as evidenced by dissimilarities between closely related virus genera. For instance, the closest relatives of the tymoviruses are the marafiviruses, whose genomes have a poly(A) tail in place of the TLS (van Regenmortel et al., 2000), while the bromoviruses are closely related to AMV yet do not exhibit genome activation by coat protein. Since it seems likely that all positive strand viral RNAs are translated in the closed loop format, the variety among 3′-UTR properties predicts a variety of 5′–3′ bridging interactions.
Many viruses with capped mRNAs harbor elements that enhance cap-dependent translation, independent of the nature of the 3′-UTR. Examples include the 5′-UTRs of the coat protein mRNA of AMV (Jobling and Gehrke, 1987) and the genomic RNA of Potato virus X (Zelenina et al., 1992). One of the best characterized examples is the 68 nt 5′-UTR of TMV, known as the omega (Ω) sequence (Gallie et al., 1987a, 1988). Ω is a cap-dependent enhancer of translation, increasing expression from capped mRNAs on the order of 10-fold in plant and animal systems in vitro and in vivo (Gallie, 2002). It is an unstructured sequence almost devoid of guanosine residues, featuring variations on a CAA repeat motif. The translational enhancement activity of Ω likely derives from two sources: (i) the lack of secondary structure reducing the dependence on initiation factors such as the helicase eIF4A and (ii) its ability to bind heat shock protein HSP101 (Wells et al., 1998). By unknown means, the presence of HSP101 recruits eIF4G (Gallie, 2002) (Fig. 1D). The presence of a potent translational enhancer in the 5′-UTR may facilitate co-translational disassembly, in which ribosome traffic drives RNA uncoating in the 5′ to 3′ direction (Gallie et al., 1987b). It seems possible that interaction between HSP101, a member of the HSP100 family of oligomeric proteins (Lee et al., 2004), bound to both the 5′- and 3′-UTRs could contribute to closed loop formation for TMV RNA. Interestingly, the translation regulating properties of 5′-UTR elements are apparently not in all cases enhancing. A 31-nt element in the 5′-UTR of BMV RNA 2 was shown to reduce translation of the polymerase gene in yeast cells, perhaps serving to limit expression of this protein (Noueiry et al., 2000).
Although tobamovirus genomes have a 5′-cap, the genome of Crucifer-infecting tobamovirus (CrTMV) harbors two internal IRESes. The 75-nt sequence called , upstream of the movement protein gene, and the 148-nt sequence, , upstream of and including the first 25 codons of the coat protein ORF, function as cap-independent translation elements (Ivanov et al., 1997). These elements apparently facilitate translation of the viral subgenomic RNAs, which may not be capped. was reported to facilitate highly efficient translation of a downstream ORF in a bicistronic reporter mRNA in plant and animal cells (Dorokhov et al., 2002). The activity of the IRES resides in an A-rich tract of purines, and (GAAA)16 had maximum activity (Dorokhov et al., 2002). Its mechanism of action is unclear, but it reiterates the recurring theme that plant translational control signals tend to be more compact than those in animal viral RNAs.
Viruses with genome-linked proteins
The genomes of one-fourth of the plant positive strand RNA viral genera, including the largest family of plant viruses, the Potyviridae, have a small protein (VPg) covalently attached to the 5′-end instead of an m7G(5′)ppp(5′)N cap structure. Therefore, they must undergo some type of cap-independent translation with novel strategies for closed loop formation. Although potyviruses and especially comoviruses resemble picornaviruses in genome structure and in the presence of a VPg, their mechanisms of ribosome recruitment for initiation appear to be quite different. The VPg-linked plant viral RNAs lack the long, highly structured 5′-UTRs with embedded AUG triplets that are typical of the well-characterized picornaviral IRES elements that confer cap-independent translation (Belsham and Jackson, 2000). For a more detailed review of plant virus cap-independent translation, see Pettit Kneller et al. (in press).
The 5′-untranslated regions of Tobacco etch potyvirus (TEV) (Carrington and Freed, 1990) and Turnip mosaic potyvirus (Basso et al., 1994b) have been shown to confer cap-independent translation. These 5′-UTRs do function as IRESes when placed downstream of a structure or ORF that blocks ribosome scanning from the 5′-end (Basso et al., 1994b; Niepel and Gallie, 1999), but their action is relatively weak, suggesting a role for the 5′-terminus in ribosome loading. The 143-nt-long AU-rich 5′-UTR of TEV RNA is predicted to contain a pseudoknot that has been implicated in translational enhancement (Zeenko and Gallie, 2005), although the presence of alternative structures makes the identification of critical features uncertain. A loop within this pseudoknot that can base-pair directly to 18S rRNA may help to recruit 40S ribosome subunits. Immediately upstream of the pseudoknot is a CAA-rich sequence reminiscent of the TMV Ω element that also contributes to cap-independent translation (Zeenko and Gallie, 2005). Cap-independent translation supported by the TEV IRES is eIF4G-dependent (Gallie, 2001), raising the possibility that this initiation factor is directly or indirectly recruited to the IRES, stabilizing the closed loop format (Fig. 1E).
There appear to have been no studies that compare the translatability of potyviral RNA with and without covalently bound VPg, although significant translation is clearly possible in the absence of the VPg (Basso et al., 1994a; Niepel and Gallie, 1999). The issue is relevant because of several lines of evidence indicating an involvement of the cap-binding translation initiation factor, eIF4E, with potyviral infections. Direct interactions have been detected between eIF4E and potyviral VPgs (Leonard et al., 2000, 2004; Schaad et al., 2000), suggesting an additional route for eIF4G recruitment to the 5′-UTR. Among animal viruses, caliciviral RNA is translated more efficiently with VPg intact (Herbert et al., 1997). While the molecular consequence of an interaction between VPg and eIF4E or eIFiso4E is unclear, the interaction is essential for infection (Gao et al., 2004; Kang et al., 2005). Thus, mutations in host genes encoding eIF4E or eIFiso4E confer resistance to numerous potyviruses (Kang et al., 2005; Lellis et al., 2002; Nicaise et al., 2003; Ruffel et al., 2002; Stein et al., 2005). An analogous interaction, between the viral proteinase–VPg fusion protein and eIF-iso4E, has been reported for Tomato ringspot nepovirus (Leonard et al., 2002).
There has been speculation that the VPg–eIF4E interaction facilitates cap-independent translation (Gao et al., 2004; Lellis et al., 2002; Thivierge et al., 2005), although it is possible that the VPg–eIF4E interaction inhibits eIF4E-dependent (i.e., cap-dependent) translation of host mRNA, freeing ribosomes for viral RNA translation. Alternatively, or in addition, the interaction may be relevant to other aspects of the infection, such as facilitating cell-to-cell movement of viral RNA (Gao et al., 2004).
Other viruses whose genomes are VPg-linked have been less studied. While the 5′-UTRs of genomic and subgenomic RNAs of Potato leafroll polerovirus appear to lack translation enhancement activity (Juszczuk et al., 2000), an IRES has been detected in a coding region. This IRES, in a highly unusual location 22 nt downstream of the start codon, directs translation of replication-associated protein 1 (Rap1) from a small ORF that overlaps with ORF 1 and is located over 1 kb from the 5′-end of the genome (Jaag et al., 2003). The sequence GGAGAGAGAGG is an essential part of this IRES and, by its purine-rich nature, resembles the CrTMV IRES discussed above. Unexpectedly, no IRES activity has been detected in the 5′-UTR of Plum pox potyvirus, and cap-independent initiation was preserved despite extensive deletion of the 5′-UTR (Simon-Buela et al., 1997), suggesting that a variety of translation initiation mechanisms may be used in the translation of plant viral VPg-linked RNAs.
Viral genomes with unmodified 5′- and 3′-ends
RNAs of viruses in the Tombusviridae and the Luteoviridae families have neither a cap nor a poly(A) tail. The Luteoviridae family is quite molecularly divergent, as the RNAs of viruses in genus Luteovirus (hereafter called luteoviruses) have an unmodified 5′-end, while those in genus Polerovirus (also of the Luteoviridae family) have a 5′ VPg (above). In general, replication genes and many gene expression control signals of the luteoviruses, but not the poleroviruses, resemble those of the Tombusviridae (Miller et al., 2002). In particular, the translation control signals of luteoviruses and those of selected genera in the Tombusviridae are strikingly similar. Perhaps the most remarkable shared characteristic is the mediation of cap-independent translation by sequences in the 3′-UTR.
