Summary
Light deprivation lowers the threshold for long-term depression (LTD) and long-term potentiation (LTP) in visual cortex by a process termed metaplasticity, but the mechanism is unknown. The decreased LTD/P threshold correlates with a decrease in the ratio of NR2A to NR2B subunits of cortical NMDA receptors (NMDARs) and a slowing of NMDAR-mediated excitatory postsynaptic currents (EPSCs). However, whether and how changes in NR2 subunit expression contribute to LTD and LTP remains controversial. In the present study, we used an NR2A knockout (KO) mouse to examine the role of this subunit in the experience-dependent modulation of NMDAR properties, LTD, and LTP. In wild-type (WT) mice, we found what has previously been shown in rats, that dark rearing alters NMDAR EPSC kinetics and temporal summation, LTP, and LTD in layer 2/3 of visual cortex. Deletion of NR2A mimicked the effect of dark-rearing on NMDAR EPSCs, and there was no additional effect of dark-rearing in the NR2A KO mice. Thus, deletion of NR2A abrogates the effects of visual experience on NMDAR EPSCs. Moreover, metaplasticity of LTP and LTD was completely lost in the absence of NR2A. These data support the hypothesis that experience-dependent changes in NR2A/B are functionally significant and yield a mechanism for an adjustable synaptic modification threshold in visual cortex.
Introduction
Metaplasticity—the plasticity of synaptic plasticity—is a change in the properties of synaptic modification as a function of the recent history of cellular or synaptic activation (Abraham and Bear, 1996). A robust demonstration of metaplasticity is the change in the properties of long-term potentiation (LTP) and long-term depression (LTD) in visual cortex of animals deprived of vision (Kirkwood et al., 1996; Philpot et al., 2003). After a period of reduced cortical activity caused by dark-rearing (Czepita et al., 1994; Maffei et al., 2006), LTP is enhanced and LTD is reduced over a range of stimulation frequencies compared to light-reared (LR) animals. These changes are rapidly reversed by light exposure of the dark-reared (DR) animals. Such findings are in accordance with the theoretical proposal of a synaptic modification threshold that “slides” as a function of the average activity of cortical neurons (Bienenstock et al., 1982). A sliding modification threshold enables the synaptic competition that yields stimulus-selective neuronal responses, and contributes to homeostasis by keeping the network of modifiable synapses within a useful dynamic range (Bear, 2003).
The forms of LTP and LTD in visual cortex that are subject to metaplastic regulation are triggered by strong and weak activation of postsynaptic NMDA receptors (NMDARs), respectively. A mechanism for metaplasticity is suggested by the observations both in vivo and in vitro that NMDAR function is enhanced in visual cortex of DR animals and diminished after brief light exposure (Fox et al., 1992; Philpot et al., 2003; Tsumoto et al., 1987). Such changes in NMDAR function would be expected to lower and raise LTD/P thresholds, respectively.
While there are many ways to modulate NMDAR effectiveness, one appealing mechanism is the activity-dependent regulation of NMDAR subunit expression and assembly. NMDARs consist of the obligatory NR1 subunit in combination with NR2A-D and NR3A-B subunits (Perez-Otano et al., 2001). NR2A and NR2B subunits, which predominate in postnatal cortex (Monyer et al., 1994; Sheng et al., 1994; Watanabe et al., 1993), exhibit several important differences that can influence NMDAR-mediated plasticity. First, NR2B-containing NMDARs have longer current durations than NR2A-containing receptors, due to a higher affinity for glutamate and slower rates of desensitization (Laurie and Seeburg, 1994; Monyer et al., 1992; Priestley et al., 1995; Vicini et al., 1998). Second, NR2B-containing NMDARs carry more calcium charge per unit current than NR2A subtypes (Sobczyk et al., 2005). Third, NR2A and NR2B subunits have distinct intracellular binding partners (Barria and Malinow, 2005; Gardoni et al., 2001; Husi et al., 2000; Sans et al., 2000; Steigerwald et al., 2000; Vissel et al., 2002; Yoshii et al., 2003).
Complementary biochemical, biophysical, and pharmacological studies have shown that dark-rearing leads to a decreased NR2A/B ratio in synaptic NMDARs which is rapidly reversed by light exposure (Chen and Bear, 2006; Philpot et al., 2001a; Quinlan et al., 1999a; Quinlan et al., 1999b). The time-course of these bidirectional changes in NMDAR subunit composition correlates well with the changes in LTD/P thresholds in visual cortex. Thus, it has been hypothesized that inactivity caused by light deprivation gradually decreases the NR2A/B ratio which, in turn, lowers the LTD/P threshold. Conversely, restoring visual experience rapidly increases the NR2A/B ratio, which raises the LTD/P threshold. This model is attractive since a lowered NR2A/B ratio has the effects of prolonging and increasing calcium currents through NMDARs and recruiting calcium/calmodulin-dependent protein kinase II—changes that would be expected to lower the threshold level of NMDAR activation required to trigger LTP.
Other than the correlations mentioned above, however, there is no direct evidence that NMDAR subunit composition contributes to metaplasticity in visual cortex. Resolving this question has taken on new importance with the publication of recent reports suggesting an entirely different view of how NR2A and NR2B subunits contribute to LTP and LTD, respectively (Liu et al., 2004; Massey et al., 2004). According to these studies, decreasing the NR2A/B ratio should promote LTD over LTP. To distinguish among these very different hypotheses, and to determine the contribution of NR2 subunit expression to metaplasticity in visual cortex, we have studied the effects of visual experience and deprivation on the properties of NMDAR-mediated synaptic transmission, LTP, and LTD in visual cortex of mice with a genetic ablation of NR2A.