The 5′-end of the 3′-UTR of Barley yellow dwarf luteovirus (BYDV) RNA harbors an approximately 100-nt sequence that facilitates highly efficient cap-independent translation initiation at the 5′-proximal AUG of the mRNA (Guo et al., 2000; Wang et al., 1997). This BYDV-(like) translation element (BTE) is conserved in all luteoviruses (not poleroviruses) and in the Dianthovirus (Mizumoto et al., 2003) and Necrovirus (Meulewaeter et al., 2004; Shen and Miller, 2004a) genera of the Tombusviridae. The BTE is characterized by a 17-nt conserved sequence, GGAUCCUGGGAAACAGG, that forms a stem-loop (paired bases are underlined), and by at least one additional stem-loop, whose loop base-pairs to the 5′-UTR (Guo et al., 2001; Pettit Kneller et al., in press). A tract in this element (in italics) also has potential to base-pair near the 3′-end of 18S rRNA (Wang et al., 1997). This may contribute to recruitment of the ribosome to the BTE.
Other viruses of the Tombusviridae harbor cap-independent translation elements in the 3′-UTR that do not resemble a BTE (Fabian and White, 2004; Meulewaeter et al., 1998b; Timmer et al., 1993). Satellite tobacco necrosis virus (STNV) RNA has a 3′-translation enhancer domain (TED) that functions like a BTE (Meulewaeter et al., 1998a; Meulewaeter et al., 1998b; Timmer et al., 1993) but bears no primary or secondary structural similarity to a BTE. The TED binds specifically to translation initiation factors eIF4E or eIFiso4E, and this binding correlates with cap-independent translation function (Gazo et al., 2004). The BTE of BYDValso interacts with cap-binding factors (E. Allen, E. Pettit, W.A. Miller, unpublished results). Thus, TED and BTE elements may recruit the translation initiation machinery by binding canonical cap-binding factors, leading to recruitment of the viral RNA to the ribosome (Figs. 1F and G). The ribosome is likely placed in the vicinity of the 5′-end via long distance base-pairing between the 3′ BTE and a single-stranded region in the 5′-UTR. Such 3′-UTR–5′-UTR base-pairing has been demonstrated for the BTEs of BYDV (Guo et al., 2001) and TNV (Shen and Miller, 2004a) and for a non-BTE cap-independent translation element of Tomato bushy stunt virus (Fabian and White, 2004); it has been predicted for all Tombusviridae (Fabian and White, 2004) and luteoviruses (Guo et al., 2001). Guo et al. (2001) provided evidence that the ribosome must scan from the 5′-end of the mRNA to the first AUG, as for normal cap-dependent translation. Thus, the BTE demonstrates that a cap-independent translation element need not be an IRES.
The 3′ location of translation initiation elements may provide a molecular switch to facilitate the shift from translation to replication, competing processes that occur on the same molecule for positive strand RNA viruses. Long-distance base-pairing and active cap-independent translation are postulated to be the default status in the absence of viral protein at the beginning of the infection. Once the viral replicase has appeared as a result of this translational activity, it could begin to transcribe the viral RNA from the 3′-end, moving in the 5′ direction on the template. The formation of full-length minus strand product would be unlikely, however, because of interference by translating ribosomes (Gamarnik and Andino, 1998). We postulate that this scenario is avoided when the passage of the replicase disrupts both the structure of the BTE and its base-pairing to the 5′-UTR. This would shut off ribosome recruitment and delivery to the 5′-end (Barry and Miller, 2002). The binding of eEF1A to the TLS at the 3′-end of TYMV RNA is thought to accomplish an analogous regulation, serving as a switch between translation and replication. When eEF1A is bound, translation is facilitated (see above), while dissociation permits access by the replicase to its CCA initiation box at the 3′-terminus (Matsuda et al., 2004b).
The BTE element serves as a 5′-cap mimic and can be functionally replaced by a cap but not by a poly(A) tail (Wang et al., 1997). It is positioned well upstream of the 3′-terminus and does not functionally replace the poly(A) tail. Indeed, the BTE is insufficient for translation in vivo and must be augmented by other sequences from the viral 3′-UTR or an artificial poly(A) tail (Guo et al., 2000). Recent studies have shown that various portions of the 869-nt-long BYDV 3′-UTR contribute to poly(A) independent translation. A distinctive stem-loop followed by the sequence RCCC (R = purine) forms the 3′-terminus of all luteovirus and Tombusviridae RNAs. In each virus, the RCCC can exist in two conformations, either single stranded, or base-paired to a site upstream of the terminal stem-loop (Koev et al., 2002; Pogany et al., 2003; Zhang et al., 2004). The base-paired conformation acts as a replication silencer, preventing access by the replicase to the 3′-end (Pogany et al., 2003) and perhaps also protects the 3′-end from exonucleases. If this is also the conformation that enhances translation as a poly(A) tail mimic, then the conformational switch of the RCCC nucleotides selects between translation (base-paired RCCC) and replication (single-stranded RCCC) much like the eEF1A binding to TYMV RNA discussed above.
Strategic consequences of genome compression: leaky scanning, readthrough and frameshifting
Overlapping open reading frames and leaky scanning
Many positive strand RNA plant viral genomes have evolved to expand their genetic repertoire through overlapping ORFs. Tymoviruses have the longest tracts of overlapping ORFs. Some 1.9 kb of TYMV RNA simultaneously encodes the 69-kDa movement protein and 206-kDa replication polyprotein. A further 0.4 kb overlap exists between the coat protein ORF and a readthrough domain of the 206-kDa ORF (Bransom et al., 1995). Overlapping ORFs are found in many other viruses and are particularly evident in the triple gene block arrays of movement protein genes.
In most cases, such as with the TYMV 69-kDa and 206-kDa ORFs and the overlapping triple gene block ORFs, both proteins are decoded from a single mRNA by the use of alternative initiation sites. The paradigm for such decoding is the phenomenon of leaky scanning (Kozak, 2002) (Fig. 2B). Initiation typically occurs at the 5′-most AUG triplet, but its efficiency is influenced by the surrounding nucleotides or ‘‘context.’’ For plants, optimum context is (A/G)aaAUGGC for dicots and (A/G)ccAUGGC for monocots (Joshi et al., 1997; Lukaszewicz et al., 2000; Lutcke et al., 1987). Two types of suboptimal initiation sites result in initiation by only a fraction of scanning ribosomes: AUG triplets with pyrimidines at the −3 and +4 positions (Fig. 2B) or certain non-AUG triplets, such as CUG (Shirako, 1998), that are surrounded by optimal or near-optimal context. ‘‘Leaky’’ ribosomes that fail to initiate at these sites continue to scan for an appropriate initiation site. Such leaky scanning is distinct from shunting, in which ribosomes bypass part of the 5′-UTR by interrupting their linear scanning. Shunting occurs in the expression of Cauliflower mosaic virus proteins (Ryabova and Hohn, 2000).
Leaky scanning is a process involving sequential initiation decisions made as 40S ribosome subunits scan in the 5′ to 3′ direction (Kozak, 2002). Leaky scanning is thus revealed by observing that downstream initiation is blocked either by optimization of the upstream initiation site or by the insertion of a new upstream AUG in a strong context. By at least one of these criteria, the third triple gene block ORF of Barley stripe mosaic virus (Zhou and Jackson, 1996) (Fig. 3) and Potato virus X (Verchot et al., 1998) is expressed by leaky scanning. By similar criteria, the second ORF of Peanut clump virus (PCV) RNA2 is also expressed by leaky scanning (Herzog et al., 1995), despite the presence of about 600 intervening nucleotides (lacking any AUG triplets) (Fig. 3). In such cases, it is important to perform experiments that preclude alternative translation mechanisms such as shunting, internal ribosome entry, or initiation by ribosomes that have completed the translation of an upstream ORF and resumed scanning (reinitiation) (Kozak, 1999). By inserting several different upstream AUG codons, altering the positional relationship between the upstream ORF and the downstream AUG, and by using a stable hairpin placed at the 5′-terminus to demonstrate that ribosomes initiate scanning by interacting with the 5′-end, these issues were addressed in the PCV study (Herzog et al., 1995).
Fig. 3.
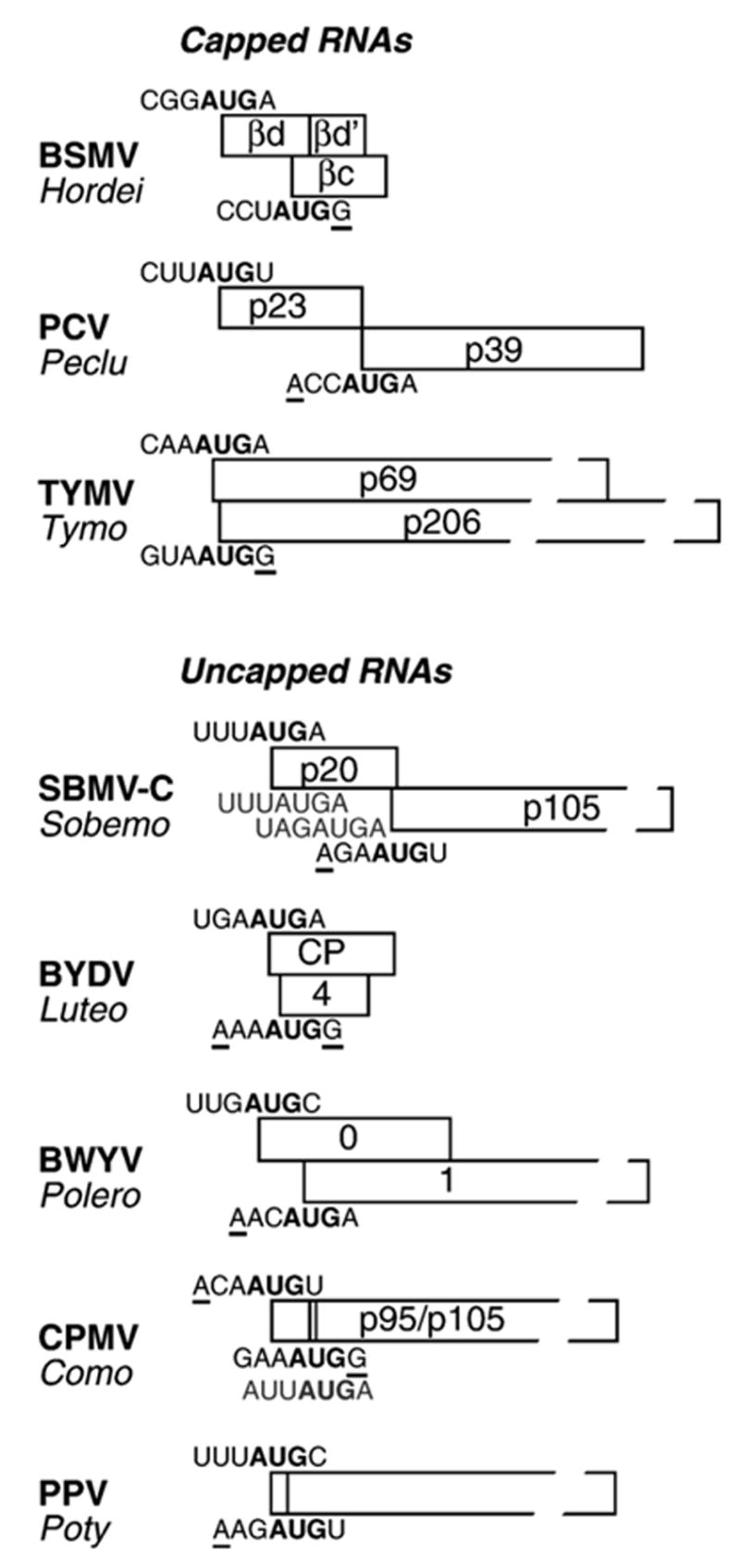
Examples of viral genes translated by leaky scanning. Only the ORFs involved in leaky scanning are shown. The genus to which each virus belongs is listed below the virus acronym. Gaps in box outline indicate ORFs that are not shown to scale. Initiation codon contexts are shown above (first ORF) or below (second ORF) the translation start site. Bases at −3 and +4 positions relative to the start codon (bold AUG), that fit the optimal context (G at +4, A at −3) are underlined. In all cases, the second ORF start codon is in a better initiation context than the upstream AUGs. The p20 ORF of SBMV-C contains two AUG codons in weak contexts that do not act as initiation codons (gray sequences below p20 ORF) (Sivakumaran and Hacker, 1998). The second start codons of CPMVand PPVare in the same reading frame as the first, yielding an N-terminally truncated protein. The CPMV ORF has an in-frame AUG (gray sequence) 12 nt downstream of the second start codon that can act as a start codon in artificial contexts, but is unlikely to function under usual conditions (Holness et al., 1989). See text for additional explanation and references.
Some further examples of leaky scanning have been demonstrated for TYMV RNA (Weiland and Dreher, 1989), subgenomic RNA1 of Barley yellow dwarf virus (BYDV) (Dinesh-Kumar and Miller, 1993), the 0.9-kb subgenomic mRNA of Cucumber necrosis virus (Johnston and Rochon, 1996), Southern bean mosaic virus (cowpea strain) (SBMV-C) RNA (Sivakumaran and Hacker, 1998), and Plum pox virus (PPV) RNA (Simon-Buela et al., 1997) (Fig. 3). Note that the observation of leaky scanning associated with BYDV, CNV, SBMV-C, and PPV RNAs indicates that this expression mechanism is not limited to capped mRNAs.
The two initiation AUGs of TYMV RNA are separated by only four intervening nucleotides. Consistent with normal leaky scanning, mutational inactivation of the upstream AUG resulted in increased initiation from the downstream AUG (Matsuda et al., 2004a). However, the close proximity of the two AUGs represents a special situation in which ribosomes seem to choose quasi-simultaneously between the alternative start sites. Initiation site selection depends on the AUG contexts, but the close spacing potentiates expression from the downstream initiation site (D. Matsuda and T. Dreher, unpublished). As with TYMV RNA, expression from the 5′-most AUG of BYDV-PAV sgRNA1 increased upon inactivation of the next AUG, 41 nt downstream. It was proposed that a following ribosome bumps up against a ribosome paused during initiation at the downstream AUG, arresting it in the vicinity of the upstream AUG and increasing the likelihood of initiation (Dinesh-Kumar and Miller, 1993). Alternatively, the downstream ribosome could affect upstream initiation by melting part of the rather extensively folded 5′-UTR. The leaky scanning that occurs on SBMV-C RNA is unusual in bypassing two intervening AUG codons (Sivakumaran and Hacker, 1998). Several of these examples present properties that differ from the standard form of leaky scanning (Kozak, 1989), suggesting that a variety of idiosyncratic expression mechanisms likely awaits discovery as more viruses are investigated in depth. Not only does leaky scanning allow expression of overlapping ORFs but it controls the relative amounts of protein synthesized from each ORF. For example, alteration of the start codon of the first ORF (P0, suppressor of silencing) of Beet western yellows polerovirus (Fig. 3) to a more efficient or less efficient context prevents virus replication (Pfeffer et al., 2002).
Translational recoding: readthrough and frameshifting
Recoding is dynamic reprogramming of translation so that the genetic code is temporarily redefined at specific codons, generally at low frequency (Gesteland et al., 1992; Baranov et al., 2002). Here, we discuss examples of stop codon readthrough and ribosomal frameshifting. The structure of the viral mRNA contains complete information in the form of cis-acting sequences to induce these recoding events. Many viruses employ in-frame readthrough of stop codons to express low levels of a C-terminally extended version of a protein (Fig. 2C). The best characterized example is the expression of the 126 and 183 kDa ORFs of TMV. Expression of the catalytic domain of the RNA-dependent RNA polymerase (RdRp), which is located in the C terminal portion of P183, requires readthrough of the P126 ORF stop codon (Pelham, 1978). This results in expression of large quantities of P126, which includes helicase and methyl transferase (capping enzyme) domains, and much smaller amounts of the RdRp (P183), which consists of P126 with a 57-kDa C-terminal extension. Translation studies in which expression of a reporter gene depended on readthrough revealed that the sequence UAGCARYYA (UAG is the P126 stop codon, R = purine, Y = pyrimidine) was sufficient for wild-type levels of readthrough (Fig. 2C), which was about 5% (Namy et al., 2001; Skuzeski et al., 1991).
Unrelated viruses also employ readthrough to express the catalytic domain of the RdRp. These include most genera of the Tombusviridae and various rod-shaped fungus-transmitted viruses. The readthrough signals of these viruses are poorly characterized.
The stop codons of the major CP gene of viruses in the Luteoviridae, furoviruses, and Beet necrotic yellow vein virus (BNYVV) are leaky, resulting in a C-terminal extension of the CP that is larger than the CP itself. In Luteoviridae, the CP alone is sufficient to form infectious virions, but the readthrough domain (C-terminal extension) is required for aphid transmission (Brault et al., 1995, 2005). For rod-shaped fungally transmitted viruses, such as BNYVV (Tamada et al., 1996) and Potato mop-top virus (Reavy et al., 1998), the readthrough domain facilitates fungal transmission. In contrast to the luteoviruses, the readthrough domain is required for BNYVV virion assembly (Schmitt et al., 1992).
In the Luteoviridae, the cis-acting signals that cause the ribosome to read through the stop codon include a cytidine-rich repeat (CCNNNN)8 – 16 beginning about 20 nt downstream of the stop codon and an essential sequence located over 700 nt downstream (Brown et al., 1996). Thus, the luteovirus readthrough signal is totally different from that of TMV. Readthrough occurs when a non-cognate aminoacyl-tRNA base-pairs to the stop codon in the ribosomal A site, followed by peptidyl transfer rather than insertion of release factor in the A site that facilitates termination. It is not known how the diverse viral readthrough signals cause a fraction of the ribosomes to be reprogrammed in this way.