Results
NR1, NR2B, and GluR1 levels are unchanged in juvenile NR2A KO mice
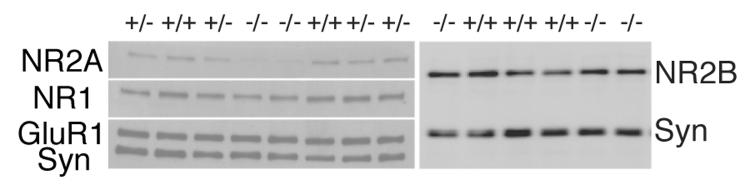
To test for possible compensatory alterations of glutamate receptor expression in NR2A-deficient mice, we first performed an immunoblot analysis of NMDA (NR1, NR2A, and NR2B) and AMPA (GluR1) receptor subunit expression in synaptoneurosomes prepared from visual cortex of +/+, +/−, and −/− mice (Figure 1). To control for protein loading and the degree of synaptic enrichment, we normalized receptor subunit protein levels to the synaptic vesicle protein synapsin (Table 1). As expected, NR2A levels were significantly reduced in NR2A deficient mice (n's = 6, 5, and 7 animals for +/+, +/−, and −/− mice, respectively; F(2,17) = 17.83, p < 0.0002). Consistent with previous observations, NR2A levels in +/− mice were approximately half that of wild-types (Kutsuwada et al., 1996; Sakimura et al., 1995). Despite reductions in NR2A levels in −/− and +/− mice, levels of the obligatory NR1 subunit were unchanged in NR2A deficient mice (F(2,17) = 1.55, p = 0.24). There was also no significant effect on NR2B levels (F(1,5) = 0.34, p = 0.59) as observed previously (Morikawa et al., 1998). Levels of the GluR1 AMPA receptor subunit were not changed in the mutant mice (F(2,17) = 0.88, p = 0.44). These data indicate that there were no gross abnormalities in the expression of ionotropic glutamate receptors at the synapse, other than the expected loss of NR2A expression in +/− and −/− mice. Our observation that other ionotropic glutamate receptor subunit levels remain unchanged in the visual cortex of NR2A-deficient mice is similar to previous findings demonstrating that NR2B, NR1, and GluR1 levels in the immature somatosensory cortex are unaltered by the loss of NR2A (Lu et al., 2001).
Figure 1.
NR2A-deficient mice express normal levels of synaptic NR1, NR2B, and GluR1 subunits. The Western blot illustrates representative examples of NR2A, NR1, GluR1, NR2B, and synapsin (Syn) immunoreactive bands from the synaptoneurosome preparation in +/+, +/−, and −/− mice aged P21-P29. For quantification, receptor subunit levels were divided by synapsin levels to control for loading and efficiency of synaptic enrichment in the synaptoneurosome preparation, and the resulting ratio was normalized to the average value for +/+ mice (see Table 1). Note that NR2A levels were reduced to roughly half of control levels in +/− mice and absent in −/− animals, while NR1, NR2B, and GluR1 levels were not affected by the genetic manipulation.
Table 1.
| NR2A genotype |
NR2A protein |
NR1 protein |
GluR1 protein |
NR2B protein |
|---|---|---|---|---|
| +/+ | 1.00 ± 0.05 | 1.00 ± 0.01 | 1.00 ± 0.06 | 1.00 ± 0.13 |
| +/− | 0.52 ± 0.25* | 0.94 ± 0.11 | 1.08 ± 0.10 | N.A. |
| −/− | 0.03 ± 0.05* | 0.83 ± 0.04 | 0.94 ± 0.06 | 1.16 ± 0.25 |
Normalized synaptic protein levels expressed as fraction of wild-type mean ± SEM.
P < 0.05
Loss of NR2A mimics and occludes the effect of dark-rearing on NMDAR EPSCs
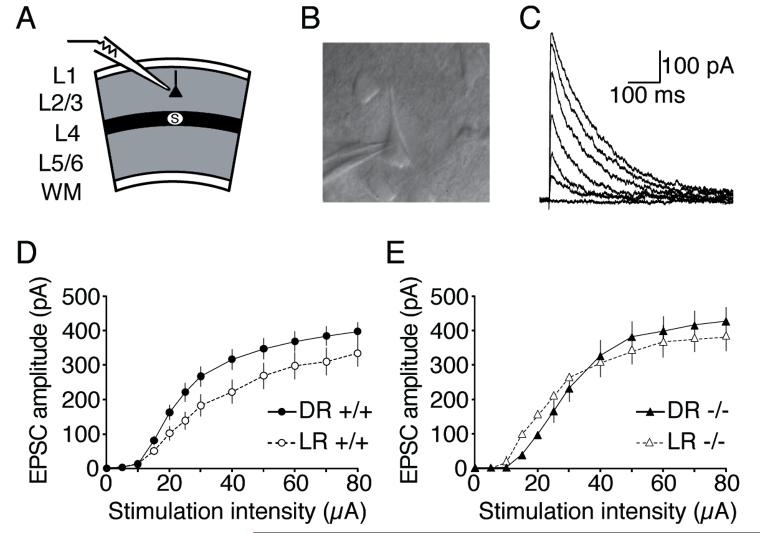
To test for gross differences in NMDA receptor function in layers 2/3 of visual cortex, we generated input-output curves of pharmacologically-isolated NMDA receptor-mediated EPSCs in +/+, +/−, and −/− mice reared normally or in complete darkness (with sample sizes between 15 and 35 cells for each of the six groups; Figure 2; data from heterozygotes were excluded from this figure for clarity but were included in all statistics). In this and all subsequent comparisons, the experimenters were blind to genotype. Because ANOVA revealed a significant main effect (F(5, 1590) = 3.2, p < 0.008), post-hoc analyses were used to test for group differences. This analysis revealed that dark-rearing significantly enhanced the input-output relationship of NMDAR responses in wild-type (WT) mice (Figure 2D; p < 0.0007). However, dark-rearing failed to alter the input-output curves of NMDAR responses in NR2A knockout (KO) mice (Figure 2E; p = 0.79). Consistent with our observation that NR1 levels were unchanged in NR2A-deficient mice, we detected no reduction in the evoked NMDAR currents in NR2A-deficient mice at this age. Our direct measurements of the relationship between stimulation intensity and NMDAR EPSC suggest that there is no net loss in the number of NMDARs functionally expressed at the synapse in NR2A-deficient mice. This finding is consistent with a report from Lu and colleagues (2001) who demonstrated that the ratio of NMDAR:AMPAR synaptic currents in the immature somatosensory cortex are not influenced by genetic deletion of NR2A. Coupled with our biochemical data demonstrating that there is no gross change in NR2B levels, our electrophysiological observations suggest that NR2B-containing NMDARs that would normally be targeted to perisynaptic sites are likely targeted to synaptic sites in the absence of NR2A. Thus, at least at the ages examined, NMDAR current amplitudes are not impaired in the absence of NR2A.
Figure 2.
Experience-dependent enhancement of NMDAR currents evoked by layer 4 stimulation of layer 2/3 pyramidal cells is absent in NR2A KO mice. A) Schematic of recording configuration. B) Example of IR-DIC image of a whole-cell recording from a layer 2/3 pyramidal cell. C) Example demonstrating pharmacologically-isolated NMDAR EPSCs evoked by increasing layer 4 stimulation. D) Dark-rearing (DR) enhanced the input-output relationship of NMDAR-mediated events compared to responses recorded from light-reared (LR) wild-type mice (+/+). E) NR2A-deficient mice maintain strong evoked NMDAR-mediated responses. Experience-dependent modifications of the input-output relationship of NMDAR EPSCs failed to occur in these NR2A KO mice.