A smaller set of viruses employs an entirely different recoding event to achieve a similar end result as readthrough. Instead of reading through a stop codon, ribosomes are induced to change reading frames during the elongation phase of translation. Like readthrough, frameshifting generally occurs for less than 5% of transiting ribosomes. In most plant viruses, the frameshift causes the ribosome to bypass a stop codon, providing a C-terminal extension to the protein generated by canonical translation (Fig. 2D). In most plant viruses known to undergo frameshifting, it is the catalytic domain of the RdRp that is expressed by frameshift. The Luteoviridae, the Dianthovirus genus of the Tombusviridae, and some sobemoviruses (Makinen et al., 1995) all employ −1 frameshifting, i.e., the ribosomes back up one base after pausing during translation of the first ORF, thereby shifting into the second ORF. As with readthrough, this appears to be a regulatory strategy to express the RdRp at low levels. One exception appears to be SBMV, in which the frameshift causes the ribosome to shift out of the RdRp reading frame in order to translate an ORF needed for virus cell-to-cell movement (Sivakumaran et al., 1998).
Plant viruses share features with the canonical −1 frameshift signals present in the polymerase genes of the animal-infecting nidoviruses and retroviruses. This includes an XXxNNNZ motif at the shifty site, where X is any base, N is usually A or U, and Z is any base except G (lower case indicates a consensus with occasional exceptions). The shifty site is followed by a highly structured region, usually a pseudoknot, beginning 5–6 nt downstream (Fig. 2D). The three-dimensional structure of the small, 28 nt frameshift-inducing pseudoknot of poleroviruses has been characterized at high resolution by X-ray crystallography (Su et al., 1999) and nuclear magnetic resonance (Cornish et al., 2005; Giedroc et al., 2003) and subjected to saturation mutagenesis (Kim et al., 2000; Kim et al., 1999). It is a remarkably compact structure featuring base triplets between the first stem of the pseudoknot and an A tract in the second loop. In contrast, the secondary structures that induce frameshifting on Red clover necrotic mosaic dianthovirus (RCNMV) (Kim and Lommel, 1998) and BYDV RNAs consist of a long bulged stem-loop, which, in the case of BYDV, must base-pair to a sequence 4 kb downstream (Barry and Miller, 2002). Despite being members of different families, the primary and secondary structures of RCNMV and BYDV frameshift elements are quite similar, as are the amino acid sequences of the polymerase genes.
Regarding mechanism, it has been proposed that the downstream pseudoknot slows or inhibits the advancing ribosome, perhaps even forcing it back one base, precisely when the shifty site codons are in the A and P sites of the ribosome (Plant et al., 2003; Yusupova et al., 2001). The tRNAs in the A and P sites simultaneously slip back one base on the mRNA and then re-pair. The shifty site sequence usually allows five of the six bases in the anticodons of the tRNAs in the A and P sites to pair to the codons in the −1 reading frame of the mRNA.
Other less well-characterized frameshift events may be unique to plant viruses. Translation of the Potato virus M 12-kDa ORF requires a −1 frameshift to occur when ribosomes reach the CP stop codon (Gramstat et al., 1994). This event requires only four slippery bases that are followed immediately by any stop codon. The stop codon presumably induces pausing, and slippage occurs with a tRNA in only the P site. The sequences of the closterovirus genomes suggest that a net reading frame shift of +1 must occur to express the RdRp (Karasev et al., 1995). If indeed +1 frameshifting occurs, this would be of interest because +1 frameshifting or other events that cause a net +1 frame change have not been demonstrated in any eukaryotic virus genome (Baranov et al., 2001).
Future perspectives
Research over the next few years on the topics we have described in this review will bring a better understanding of the range of regulatory elements present in plant viruses that appear to be quite distinct from those found in animal viruses, including the 3′-translational enhancers and IRES elements. A number of other fruitful research directions can be anticipated. More information is needed on the features that regulate the competition between coexisting viral mRNAs and the levels of the different gene products encoded by a given virus. We have described the expanded coding possibilities afforded by overlapping open reading frames, readthrough, and frameshifting, and mentioned the fact that recoding results in low expression levels for one of the proteins. But we do not fully understand the importance of particular expression ratios nor all the factors that influence those ratios, especially when proteins are made from different mRNAs, including subgenomic RNAs. For instance, we do not understand why subgenomic RNAs encoding coat proteins often translationally out-compete genomic RNAs encoding replication proteins (Pyne and Hall, 1979; Wang et al., 1999). The regulated timing and quantity of viral protein accumulation likely are an important ingredient for a successful infection (Shen and Miller, 2004a, 2004b).
A question that has been insufficiently addressed for plant viruses is the effect of virus infection on host translation. It is generally believed that plant viruses, unlike most animal viruses, do not globally shut off host gene expression (Hull, 2002). During animal virus infections, there is active parrying at the level of the translation machinery in which the host attempts to limit the translation of viral proteins while the virus attempts to establish optimal conditions for the selective translation of viral mRNAs (Gale et al., 2000). For instance, picornaviruses, whose RNAs lack a 5′-cap, turn that distinction to their advantage by undermining cap-dependent translation. It is not known to what extent virus–host interactions of this type occur during plant virus infections, but it is important to clarify this matter. In one set of studies, host gene expression was transiently reduced at the virus infection front (Wang and Maule, 1995), though this appeared not to occur at the level of translation (Aranda and Maule, 1998). The regulation of translation is an innate response that is a valuable part of the antiviral arsenal available to animal cells (Schneider and Mohr, 2003). In plants, understanding the involvement of similar responses is only just beginning (Bilgin et al., 2003), but there is the possibility that the powerful role of post-transcriptional gene silencing in plants has diminished reliance on antiviral regulation at the translational level.
A fertile field for future research should be elucidating the transition from translation to replication during the infection. This is expected to require a clearance of ribosomes from the mRNA. Two possible strategies are suggested by studies with BYDV and TYMV mentioned in this review. But the recruitment of viral RNAs to the membranous sites of RNA replication is also part of this transition (Schwartz et al., 2002). Studies with BMV indicate that the translation of viral RNAs in yeast requires a set of host proteins (Lsm1–7p and Pat1p (Noueiry et al., 2003)) that are components of P bodies containing non-translating mRNAs. One role of the P bodies is decapping and RNA degradation, but others seem likely (Teixeira et al., 2005). Viral RNAs should be able to avoid being drawn too rapidly into the degradative pathway and perhaps have evolved to co-opt P bodies to assist in escaping translation for recruitment into the sites of replication.
Acknowledgments
This work was funded in part by NIH grant R01-GM067104 to WAM and NSF grant MCB-0235563 to TWD.
References
- Aranda M, Maule A. Virus-induced host gene shutoff in animals and plants. Virology. 1998;243:261–267. doi: 10.1006/viro.1998.9032. [DOI] [PubMed] [Google Scholar]
- Baranov PV, Gurvich OL, Fayet O, Prere MF, Miller WA, Gesteland RF, Atkins JF, Giddings MC. RECODE: a database of frameshifting, bypassing and codon redefinition utilized for gene expression. Nucleic Acids Res. 2001;29:264–267. doi: 10.1093/nar/29.1.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranov PV, Gesteland RF, Atkins JF. Recording: translational bifurcations in gene expression. Gene. 2002;286:187–201. doi: 10.1016/s0378-1119(02)00423-7. [DOI] [PubMed] [Google Scholar]
- Barends S, Bink HH, van den Worm SH, Pleij CW, Kraal B. Entrapping ribosomes for viral translation: tRNA mimicry as a molecular Trojan horse. Cell. 2003;112:123–129. doi: 10.1016/s0092-8674(02)01256-4. [DOI] [PubMed] [Google Scholar]
- Barends S, Rudinger-Thirion J, Florentz C, Giege R, Pleij CW, Kraal B. tRNA-like structure regulates translation of Brome mosaic virus RNA. J Virol. 2004;78:4003–4010. doi: 10.1128/JVI.78.8.4003-4010.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry JK, Miller WA. A programmed −1 ribosomal frameshift that requires base-pairing across four kilobases suggests a novel mechanism for controlling ribosome and replicase traffic on a viral RNA. Proc Natl Acad Sci USA. 2002;99:11133–11138. doi: 10.1073/pnas.162223099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso J, Dallaire P, Charest PJ, Devantier Y, Laliberte JF. Evidence for an internal ribosome entry site within the 5′ non-translated region of turnip mosaic potyvirus RNA. J Gen Virol. 1994a;75:3157–3165. doi: 10.1099/0022-1317-75-11-3157. [DOI] [PubMed] [Google Scholar]
- Basso J, Dallaire P, Charest PJ, Devantier Y, Laliberte JF. Evidence for an internal ribosome entry site within the 5′ non-translated region of turnip mosaic potyvirus RNA. J Gen Virol. 1994b;75:3157–3165. doi: 10.1099/0022-1317-75-11-3157. [DOI] [PubMed] [Google Scholar]
- Belsham GJ, Jackson RJ. Translation initiation on picornavirus RNA. In: Sonenberg N, Hershey JWB, Mathews MB, editors. Translational Control of Gene Expression. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 2000. [Google Scholar]
- Bilgin DD, Liu Y, Schiff M, Dinesh-Kumar SP. P58(IPK), a plant ortholog of double-stranded RNA-dependent protein kinase PKR inhibitor, functions in viral pathogenesis. Dev Cell. 2003;4:651–661. doi: 10.1016/s1534-5807(03)00125-4. [DOI] [PubMed] [Google Scholar]
- Bol JF. Replication of alfamo- and ilarviruses: role of the coat protein. Annu Rev Phytopathol. 2005;43:39–62. doi: 10.1146/annurev.phyto.43.101804.120505. [DOI] [PubMed] [Google Scholar]
- Bransom KL, Weiland JJ, Tsai CH, Dreher TW. Coding density of the turnip yellow mosaic virus genome: roles of the overlapping coat protein and p206-readthrough coding regions. Virology. 1995;206:403–412. doi: 10.1016/s0042-6822(95)80056-5. [DOI] [PubMed] [Google Scholar]
- Brault V, Van den Heuvel JFJM, Verbeek M, Ziegler-Graff V, Reutenauer A, Herrbach E, Garaud JC, Guilley H, Richards K, Jonard G. Aphid transmission of beet western yellows luteovirus requires the minor capsid read-through protein P74. EMBO J. 1995;14:650–659. doi: 10.1002/j.1460-2075.1995.tb07043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brault V, Perigon S, Reinbold C, Erdinger M, Scheidecker D, Herrbach E, Richards K, Ziegler-Graff V. The polerovirus minor capsid protein determines vector specificity and intestinal tropism in the aphid. J Virol. 2005;79:9685–9693. doi: 10.1128/JVI.79.15.9685-9693.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CM, Dinesh-Kumar SP, Miller WA. Local and distant sequences are required for efficient read-through of the barley yellow dwarf virus-PAV coat protein gene stop codon. J Virol. 1996;70:5884–5892. doi: 10.1128/jvi.70.9.5884-5892.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrington JC, Freed DD. Cap-independent enhancement of translation by a plant potyvirus 5′ nontranslated region. J Virol. 1990;64:1590–1597. doi: 10.1128/jvi.64.4.1590-1597.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish PV, Hennig M, Giedroc DP. A loop 2 cytidine-stem 1 minor groove interaction as a positive determinant for pseudoknot-stimulated -1 ribosomal frameshifting. Proc Natl Acad Sci USA. 2005;102:12694–12699. doi: 10.1073/pnas.0506166102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinesh-Kumar SP, Miller WA. Control of start codon choice on a plant viral RNA encoding overlapping genes. Plant Cell. 1993;5:679–692. doi: 10.1105/tpc.5.6.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorokhov YL, Skulachev MV, Ivanov PA, Zvereva SD, Tjulkina LG, Merits A, Gleba YY, Hohn TJ, Atabekov JG. Polypurine (A)-rich sequences promote cross-kingdom conservation of internal ribosome entry. Proc Natl Acad Sci USA. 2002;99:5301–5306. doi: 10.1073/pnas.082107599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreher TW, Uhlenbeck OC, Browning KS. Quantitative assessment of EF−1alpha. GTP binding to aminoacyl-tRNAs, aminoacyl-viral RNA, and tRNA shows close correspondence to the RNA binding properties of EF-Tu. J Biol Chem. 1999;274:666–672. doi: 10.1074/jbc.274.2.666. [DOI] [PubMed] [Google Scholar]
- Fabian MR, White KA. 5′-3′ RNA-RNA interaction facilitates cap-and poly(A) tail-independent translation of tomato bushy stunt virus mRNA: a potential common mechanism for Tombusviridae. J Biol Chem. 2004;279:28862–28872. doi: 10.1074/jbc.M401272200. [DOI] [PubMed] [Google Scholar]
- Gale M, Jr, Tan SL, Katze MG. Translational control of viral gene expression in eukaryotes. Microbiol Mol Biol Rev. 2000;64:239–280. doi: 10.1128/mmbr.64.2.239-280.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallie DR. The cap and poly(A) tail function synergistically to regulate mRNA translational efficiency. Genes Dev. 1991;5:2108–2116. doi: 10.1101/gad.5.11.2108. [DOI] [PubMed] [Google Scholar]
- Gallie DR. Cap-Independent translation conferred by the 5′ leader of Tobacco etch virus is eukaryotic initiation factor 4G dependent. J Virol. 2001;75:12141–12152. doi: 10.1128/JVI.75.24.12141-12152.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallie DR. The 5′-leader of tobacco mosaic virus promotes translation through enhanced recruitment of eIF4F. Nucleic Acids Res. 2002;30:3401–3411. doi: 10.1093/nar/gkf457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallie DR, Kobayashi M. The role of the 3′-untranslated region of non-polyadenylated plant viral mRNAs in regulating translational efficiency. Gene. 1994;142:159–165. doi: 10.1016/0378-1119(94)90256-9. [DOI] [PubMed] [Google Scholar]
- Gallie DR, Walbot V. RNA pseudoknot domain of tobacco mosaic virus can functionally substitute for a poly(A) tail in plant and animal cells. Genes Dev. 1990;4:1149–1157. doi: 10.1101/gad.4.7.1149. [DOI] [PubMed] [Google Scholar]
- Gallie DR, Sleat DE, Watts JW, Turner PC, Wilson TM. The 5′-leader sequence of tobacco mosaic virus RNA enhances the expression of foreign gene transcripts in vitro and in vivo. Nucleic Acids Res. 1987a;15:3257–3273. doi: 10.1093/nar/15.8.3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallie DR, Sleat DE, Watts JW, Turner PC, Wilson TM. In vivo uncoating and efficient expression of foreign mRNAs packaged in TMV-like particles. Science. 1987b;236:1122–1124. doi: 10.1126/science.3472350. [DOI] [PubMed] [Google Scholar]
- Gallie DR, Sleat DE, Watts JW, Turner PC, Wilson T. Mutational analysis of the tobacco mosaic virus 5′-leader for altered ability to enhance translation. Nucleic Acids Res. 1988;16:883–893. doi: 10.1093/nar/16.3.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamarnik AV, Andino R. Switch from translation to RNA replication in a positive-stranded RNA virus. Genes Dev. 1998;12:2293–2304. doi: 10.1101/gad.12.15.2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z, Johansen E, Eyers S, Thomas CL, Noel Ellis TH, Maule AJ. The potyvirus recessive resistance gene, sbm1, identifies a novel role for translation initiation factor eIF4E in cell-to-cell trafficking. Plant J. 2004;40:376–385. doi: 10.1111/j.1365-313X.2004.02215.x. [DOI] [PubMed] [Google Scholar]