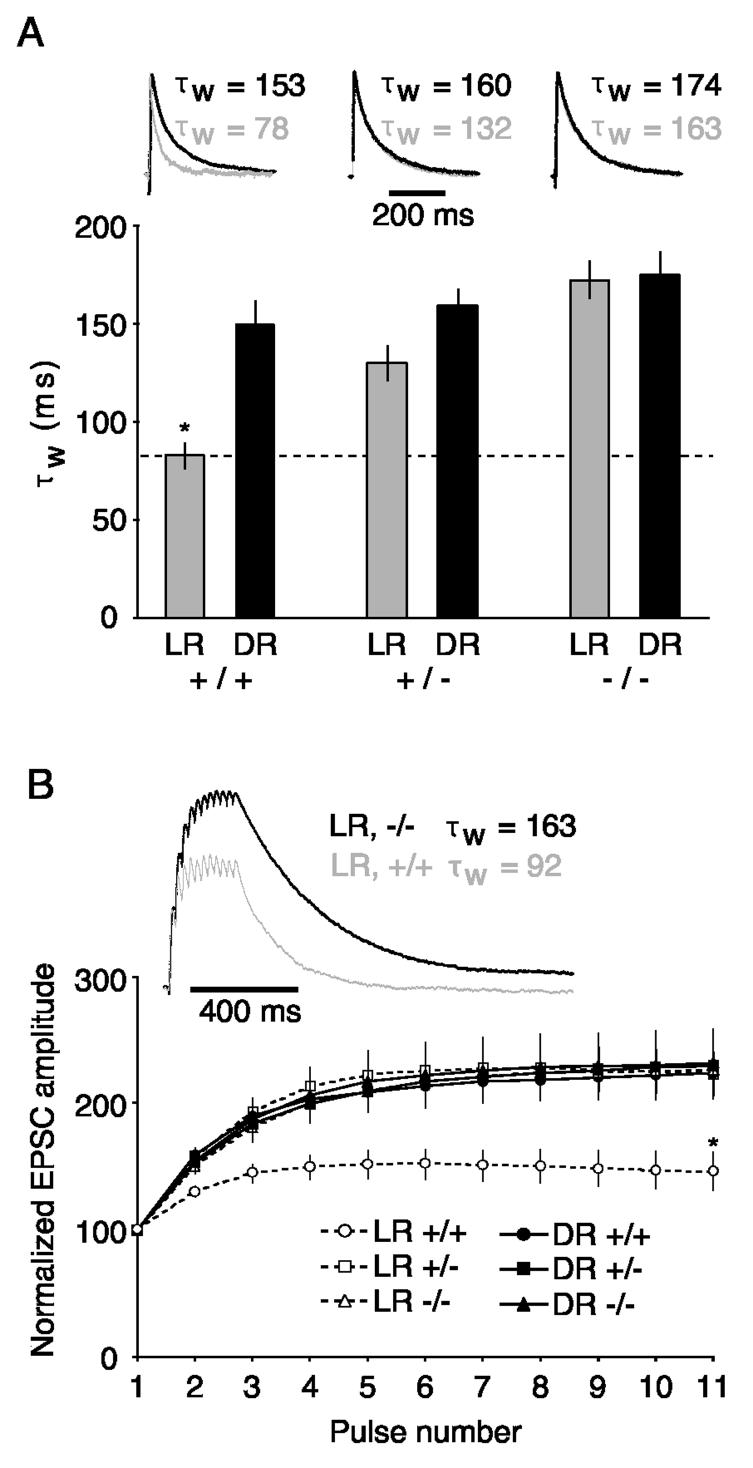
To test the contribution of NR2A subunits to experience-dependent modifications in current duration (Philpot et al., 2001a), we recorded NMDAR EPSCs in visually-identified layer 2/3 pyramidal cells. As expected, dark-rearing significantly increased NMDAR current duration in layer 2/3 pyramidal cells of normal mice (Figure 3A; F(1,127) = 13.31, p < 0.0005). The NR2A-deficiency also prolonged NMDAR current durations (F(2,127) = 13.72, p < 0.0001), and there was a significant interaction between rearing condition and genotype on EPSC duration (F(2,27) = 4.40, p < 0.02). Post-hoc analyses (Fisher's PLSD) revealed that LR, WT animals had significantly faster currents than DR animals and NR2A-deficient mice (+/−, −/−). Notably, dark-rearing had no effect in mice lacking functional NR2A subunits (p = 0.89). These data demonstrate that genetic ablation of functional NR2A subunits mimics and occludes the consequences of light deprivation.
Figure 3.
Loss of NR2A mimics and occludes the consequences of light deprivation on NMDAR EPSC duration in visual cortex layer 2/3 pyramidal cells. A) NMDAR EPSC decays were quantified by calculating a weighted time constant (τw) from the double-exponential fit (see Results). Bar graph depicts the averaged τw for NMDAR-mediated responses from light-reared (LR) or dark-reared (DR) mice (+/+, +/−, and −/−). Traces are of normalized representative EPSCs, for the corresponding genotype in the bar graph below, recorded from DR (dark trace) or LR (light trace) animals. Note that NMDAR-mediated EPSCs are prolonged in DR cortex from +/+ mice, but loss of NR2A mimics and occludes the consequences of dark-rearing. Bar graph values from left to right are: 83 ± 6.4, 150 ± 12.5, 130 ± 8.6, 159 ± 8.1, 173 ± 9.2, and 175 ± 11.5. B) Reducing NR2A expression genetically or by dark-rearing enhances the temporal summation of NMDAR EPSCs evoked at 40 Hz (11 pulses). Traces represent normalized NMDAR EPSCs from WT (light trace) and KO (dark trace) mice. Stimulus artifacts were clipped for clarity.
We have previously demonstrated that the temporal summation of NMDAR-mediated EPSCs is extremely sensitive to small changes in NMDA receptor current durations (Philpot et al., 2001a). NMDAR currents in layer 2/3 pyramidal cells were evoked by extracellular stimulation of layer 4 for 11 pulses at 40 Hz, and the total EPSC amplitude was measured after each pulse (Figure 3B). Total EPSC amplitude was described by a significant interaction between pulse number, genotype, and rearing condition (F(20,1200) = 2.4, p < 0.0007). Consistent with the observation that NMDAR current durations from LR, WT mice differed significantly from all the other groups, post-hoc analyses revealed that the only significant differences in temporal summation arose between the LR, WT mice versus all the other groups. These data demonstrate that modest increases in NMDA receptor current duration, driven by a genetic or experience-dependent reduction in NR2A levels, are sufficient to maximize temporally summated NMDAR EPSCs generated by 40 Hz stimulation. Moreover, the genetic loss of NR2A occludes further changes by dark-rearing on NMDAR EPSC temporal summation.
Deprivation-induced metaplasticity fails to occur in NR2A KO mice
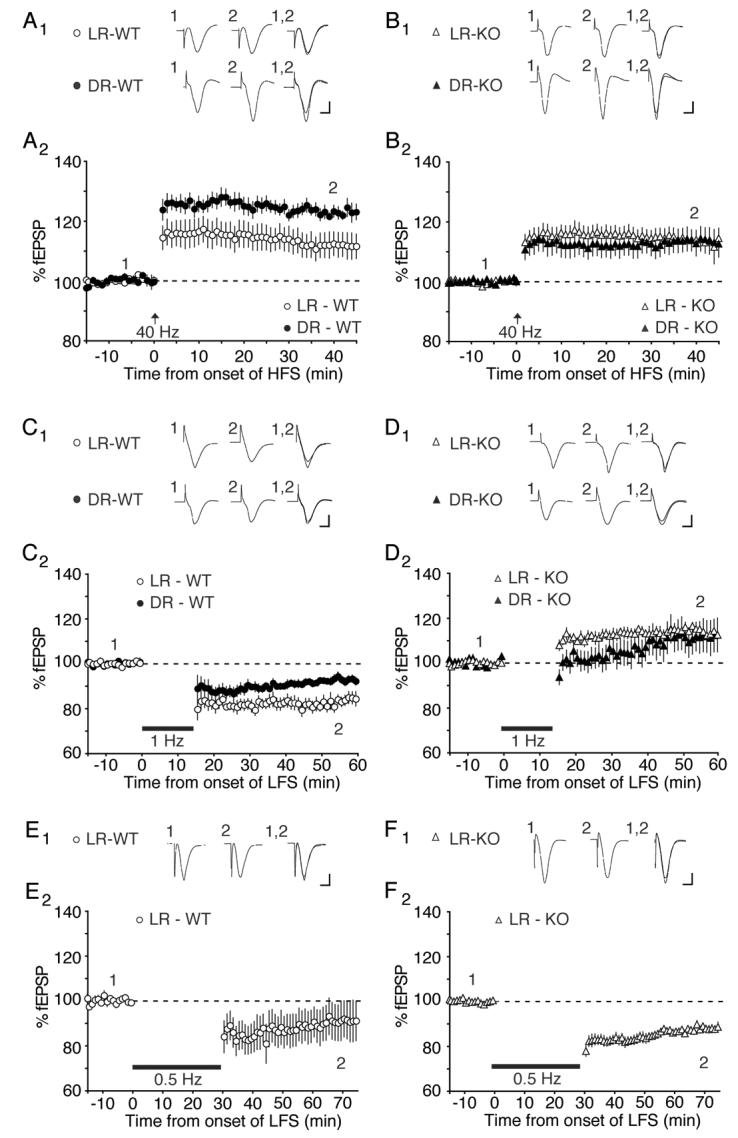
To assess differences in cortical plasticity, layer 2/3 field potentials were evoked by layer 4 stimulation in visual cortical slices of P21-28 mice (Kirkwood et al., 1996; Philpot et al., 2003). There was no effect of deleting NR2A on LTP induced in layer 2/3 of slices prepared from LR animals. Remarkably, however, the metaplasticity of LTP and LTD was completely absent in the NR2A KO mice.
In WT mice, dark-rearing significantly enhanced LTP induced with a 40 Hz tetanus, confirming previous observations in rats (Kirkwood et al., 1996). LTP in LR mice measured 111.8 ± 4.2 % of baseline (n = 13) compared to 123.1 ± 1.9 % of baseline in DR mice (n = 8; p < 0.05; Fig. 4A). In contrast, there was no effect of dark-rearing on the level of LTP induced by 40 Hz stimulation in NR2A KO mice compared with LR NR2A KO mice (LR: 114.4 ± 2.9 % of baseline, n = 14; DR: 113.6 ± 3.8 % of baseline, n = 9; p = 0.88; Fig. 4B). These data suggest that although NR2A is not required for LTP in visual cortex, it is essential for experience-dependent metaplasticity.
Figure 4.
Loss of NR2A lowers the threshold for synaptic potentiation and prevents experience-dependent modifications in LTP and LTD. A) Averaged data and representative waveforms demonstrating that dark-rearing enhances the induction of LTP at 40 Hz stimulation in WT mice. B) Visual deprivation fails to modify the magnitude of LTP induced by 40 Hz stimulation in NR2A KO mice. C) 1 Hz stimulation induces robust LTD in LR, WT mice. Dark-rearing reduces the magnitude of LTD. D) 1 Hz stimulation induces LTP in NR2A KO mice, and dark-rearing fails to modify the magnitude of the plasticity. E) 0.5 Hz induces LTD in LR, WT mice as well as in LR, KO mice (F). Scale bars: 5ms, 500μV.
In WT mice, dark-rearing significantly reduced LTD induced with a 1 Hz tetanus, again confirming previous findings in rats (Kirkwood et al., 1996; Philpot et al., 2003). LTD in LR mice measured 82.0 ± 2.7% of baseline (n = 6) compared to 92.7 ± 1.4 % of baseline in DR mice (n = 6; p < 0.003; Fig. 4C). We were surprised to find that 1 Hz stimulation in LR, NR2A KO mice produced no LTD at all; instead, responses modestly potentiated (114.9 ± 1.8 % of baseline, n = 5; Fig. 4D). Moreover, there was no longer any difference between LR and DR animals with respect to the plastic response to 1 Hz stimulation (DR: 111.3 ± 6.8 % of baseline, n = 8). These data suggest that the LTP threshold is greatly reduced in the absence of NR2A and no longer modified by the history of cortical experience.