- Gazo BM, Murphy P, Gatchel JR, Browning KS. A novel interaction of Cap-binding protein complexes eukaryotic initiation factor (eIF) 4F and eIF(iso)4F with a region in the 3′-untranslated region of satellite tobacco necrosis virus. J Biol Chem. 2004;279:13584–13592. doi: 10.1074/jbc.M311361200. [DOI] [PubMed] [Google Scholar]
- Gesteland RF, Weiss RB, Atkins JF. Recording: reprogrammed genetic decoding. Science. 1992;257:1640–1641. doi: 10.1126/science.1529352. [DOI] [PubMed] [Google Scholar]
- Giedroc DP, Cornish PV, Hennig M. Detection of scalar couplings involving 2′-hydroxyl protons across hydrogen bonds in a frameshifting mRNA pseudoknot. J Am Chem Soc. 2003;125:4676–4677. doi: 10.1021/ja029286t. [DOI] [PubMed] [Google Scholar]
- Gramstat A, Prufer D, Rohde W. The nucleic acid-binding zinc finger protein of potato virus M is translated by internal initiation as well as by ribosomal frameshifting involving a shifty stop codon and a novel mechanism of P-site slippage. Nucleic Acids Res. 1994;22:3911–3917. doi: 10.1093/nar/22.19.3911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Allen E, Miller WA. Structure and function of a cap-independent translation element that functions in either the 3′ or the 5′ untranslated region. RNA. 2000;6:1808–1820. doi: 10.1017/s1355838200001539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Allen E, Miller WA. Base-pairing between untranslated regions facilitates translation of uncapped, nonpolyadenylated viral RNA. Mol Cell. 2001;7:1103–1109. doi: 10.1016/s1097-2765(01)00252-0. [DOI] [PubMed] [Google Scholar]
- Guogas LM, Filman DJ, Hogle JM, Gehrke L. Cofolding organizes alfalfa mosaic virus RNA and coat protein for replication. Science. 2004;306:2108–2111. doi: 10.1126/science.1103399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentze MW. eIF4G: a multipurpose ribosome adapter? Science. 1997;275:500–501. doi: 10.1126/science.275.5299.500. (published erratum appears in Science 1997 Mar. 14;275 (5306):1553) [DOI] [PubMed] [Google Scholar]
- Herbert TP, Brierley I, Brown TD. Identification of a protein linked to the genomic and subgenomic mRNAs of feline calicivirus and its role in translation. J Gen Virol. 1997;78 :1033–1040. doi: 10.1099/0022-1317-78-5-1033. Pt 5. [DOI] [PubMed] [Google Scholar]
- Herzog E, Guilley H, Fritsch C. Translation of the second gene of peanut clump virus RNA 2 occurs by leaky scanning in vitro. Virology. 1995;208:215–225. doi: 10.1006/viro.1995.1145. [DOI] [PubMed] [Google Scholar]
- Holness CL, Lomonossoff GP, Evans D, Maule AJ. Identification of the initiation codons for translation of cowpea mosaic virus middle component RNA using site-directed mutagenesis of an infectious cDNA clone. Virology. 1989;172:311–320. doi: 10.1016/0042-6822(89)90133-5. [DOI] [PubMed] [Google Scholar]
- Hull R. Matthews’ Plant Virology. 4. Academic Press; London: 2002. [Google Scholar]
- Ivanov PA, Karpova OV, Skulachev MV, Tomashevskaya OL, Rodionova NP, Dorokhov Yu L, Atabekov JG. A tobamovirus genome that contains an internal ribosome entry site functional in vitro. Virology. 1997;232:32–43. doi: 10.1006/viro.1997.8525. [DOI] [PubMed] [Google Scholar]
- Jaag HM, Kawchuk L, Rohde W, Fischer R, Emans N, Prufer D. An unusual internal ribosomal entry site of inverted symmetry directs expression of a potato leafroll polerovirus replication-associated protein. Proc Natl Acad Sci USA. 2003;100:8939–8944. doi: 10.1073/pnas.1332697100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobling SA, Gehrke L. Enhanced translation of chimaeric messenger RNAs containing a plant viral untranslated leader sequence. Nature. 1987;325:622–625. doi: 10.1038/325622a0. [DOI] [PubMed] [Google Scholar]
- Johnston JC, Rochon DM. Both codon context and leader length contribute to efficient expression of two overlapping open reading frames of a cucumber necrosis virus bifunctional subgenomic mRNA. Virology. 1996;221:232–239. doi: 10.1006/viro.1996.0370. [DOI] [PubMed] [Google Scholar]
- Joshi CP, Zhou H, Huang X, Chiang VL. Context sequences of translation initiation codon in plants. Plant Mol Biol. 1997;35:993–1001. doi: 10.1023/a:1005816823636. [DOI] [PubMed] [Google Scholar]
- Juszczuk M, Paczkowska E, Sadowy E, Zagorski W, Hulanicka DM. Effect of genomic and subgenomic leader sequences of potato leafroll virus on gene expression. FEBS Lett. 2000;484:33–36. doi: 10.1016/s0014-5793(00)02122-0. [DOI] [PubMed] [Google Scholar]
- Kang BC, Yeam I, Frantz JD, Murphy JF, Jahn MM. The pvr1 locus in Capsicum encodes a translation initiation factor eIF4E that interacts with Tobacco etch virus VPg. Plant J. 2005;42:392–405. doi: 10.1111/j.1365-313X.2005.02381.x. [DOI] [PubMed] [Google Scholar]
- Karasev AV, Boyko VP, Gowda S, Nikolaeva OV, Hilf ME, Koonin EV, Niblett CL, Cline K, Gumpf DJ, Lee RF, et al. Complete sequence of the citrus tristeza virus RNA genome. Virology. 1995;208:511–520. doi: 10.1006/viro.1995.1182. [DOI] [PubMed] [Google Scholar]
- Kim KH, Lommel SA. Sequence element required for efficient −1 ribosomal frameshifting in red clover necrotic mosaic dianthovirus. Virology. 1998;250:50–59. doi: 10.1006/viro.1998.9358. [DOI] [PubMed] [Google Scholar]
- Kim YG, Su L, Maas S, O’Neill A, Rich A. Specific mutations in a viral RNA pseudoknot drastically change ribosomal frameshifting efficiency. Proc Natl Acad Sci USA. 1999;96:14234–14239. doi: 10.1073/pnas.96.25.14234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YG, Maas S, Wang SC, Rich A. Mutational study reveals that tertiary interactions are conserved in ribosomal frameshifting pseudoknots of two luteoviruses. RNA. 2000;6:1157–1165. doi: 10.1017/s1355838200000510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koev G, Liu S, Beckett R, Miller WA. The 3′-terminal structure required for replication of barley yellow dwarf virus RNA contains an embedded 3′ end. Virology. 2002;292:114–126. doi: 10.1006/viro.2001.1268. [DOI] [PubMed] [Google Scholar]
- Kozak M. The scanning model for translation: an update. J Cell Biol. 1989;108:229–241. doi: 10.1083/jcb.108.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Initiation of translation in prokaryotes and eukaryotes. Gene. 1999;234:187–208. doi: 10.1016/s0378-1119(99)00210-3. [DOI] [PubMed] [Google Scholar]
- Kozak M. Pushing the limits of the scanning mechanism for initiation of translation. Gene. 2002;299:1–34. doi: 10.1016/S0378-1119(02)01056-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krab IM, Caldwell C, Gallie DR, Bol JF. Coat protein enhances translational efficiency of Alfalfa mosaic virus RNAs and interacts with the eIF4G component of initiation factor eIF4F. J Gen Virol. 2005;86:1841–1849. doi: 10.1099/vir.0.80796-0. [DOI] [PubMed] [Google Scholar]
- Le H, Tanguay RL, Balasta ML, Wei CC, Browning KS, Metz AM, Goss DJ, Gallie DR. Translation initiation factors eIF-iso4G and eIF-4B interact with the poly(A)-binding protein and increase its RNA binding activity. J Biol Chem. 1997;272:16247–16255. doi: 10.1074/jbc.272.26.16247. [DOI] [PubMed] [Google Scholar]
- Leathers V, Tanguay R, Kobayashi M, Gallie DR. A phylogenetically conserved sequence within viral 3′ untranslated RNA pseudoknots regulates translation. Mol Cell Biol. 1993;13:5331–5347. doi: 10.1128/mcb.13.9.5331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Sowa ME, Choi JM, Tsai FT. The ClpB/Hsp104 molecular chaperone-a protein disaggregating machine. J Struct Biol. 2004;146:99–105. doi: 10.1016/j.jsb.2003.11.016. [DOI] [PubMed] [Google Scholar]
- Lellis AD, Kasschau KD, Whitham SA, Carrington JC. Loss-of-susceptibility mutants of Arabidopsis thaliana reveal an essential role for eIF(iso)4E during potyvirus infection. Curr Biol. 2002;12:1046–1051. doi: 10.1016/s0960-9822(02)00898-9. [DOI] [PubMed] [Google Scholar]
- Leonard S, Plante D, Wittmann S, Daigneault N, Fortin MG, Laliberte JF. Complex formation between potyvirus VPg and translation eukaryotic initiation factor 4E correlates with virus infectivity. J Virol. 2000;74:7730–7737. doi: 10.1128/jvi.74.17.7730-7737.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard S, Chisholm J, Laliberte JF, Sanfacon H. Interaction in vitro between the proteinase of Tomato ringspot virus (genus Nepovirus) and the eukaryotic translation initiation factor iso4E from Arabidopsis thaliana. J Gen Virol. 2002;83:2085–2089. doi: 10.1099/0022-1317-83-8-2085. [DOI] [PubMed] [Google Scholar]