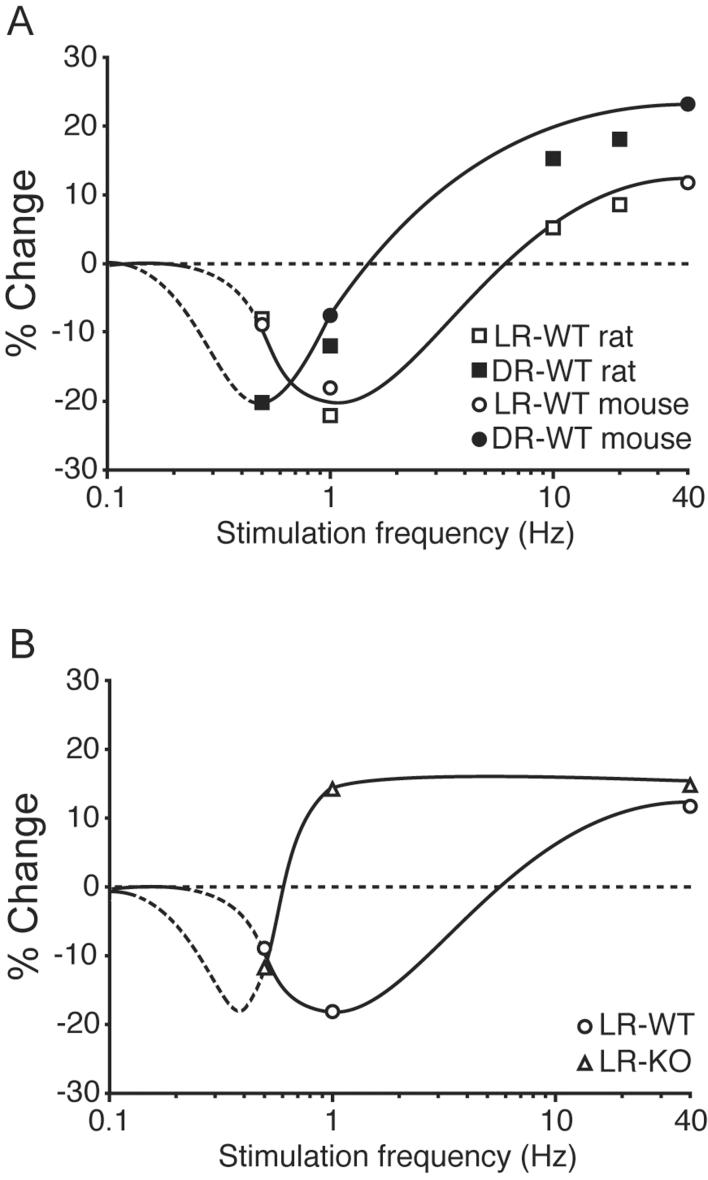
To better determine how genetic deletion of NR2A altered the shape of the frequency-response function, we tested the consequences of 30 minutes of 0.5 Hz stimulation in WT and KO mice reared normally (Figure 4E-F). We found that this manipulation produced a modest but significant depression in both KO and WT mice (WT: 91.2 ± 8.2% of baseline, n = 6; KO: 87.9 ± 1.6 % of baseline, n = 10). These data indicate that it is possible to weaken synapses in NR2A KO mice, and that the genetic deletion of NR2A produces an extreme leftward shift in the frequency-response function (see Figure 5).
Figure 5.
Comparison of the effect of dark-rearing (A) and genetic deletion of NR2A (B) on LTD/P frequency-response functions. Curves are semi-schematic, fit to data from the current study and, in panel A, also to that previously reported for rats (Kirkwood et al., 1996; Philpot et al., 2003). Dashed regions of the curves are extrapolated from existing data and remain to be confirmed experimentally.
Discussion
To summarize the major findings of this study, our data show that visual experience regulates NMDAR EPSC kinetics and temporal summation, LTP, and LTD in layer 2/3 of mouse visual cortex as previously reported in rats (Carmignoto and Vicini, 1992; Kirkwood et al., 1996; Philpot et al., 2003; Philpot et al., 2001a), and that these effects of experience require the NMDAR NR2A subunit. In the absence of NR2A, the NMDAR EPSC kinetics and summation resemble those observed in DR WT mice, and there is no additional effect of dark-rearing in the NR2A KO mice. Metaplasticity of LTP and LTD is completely lost in the absence of NR2A, and the loss of NR2A appears to greatly lower the threshold for inducing LTP with low-frequency stimulation. Taken together, the data support the hypothesis that experience-dependent changes in NR2A/B, documented previously in visual cortex (Chen and Bear, 2006; Quinlan et al., 1999a; Quinlan et al., 1999b), are functionally significant and provide a mechanism for an adjustable synaptic modification threshold (reviewed by (Bear, 2003).
Our data provide the first direct evidence that experience-dependent modifications in NMDAR current duration are due to a change in NR2 subunit composition. Previous studies demonstrated a strong correlation between an increase in sensory experience, an increase in the NR2A/B ratio, and faster NMDAR current durations (Carmignoto and Vicini, 1992; Crair and Malenka, 1995; Flint et al., 1997; Hestrin, 1992; Nase et al., 1999; Philpot et al., 2001a; Quinlan et al., 1999b; Stocca and Vicini, 1998). The correlation between the expression of NR2A and NMDAR kinetics in hippocampus (Kirson and Yaari, 1996), visual cortex (Quinlan et al., 1999b)(present study), and somatosensory cortex (Flint et al., 1997; Lu et al., 2001) suggested that changes in NMDAR composition underlie the developmental and experience-dependent modifications in NMDAR current duration (although see (Barth and Malenka, 2001), an idea supported by the absence of developmental shortening of NMDAR currents in neurons lacking NR2A (Fu et al., 2005). However, other mechanisms also contribute to the developmental shortening of NMDARs. For example, calcineurin activity attenuates NMDAR kinetics in the developing superior colliculus (Shi et al., 2000) and the presence of exon 5 on NR1 is known to shorten NMDAR kinetics in cerebellar cells (Prybylowski et al., 2000; Rumbaugh et al., 2000). The present study provides direct evidence for the importance of NR2A for experience-dependent modifications in NMDAR currents, because the consequences of dark-rearing on NMDAR current duration are mimicked and occluded by the genetic loss of NR2A function.
While our data clearly show that the presence of NR2A is a molecular requirement for experience-dependent modifications in NMDAR current duration in visual cortex, it remains to be seen if NMDAR current duration is modified at the level of NR2A expression itself or through posttranslational modifications occurring through NR2A. For example, Shi and colleagues (2000) suggested that calcineurin activity can shorten NMDAR kinetics. Because the phosphatase can act through NR2A (e.g. (Krupp et al., 2002), we cannot rule out the possibility that posttranslational modifications specific to NR2A are responsible for experience-dependent modifications in NMDAR current. Given evidence that NMDARs are largely unaffected by genetic deletion of calcineurin (Zeng et al., 2001), we favor the idea that the ratio of NR2A/B is the major underlying factor for experience-dependent modifications in visual cortex. Regardless, our data indicate that experience-dependent modifications in NMDAR kinetics within visual cortex require the presence of NR2A and are unlikely to occur through altered expression of NR1 splice variants or posttranslational modifications of NR1 or NR2B.
The importance of the NR2B to NR2A subunit switch for synaptic plasticity has remained a matter of controversy. Several influential papers suggest that NR2A and NR2B subunit-containing NMDARs have a separable role in synapse modification, with NR2A subtypes regulating LTP and NR2B subtypes regulating LTD (Liu et al., 2004; Massey et al., 2004). The validity of these findings is now being questioned because of suggestions that these studies were conducted using non-specific concentrations of NR2A-selective antagonists and/or because of difficulty replicating the initial findings (Berberich et al., 2005; Morishita et al., 2006; Neyton and Paoletti, 2006). Recent data contradict the initial findings that NR2A and NR2B play distinct roles in regulating the polarity of synaptic plasticity, and instead suggest that either subunit is capable of inducing LTD and LTP (Berberich et al., 2005; Weitlauf et al., 2005; Zhao et al., 2005). While much of the confusion surrounding the role of NMDAR subtypes in synaptic plasticity may be the result of regional and developmental differences in NMDAR expression (see (Philpot et al., 2001b), our data support an alternative interpretation of the function of the NMDAR subunit switch. We suggest that the NR2A/B ratio reflects—and is set by—the amount of ongoing cortical activity, which normally increases during postnatal development. The NR2A/B ratio establishes the threshold for subsequent activity-dependent synaptic modifications. We favor the idea that this threshold is set directly by the biophysical and biochemical properties of NMDARs at modifiable synapses, but additional sequelae, such as a change in inhibitory tone (Fagiolini et al., 2003; Steele and Mauk, 1999), may also contribute.
Although we observed an extreme reduction in the stimulation frequency required to induce LTP in the NR2A KO mice (see Fig 5), the absence of NR2A failed to enhance the magnitude of LTP that can be induced, consistent with previous observations (Lu et al., 2001). This finding suggests that there may be separable mechanisms for regulating the threshold for inducing synaptic plasticity and the magnitude of the expressed plasticity. A parsimonious explanation for the lack of enhanced LTP in NR2A KO mice may be that there are limited resources available to make synapses stronger, and that these may be tightly and independently regulated in a homeostatic manner. Consistent with this idea, potentiated synapses have been observed to compete for a limited supply of “plasticity factors”, which can limit the total synaptic expression of LTP (Fonseca et al., 2004).
Taken together, the data suggest that during visual cortical development the progressive increase in the NR2A/B ratio in a normal visual environment raises the threshold for inducing LTP such that only the most strongly correlated inputs are maintained while less correlated inputs are more likely to be weakened. This experience-dependent adjustment in the plasticity threshold normally allows neurons to become progressively more tuned to select features of the environment (Bienenstock et al., 1982). Thus, the loss of orientation selectivity and incomplete expression of ocular dominance plasticity previously reported in the NR2A KO mouse (Fagiolini et al., 2003) is likely to be a manifestation of impaired metaplasticity in vivo.
Experimental Procedures
Subjects
Mice deficient in NR2A were generously supplied by S. Nakanishi. The mice were developed by replacing the region spanning the M2 transmembrane segment of NR2A subunits with the neomycin resistance gene as previously described (Kadotani et al., 1996). A pathogen-free line was rederived on a C57BL/6 background by Charles River Laboratories. Wild-type (+/+), heterozygote (+/−), and NR2A KO (−/−) mice were used between postnatal days (P) 21-28. Subjects were fed ad libitum and reared in normal lighting conditions (12/12 light/dark cycle) or in complete darkness.
Cortical Slice Preparation
Following an overdose of barbiturates (i.p.), mice were decapitated upon disappearance of corneal reflexes in compliance with the U.S. Department of Health and Human Services. The brain was rapidly removed and immersed in ice-cold dissection buffer (composition in mM: NaCl, 87; KCl, 2.5; NaH2PO4, 1.25; NaHCO3, 25; sucrose, 75; dextrose, 10; ascorbic acid, 1.3; MgCl2, 7; and CaCl2, 0.5) bubbled with 95% O2 and 5% CO2. The visual cortex was rapidly removed and 350 μm coronal slices were cut using a vibrating microtome (Leica VT100S). Slices recovered for 15 min in a submersion chamber at 32° C filled with warmed artificial cerebral spinal fluid (ACSF; 124 mM NaCl, 5 mM KCl, 1.25 mM Na2PO4, 26 mM NaHCO3, 1 mM MgCl2, 2 mM CaCl2, and 10 mM dextrose, saturated with 95% O2, 5% CO2) and then cooled gradually to room temperature until use.
Extracellular Electrophysiology
Slices were transferred to an interface recording chamber maintained at 30°C and perfused with ACSF at a rate of 2.5 mL/min. A stimulation electrode (concentric bipolar tungsten) was positioned in layer 4, and a glass recording electrode (∼ 1 MΩ) filled with ACSF was positioned in layers 2/3. The magnitude of responses evoked by a 200 μs pulse was monitored by the amplitude of the field potential. Stimulation intensity was adjusted to elicit half the maximal response, and stable baseline responses were elicited every 30 sec. The resulting signals were filtered between 0.1 Hz and 3 kHz, amplified 1000 times, and captured at 10 kHz on an IBM-compatible computer using pCLAMP 9.2 software (Molecular Devices). After achieving a stable baseline (< 5% drift) for 15 minutes, slices were stimulated with either 40 Hz stimulation for 1 second, repeated three times with a ten second interval, or 900 pulses at 1 Hz, or 900 pulses at 0.5 Hz. Field excitatory postsynaptic potential (fEPSP) amplitudes were recorded every 30 seconds for 45 minutes following the cessation of the stimulation protocol. Control and experimental subjects were run in an interleaved fashion. The data were normalized, averaged, and reported as means ± SEM. Changes in synaptic strength were measured by comparing the average response amplitude 35-40 minutes after conditioning stimulation to the pre-conditioning baseline response.