- Leonard S, Viel C, Beauchemin C, Daigneault N, Fortin MG, Laliberte JF. Interaction of VPg-Pro of turnip mosaic virus with the translation initiation factor 4E and the poly(A)-binding protein in planta. J Gen Virol. 2004;85:1055–1063. doi: 10.1099/vir.0.19706-0. [DOI] [PubMed] [Google Scholar]
- Lukaszewicz M, Feuermann M, Jerouville B, Stas A, Boutry M. In vivo evaluation of the context sequence of the translation initiation codon in plants. Plant Sci. 2000;154:89–98. doi: 10.1016/s0168-9452(00)00195-3. [DOI] [PubMed] [Google Scholar]
- Lutcke HA, Chow KC, Mickel FS, Moss KA, Kern HF, Scheele GA. Selection of AUG initiation codons differs in plants and animals. EMBO J. 1987;6:43–48. doi: 10.1002/j.1460-2075.1987.tb04716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makinen K, Naess V, Tamm T, Truve E, Aaspollu A, Saarma M. The putative replicase of cocksfoot mottle sobemovirus is translated as a part of the polyprotein by −1 ribosomal frameshift. Virology. 1995;207:566–571. doi: 10.1006/viro.1995.1118. [DOI] [PubMed] [Google Scholar]
- Mans RM, Pleij CW, Bosch L. tRNA-like structures. Structure, function and evolutionary significance. Eur J Biochem. 1991;201:303–324. doi: 10.1111/j.1432-1033.1991.tb16288.x. [DOI] [PubMed] [Google Scholar]
- Matsuda D, Dreher TW. The tRNA-like structure of Turnip yellow mosaic virus RNA is a 3′-translational enhancer. Virology. 2004;321:36–46. doi: 10.1016/j.virol.2003.10.023. [DOI] [PubMed] [Google Scholar]
- Matsuda D, Bauer L, Tinnesand K, Dreher TW. Expression of the two nested overlapping reading frames of turnip yellow mosaic virus RNA is enhanced by a 5′ cap and by 5′ and 3′ viral sequences. J Virol. 2004a;78:9325–9335. doi: 10.1128/JVI.78.17.9325-9335.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda D, Yoshinari S, Dreher TW. eEF1A binding to aminoacylated viral RNA represses minus strand synthesis by TYMV RNA-dependent RNA polymerase. Virology. 2004b;321:47–56. doi: 10.1016/j.virol.2003.10.028. [DOI] [PubMed] [Google Scholar]
- Meulewaeter F, Danthinne X, Van Montagu M, Cornelissen M. 5′-and 3′-sequences of satellite tobacco necrosis virus RNA promoting translation in tobacco. Plant J. 1998a;14:169–176. doi: 10.1046/j.1365-313x.1998.00104.x. (published erratum appears in Plant J. 1998 Jul;15(1):153–4) [DOI] [PubMed] [Google Scholar]
- Meulewaeter F, Van Montagu M, Cornelissen M. Features of the autonomous function of the translational enhancer domain of satellite tobacco necrosis virus. RNA. 1998b;4:1347–1356. doi: 10.1017/s135583829898092x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meulewaeter F, van Lipzig R, Gultyaev AP, Pleij CW, Van Damme D, Cornelissen M, van Eldik G. Conservation of RNA structures enables TNV and BYDV 5′ and 3′ elements to cooperate synergistically in cap-independent translation. Nucleic Acids Res. 2004;32:1721–1730. doi: 10.1093/nar/gkh338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller WA, Liu S, Beckett R. Barley yellow dwarf virus: Luteoviridae or Tombusviridae? Mol Plant Pathol. 2002;3:177–183. doi: 10.1046/j.1364-3703.2002.00112.x. [DOI] [PubMed] [Google Scholar]
- Mizumoto H, Tatsuta M, Kaido M, Mise K, Okuno T. Cap-independent translational enhancement by the 3′ untranslated region of red clover necrotic mosaic virus RNA1. J Virol. 2003;77:12113–12121. doi: 10.1128/JVI.77.22.12113-12121.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namy O, Hatin I, Rousset JP. Impact of the six nucleotides downstream of the stop codon on translation termination. EMBO Rep. 2001;2:787–793. doi: 10.1093/embo-reports/kve176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neeleman L, Olsthoorn RC, Linthorst HJ, Bol JF. Translation of a nonpolyadenylated viral RNA is enhanced by binding of viral coat protein or polyadenylation of the RNA. Proc Natl Acad Sci USA. 2001;98:14286–14291. doi: 10.1073/pnas.251542798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neeleman L, Linthorst HJ, Bol JF. Efficient translation of alfamovirus RNAs requires the binding of coat protein dimers to the 3′ termini of the viral RNAs. J Gen Virol. 2004;85:231–240. doi: 10.1099/vir.0.19581-0. [DOI] [PubMed] [Google Scholar]
- Nicaise V, German-Retana S, Sanjuan R, Dubrana MP, Mazier M, Maisonneuve B, Candresse T, Caranta C, LeGall O. The eukaryotic translation initiation factor 4E controls lettuce susceptibility to the potyvirus lettuce mosaic virus. Plant Physiol. 2003;132:1272–1282. doi: 10.1104/pp.102.017855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niepel M, Gallie DR. Identification and characterization of the functional elements within the tobacco etch virus 5′ leader required for cap-independent translation. J Virol. 1999;73:9080–9088. doi: 10.1128/jvi.73.11.9080-9088.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noueiry AO, Chen J, Ahlquist P. A mutant allele of essential, general translation initiation factor DED1 selectively inhibits translation of a viral mRNA. Proc Natl Acad Sci USA. 2000;97:12985–12990. doi: 10.1073/pnas.240460897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noueiry AO, Diez J, Falk SP, Chen J, Ahlquist P. Yeast Lsm1p-7p/Pat1p deadenylation-dependent mRNA-decapping factors are required for brome mosaic virus genomic RNA translation. Mol Cell Biol. 2003;23:4094–4106. doi: 10.1128/MCB.23.12.4094-4106.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham HR. Leaky UAG termination codon in tobacco mosaic virus RNA. Nature. 1978;272:469–471. doi: 10.1038/272469a0. [DOI] [PubMed] [Google Scholar]
- Pestova TV, Kolupaeva VG, Lomakin IB, Pilipenko EV, Shatsky IN, Agol VI, Hellen CU. Molecular mechanisms of translation initiation in eukaryotes. Proc Natl Acad Sci USA. 2001;98:7029–7036. doi: 10.1073/pnas.111145798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettit Kneller EJ, Rakotondrafara AM, Miller WA. Cap-independent translation of plant viral RNAs. Virus Res. doi: 10.1016/j.virusres.2005.10.010. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer S, Dunoyer P, Heim F, Richards KE, Jonard G, Ziegler-Graff V. P0 of Beet western yellows virus is a suppressor of posttranscriptional gene silencing. J Virol. 2002;76:6815–6824. doi: 10.1128/JVI.76.13.6815-6824.2002. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Plant EP, Jacobs KL, Harger JW, Meskauskas A, Jacobs JL, Baxter JL, Petrov AN, Dinman JD. The 9-A solution: how mRNA pseudoknots promote efficient programmed −1 ribosomal frameshifting. RNA. 2003;9:168–174. doi: 10.1261/rna.2132503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogany J, Fabian MR, White KA, Nagy PD. A replication silencer element in a plus-strand RNA virus. EMBO J. 2003;22:5602–5611. doi: 10.1093/emboj/cdg523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyne JW, Hall TC. Efficient ribosome binding of brome mosaic virus (BMV) RNA4 contributes to its ability to outcompete the other BMV RNAs for translation. Intervirology. 1979;11:23–29. doi: 10.1159/000149008. [DOI] [PubMed] [Google Scholar]
- Reavy B, Arif M, Cowan GH, Torrance L. Association of sequences in the coat protein/readthrough domain of potato mop-top virus with transmission by Spongospora subterranea. J Gen Virol. 1998;79:2343–2347. doi: 10.1099/0022-1317-79-10-2343. Pt 10. [DOI] [PubMed] [Google Scholar]
- Ruffel S, Dussault MH, Palloix A, Moury B, Bendahmane A, Robaglia C, Caranta C. A natural recessive resistance gene against potato virus Y in pepper corresponds to the eukaryotic initiation factor 4E (eIF4E) Plant J. 2002;32:1067–1075. doi: 10.1046/j.1365-313x.2002.01499.x. [DOI] [PubMed] [Google Scholar]
- Ryabova LA, Hohn T. Ribosome shunting in the cauliflower mosaic virus 35S RNA leader is a special case of reinitiation of translation functioning in plant and animal systems. Genes Dev. 2000;14:817–829. [PMC free article] [PubMed] [Google Scholar]
- Sachs AB, Sarnow P, Hentze MW. Starting at the beginning, middle, and end: translation initiation in eukaryotes. Cell. 1997;89:831–838. doi: 10.1016/s0092-8674(00)80268-8. [DOI] [PubMed] [Google Scholar]
- Schaad MC, Anderberg RJ, Carrington JC. Strain-specific interaction of the tobacco etch virus NIa protein with the translation initiation factor eIF4E in the yeast two-hybrid system. Virology. 2000;273:300–306. doi: 10.1006/viro.2000.0416. [DOI] [PubMed] [Google Scholar]