Voltage-clamp Recordings
Slices were placed in a submersion chamber, maintained at 31°C, and perfused at 2 mL/min with oxygenated ACSF containing 4 mM MgCl2, 4 mM CaCl2, 1 μM glycine, 50 μM picrotoxin (Fluka), and 20 μM CNQX. These conditions are sufficient to pharmacologically isolate NMDAR-mediated responses (Philpot et al., 2001a; Philpot et al., 2001b; Quinlan et al., 1999b). Layer 2/3 pyramidal cells were visually identified using a Nikon E600FN microscope equipped with IR-DIC optics. Patch pipettes were pulled from thick-walled borosilicate glass. Open tip resistances were 3-5 MΩ when pipettes were filled with the internal solution containing (in mM): 102 cesium gluconate, 5 TEA-chloride, 3.7 NaCl, 20 Hepes, 0.3 sodium guanosine triphosphate, 4 magnesium adenosine triphosphate, 0.2 EGTA, 10 BAPTA, and 5 QX-314 chloride (Alomone Labs, Jerusalem, Israel) with pH adjusted to 7.2 and osmolarity adjusted to ∼300 mmol/kg with sucrose or ddH2O. Cells were voltage-clamped at +40 mV in the whole cell configuration using a patch-clamp amplifier (Axoclamp 1D, Axon Instruments), and data were acquired and analyzed using a system from DataWave Technologies Inc. (Boulder, CO). Pipette seal resistances were > 1 GΩ, and pipette capacitive transients were minimized prior to breakthrough. Series resistance was monitored throughout the experiment by giving a test pulse and measuring the amplitude of the capacitive current filtered at 30 kHz. Only cells with series resistance < 30 MΩ were included in this study. No series resistance compensation was applied. Input resistance was monitored throughout the experiment by measuring the amplitude of the steady-state current, filtered at 2 kHz, evoked from a test pulse. Excitatory postsynaptic currents (EPSCs) were evoked from a stimulating electrode (concentric bipolar stimulating; ∼ 200 μM tip separation) placed in layer 4, and stimulation intensity was adjusted to evoke ∼100 pA response. Stimulation was given for 200 μsec every 6 sec. To examine functional changes in the summation of NMDAR-mediated currents, 11 pulses of 40 Hz trains of stimulation were given every 6 sec for 3 min. In addition, input-output curves were generated by systematically adjusting the stimulation intensity from 0 to 80 μA. The deactivation kinetics of NMDAR-mediated EPSCs were described by averaging 30 evoked responses and fitting the current decay using the following formula:
where “I” is the current amplitude, “t” is time, “If” and “Is” are the peak amplitudes of the fast and slow components, respectively, and “τf” and “τs” are their respective time constants. A nonlinear regression in GraphPad Prism software (San Diego, CA) was used to fit decay curves. For quantification purposes, we used the weighted time constant (τw), calculated as:
Biochemical Analysis
Synaptoneurosomes were prepared as previously described (Hollingsworth et al., 1985; Quinlan et al., 1999b). Briefly, animals were given a lethal dose of barbiturates (i.p.) and decapitated upon disappearance of corneal reflexes. The brain was quickly removed into ice cold dissection buffer (212.7 mM sucrose, 2.6 mM KCl, 1.23 mM NaH2PO4, 26 mM Na HCO3, 10 mM dextrose, 1 mM MgCl2, 0.5 mM CaCl2, 0.02 mM CNQX, 0.1 mM AP5) saturated with 95% O2, 5% CO2. Visual cortices were bilaterally removed and placed in homogenization buffer consisting of 10 mM HEPES, 2 mM EDTA, 2 mM EGTA, 0.5 mM DTT, 0.1 mM PMSF, 10 mg/L leupeptin, and 100 nM microcystin. The samples were homogenized using two mL glass tissue homogenizers (Kontes, Vineland, New Jersey), filtered through a double layer of 105 μm pore nylon mesh filter, and finally passed through 5 μm pore filter paper. Homogenized tissue was centrifuged at 3,600 RPMs for 10 minutes at 4°C. The sediment containing a high density of synaptic protein was then resuspended in 1% boiling SDS and stored at −20°C. An optical densitometric assay (BCA Assay, Pierce) was used to measure the concentration of synaptic proteins. Protein concentrations across samples were normalized and preserved from decay with sample buffer. 10 μg of protein were loaded per lane and samples were run on 7.5% polyacrylamide gels using BioRad mini-protean II and III cells. Gels were then transferred to nitrocellulose membranes (BioRad) and probed against NR2A (Molecular Probes, 1:250), NR2B (Santa Cruz, 1:250), synapsin (Chemicon, 1:1500), GluR1 (Oncogene Research, 1:1000), or NR1 (Chemicon, 1:1000). Samples were next probed with the appropriate secondary antibody conjugated to horseradish peroxidase in Tris-buffered saline (pH 7.4) containing 0.1% Triton X-100. To visualize the immunoblots, enhanced chemiluminescence (Amersham ECL) was used with autoradiographic film (Amersham Hyper ECL). Developed autoradiographs were scanned using Alpha Imager software. Samples from +/+, +/−, and −/− were run simultaneously on each gel to control for antibody penetration and exposure time. Immunoblot bands were quantified by densitometric analysis using NIH Image software. Notably, long exposure times revealed a faint NR2A band in NR2A-KO mice, suggesting some cross-reactivity of the NR2A polyclonal antibodies with NR2B. For quantifying NR1, NR2A, and GluR1 levels, densitometric intensities were expressed as a ratio of synapsin levels and normalized to the averaged protein levels in +/+ mice.
Statistics
Either multiple factor ANOVA's or mixed effect ANOVA's with a repeated measures factor were run with post hoc analyses (Fisher's PLSD) to test for statistical significance between multiple groups. Data are expressed as means ± SEM, and significance was placed at p < 0.05.
Drugs
Unless otherwise noted, drugs were purchased from Sigma.