- Schmitt C, Balmori E, Jonard G, Richards KE, Guilley H. In vitro mutagenesis of biologically active transcripts of beet necrotic yellow vein virus RNA 2: evidence that a domain of the 75-kDa readthrough protein is important for efficient virus assembly. Proc Natl Acad Sci USA. 1992;89:5715–5719. doi: 10.1073/pnas.89.13.5715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider RJ, Mohr I. Translation initiation and viral tricks. Trends Biochem Sci. 2003;28:130–136. doi: 10.1016/S0968-0004(03)00029-X. [DOI] [PubMed] [Google Scholar]
- Schwartz M, Chen J, Janda M, Sullivan M, den Boon J, Ahlquist P. A positive-strand RNA virus replication complex parallels form and function of retrovirus capsids. Mol Cell. 2002;9:505–514. doi: 10.1016/s1097-2765(02)00474-4. [DOI] [PubMed] [Google Scholar]
- Shen R, Miller WA. The 3′ untranslated region of tobacco necrosis virus RNA contains a barley yellow dwarf virus-like cap-independent translation element. J Virol. 2004a;78:4655–4664. doi: 10.1128/JVI.78.9.4655-4664.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen R, Miller WA. Subgenomic RNA as a riboregulator: negative regulation of RNA replication by Barley yellow dwarf virus subgenomic RNA 2. Virology. 2004b;327:196–205. doi: 10.1016/j.virol.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Shirako Y. Non-AUG translation initiation in a plant RNAvirus: a forty-amino-acid extension is added to the N terminus of the soil-borne wheat mosaic virus capsid protein. J Virol. 1998;72:1677–1682. doi: 10.1128/jvi.72.2.1677-1682.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon-Buela L, Guo HS, Garcia JA. Cap-independent leaky scanning as the mechanism of translation initiation of a plant viral genomic RNA. J Gen Virol. 1997;78:2691–2699. doi: 10.1099/0022-1317-78-10-2691. [DOI] [PubMed] [Google Scholar]
- Sivakumaran K, Hacker DL. The 105-kDa polyprotein of southern bean mosaic virus is translated by scanning ribosomes. Virology. 1998;246:34–44. doi: 10.1006/viro.1998.9183. [DOI] [PubMed] [Google Scholar]
- Sivakumaran K, Fowler BC, Hacker DL. Identification of viral genes required for cell-to-cell movement of southern bean mosaic virus. Virology. 1998;252:376–386. doi: 10.1006/viro.1998.9489. [DOI] [PubMed] [Google Scholar]
- Skuzeski JM, Nichols LM, Gesteland RF, Atkins JF. The signal for a leaky UAG stop codon in several plant viruses includes the two downstream codons. J Mol Biol. 1991;218:365–373. doi: 10.1016/0022-2836(91)90718-l. [DOI] [PubMed] [Google Scholar]
- Stein N, Perovic D, Kumlehn J, Pellio B, Stracke S, Streng S, Ordon F, Graner A. The eukaryotic translation initiation factor 4E confers multiallelic recessive Bymovirus resistance in Hordeum vulgare (L.) Plant J. 2005;42:912–922. doi: 10.1111/j.1365-313X.2005.02424.x. [DOI] [PubMed] [Google Scholar]
- Su L, Chen L, Egli M, Berger JM, Rich A. Minor groove RNA triplex in the crystal structure of a ribosomal frameshifting viral pseudoknot. Nat Struct Biol. 1999;6:285–292. doi: 10.1038/6722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamatsu N, Watanabe Y, Meshi T, Okada Y. Mutational analysis of the pseudoknot region in the 3′ noncoding region of tobacco mosaic virus RNA. J Virol. 1990;64:3686–3693. doi: 10.1128/jvi.64.8.3686-3693.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamada T, Schmitt C, Saito M, Guilley H, Richards K, Jonard G. High resolution analysis of the readthrough domain of beet necrotic yellow vein virus readthrough protein: a KTER motif is important for efficient transmission of the virus by Polymyxa betae. J Gen Virol. 1996;77:1359–1367. doi: 10.1099/0022-1317-77-7-1359. [DOI] [PubMed] [Google Scholar]
- Teixeira D, Sheth U, Valencia-Sanchez MA, Brengues M, Parker R. Processing bodies require RNA for assembly and contain nontranslating mRNAs. RNA. 2005;11:371–382. doi: 10.1261/rna.7258505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thivierge K, Nicaise V, Dufresne PJ, Cotton S, Laliberte JF, Le Gall O, Fortin MG. Plant virus RNAs. coordinated recruitment of conserved host functions by (+) ssRNA viruses during early infection events. Plant Physiol. 2005;138:1822–1827. doi: 10.1104/pp.105.064105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmer RT, Benkowski LA, Schodin D, Lax SR, Metz AM, Ravel JM, Browning KS. The 5′ and 3′ untranslated regions of satellite tobacco necrosis virus RNA affect translational efficiency and dependence on a 5′ cap structure. J Biol Chem. 1993;268:9504–9510. [PubMed] [Google Scholar]
- van Regenmortel MHV, Fauquet CM, Bishop DHL, Carstens EB, Estes MK, Lemon SM, Maniloff J, Mayo MA, McGeoch DJ, Pringle CR, Wickner RB, editors. Virus Taxonomy: Seventh report of the International Committee on Taxonomy of Viruses. Academic Press; San Diego: 2000. [Google Scholar]
- Varani G, Allain FH. How a rotavirus hijacks the human protein synthesis machinery. Nat Struct Biol. 2002;9:158–160. doi: 10.1038/nsb0302-158. [DOI] [PubMed] [Google Scholar]
- Verchot J, Angell SM, Baulcombe DC. In vivo translation of the triple gene block of potato virus X requires two subgenomic mRNAs. J Virol. 1998;72:8316–8320. doi: 10.1128/jvi.72.10.8316-8320.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Maule AJ. Inhibition of host gene expression associated with plant virus replication. Science. 1995;267:229–231. doi: 10.1126/science.267.5195.229. [DOI] [PubMed] [Google Scholar]
- Wang S, Browning KS, Miller WA. A viral sequence in the 3′-untranslated region mimics a 5′ cap in facilitating translation of uncapped mRNA. EMBO J. 1997;16:4107–4116. doi: 10.1093/emboj/16.13.4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Guo L, Allen E, Miller WA. A potential mechanism for selective control of cap-independent translation by a viral RNA sequence in cis and in trans. RNA. 1999;5:728–738. doi: 10.1017/s1355838299981979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei CC, Balasta ML, Ren J, Goss DJ. Wheat germ poly(A) binding protein enhances the binding affinity of eukaryotic initiation factor 4F and (iso)4F for cap analogues. Biochemistry. 1998;37:1910–1916. doi: 10.1021/bi9724570. [DOI] [PubMed] [Google Scholar]
- Weiland JJ, Dreher TW. Infectious TYMV RNA from cloned cDNA: effects in vitro and in vivo of point substitutions in the initiation codons of two extensively overlapping ORFs. Nucleic Acid Res. 1989;17:4675–4687. doi: 10.1093/nar/17.12.4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells DR, Tanguay RL, Le H, Gallie DR. HSP101 functions as a specific translational regulatory protein whose activity is regulated by nutrient status. Genes Dev. 1998;12:3236–3251. doi: 10.1101/gad.12.20.3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusupova GZ, Yusupov MM, Cate JH, Noller HF. The path of messenger RNA through the ribosome. Cell. 2001;106:233–241. doi: 10.1016/s0092-8674(01)00435-4. [DOI] [PubMed] [Google Scholar]
- Zeenko V, Gallie DR. Cap-independent translation of tobacco etch virus is conferred by an RNA pseudoknot in the 5′-leader. J Biol Chem. 2005;280:26813–26824. doi: 10.1074/jbc.M503576200. [DOI] [PubMed] [Google Scholar]
- Zeenko VV, Ryabova LA, Spirin AS, Rothnie HM, Hess D, Browning KS, Hohn T. Eukaryotic elongation factor 1A interacts with the upstream pseudoknot domain in the 3′ untranslated region of tobacco mosaic virus RNA. J Virol. 2002;76:5678–5691. doi: 10.1128/JVI.76.11.5678-5691.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelenina DA, Kulaeva OI, Smirnyagina EV, Solovyev AG, Miroshnichenko NA, Fedorkin ON, Rodionova NP, Morozov S, Atabekov JG. Translation enhancing properties of the 5′-leader of potato virus X genomic RNA. FEBS Lett. 1992;296:267–270. doi: 10.1016/0014-5793(92)80301-v. [DOI] [PubMed] [Google Scholar]
- Zhang G, Zhang J, Simon AE. Repression and derepression of minus-strand synthesis in a plus-strand RNA virus replicon. J Virol. 2004;78:7619–7633. doi: 10.1128/JVI.78.14.7619-7633.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Jackson AO. Expression of the barley stripe mosaic virus RNA beta ‘‘triple gene block’’. Virology. 1996;216:367–379. doi: 10.1006/viro.1996.0072. [DOI] [PubMed] [Google Scholar]