Acknowledgements
Thanks to W. Chen, E. Sklar, and S. Meagher for assistance, and to R. Corlew for a critical reading of the manuscript. This work was supported by HHMI, NIH (M.F.B.), and the Whitehall Foundation (B.D.P.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham WC, Bear MF. Metaplasticity: the plasticity of synaptic plasticity. Trends Neurosci. 1996;19:126–130. doi: 10.1016/s0166-2236(96)80018-x. [DOI] [PubMed] [Google Scholar]
- Barria A, Malinow, R. NMDA receptor subunit composition controls synaptic plasticity by regulating binding to CaMKII. Neuron. 2005;48:289–301. doi: 10.1016/j.neuron.2005.08.034. [DOI] [PubMed] [Google Scholar]
- Barth AL, Malenka RC. NMDAR EPSC kinetics do not regulate the critical period for LTP at thalamocortical synapses. Nat Neurosci. 2001;4:235–236. doi: 10.1038/85070. [DOI] [PubMed] [Google Scholar]
- Bear MF. Bidirectional synaptic plasticity: from theory to reality. Philos Trans R Soc Lond B Biol Sci. 2003;358:649–655. doi: 10.1098/rstb.2002.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berberich S, Punnakkal P, Jensen V, Pawlak V, Seeburg PH, Hvalby O, Kohr G. Lack of NMDA receptor subtype selectivity for hippocampal long-term potentiation. J Neurosci. 2005;25:6907–6910. doi: 10.1523/JNEUROSCI.1905-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienenstock EL, Cooper LN, Munro PW. Theory for the development of neuron selectivity: Orientation specificity and binocular interaction in visual cortex. J Neurosci. 1982;2:32–48. doi: 10.1523/JNEUROSCI.02-01-00032.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmignoto G, Vicini S. Activity-dependent decrease in NMDA receptor responses during development of the visual cortex. Science. 1992;258:1007–1011. doi: 10.1126/science.1279803. [DOI] [PubMed] [Google Scholar]
- Chen WS, Bear MF. Activity-dependent regulation of NR2B translation contributes to metaplasticity in mouse visual cortex. Neuropharmacology. 2006 doi: 10.1016/j.neuropharm.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Crair MC, Malenka RC. A critical period for long-term potentiation at thalamocortical synapses. Nature. 1995;375:325–328. doi: 10.1038/375325a0. [DOI] [PubMed] [Google Scholar]
- Czepita D, Reid SN, Daw NW. Effect of longer periods of dark rearing on NMDA receptors in cat visual cortex. J Neurophysiol. 1994;72:1220–1226. doi: 10.1152/jn.1994.72.3.1220. [DOI] [PubMed] [Google Scholar]
- Fagiolini M, Katagiri H, Miyamoto H, Mori H, Grant SG, Mishina M, Hensch TK. Separable features of visual cortical plasticity revealed by N-methyl-D-aspartate receptor 2A signaling. Proc Natl Acad Sci U S A. 2003;100:2854–2859. doi: 10.1073/pnas.0536089100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint AC, Maisch US, Weishaupt JH, Kriegstein AR, Monyer H. NR2A subunit expression shortens NMDA receptor synaptic currents in developing neocortex. J Neurosci. 1997;17:2469–2476. doi: 10.1523/JNEUROSCI.17-07-02469.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca R, Nagerl UV, Morris RG, Bonhoeffer T. Competing for memory: hippocampal LTP under regimes of reduced protein synthesis. Neuron. 2004;44:1011–1020. doi: 10.1016/j.neuron.2004.10.033. [DOI] [PubMed] [Google Scholar]
- Fox K, Daw N, Sato H, Czepita D. The effect of visual experience on development of NMDA receptor synaptic transmission in kitten visual cortex. J Neurosci. 1992;12:2672–2684. doi: 10.1523/JNEUROSCI.12-07-02672.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Z, Logan SM, Vicini S. Deletion of the NR2A subunit prevents developmental changes of NMDA-mEPSCs in cultured mouse cerebellar granule neurones. J Physiol. 2005;563:867–881. doi: 10.1113/jphysiol.2004.079467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardoni F, Schrama LH, Kamal A, Gispen WH, Cattabeni F, Di Luca M. Hippocampal synaptic plasticity involves competition between Ca2+/calmodulin-dependent protein kinase II and postsynaptic density 95 for binding to the NR2A subunit of the NMDA receptor. J Neurosci. 2001;21:1501–1509. doi: 10.1523/JNEUROSCI.21-05-01501.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hestrin S. Developmental regulation of NMDA receptor-mediated synaptic currents at a central synapse. Nature. 1992;357:686–689. doi: 10.1038/357686a0. [DOI] [PubMed] [Google Scholar]
- Hollingsworth EB, McNeal ET, Burton JL, Williams RJ, Daly JW, Creveling CR. Biochemical characterization of a filtered synaptoneurosome preparation from guinea pig cerebral cortex: cyclic adenosine 3′:5′-monophosphate-generating systems, receptors, and enzymes. J Neurosci. 1985;5:2240–2253. doi: 10.1523/JNEUROSCI.05-08-02240.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husi H, Ward MA, Choudhary JS, Blackstock WP, Grant SG. Proteomic analysis of NMDA receptor-adhesion protein signaling complexes. Nat Neurosci. 2000;3:661–669. doi: 10.1038/76615. [DOI] [PubMed] [Google Scholar]
- Kadotani H, Hirano T, Masugi M, Nakamura K, Nakao K, Katsuki M, Nakanishi S. Motor discoordination results from combined gene disruption of the NMDA receptor NR2A and NR2C subunits, but not from single disruption of the NR2A or NR2C subunit. J Neurosci. 1996;16:7859–7867. doi: 10.1523/JNEUROSCI.16-24-07859.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood A, Rioult MG, Bear MF. Experience-dependent modification of synaptic plasticity in visual cortex. Nature. 1996;381:526–528. doi: 10.1038/381526a0. [DOI] [PubMed] [Google Scholar]
- Kirson ED, Yaari Y. Synaptic NMDA receptors in developing mouse hippocampal neurones: functional properties and sensitivity to ifenprodil. J Physiol (Lond) 1996;497:437–455. doi: 10.1113/jphysiol.1996.sp021779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupp JJ, Vissel B, Thomas CG, Heinemann SF, Westbrook GL. Calcineurin acts via the C-terminus of NR2A to modulate desensitization of NMDA receptors. Neuropharmacology. 2002;42:593–602. doi: 10.1016/s0028-3908(02)00031-x. [DOI] [PubMed] [Google Scholar]
- Kutsuwada T, Sakimura K, Manabe T, Takayama C, Katakura N, Kushiya E, Natsume R, Watanabe M, Inoue Y, Yagi T, et al. Impairment of suckling response, trigeminal neuronal pattern formation, and hippocampal LTD in NMDA receptor epsilon 2 subunit mutant mice. Neuron. 1996;16:333–344. doi: 10.1016/s0896-6273(00)80051-3. [DOI] [PubMed] [Google Scholar]
- Laurie DJ, Seeburg PH. Ligand affinities at recombinant N-methyl-D-aspartate receptors depend on subunit composition. Eur J Pharmacol. 1994;268:335–345. doi: 10.1016/0922-4106(94)90058-2. [DOI] [PubMed] [Google Scholar]
- Liu L, Wong TP, Pozza MF, Lingenhoehl K, Wang Y, Sheng M, Auberson YP, Wang YT. Role of NMDA receptor subtypes in governing the direction of hippocampal synaptic plasticity. Science. 2004;304:1021–1024. doi: 10.1126/science.1096615. [DOI] [PubMed] [Google Scholar]
- Lu HC, Gonzalez E, Crair MC. Barrel cortex critical period plasticity is independent of changes in NMDA receptor subunit composition. Neuron. 2001;32:619–634. doi: 10.1016/s0896-6273(01)00501-3. [DOI] [PubMed] [Google Scholar]
- Maffei A, Nataraj K, Nelson SB, Turrigiano GG. Potentiation of cortical inhibition by visual deprivation. Nature. 2006;443:81–84. doi: 10.1038/nature05079. [DOI] [PubMed] [Google Scholar]
- Massey PV, Johnson BE, Moult PR, Auberson YP, Brown MW, Molnar E, Collingridge GL, Bashir ZI. Differential roles of NR2A and NR2B-containing NMDA receptors in cortical long-term potentiation and long-term depression. J Neurosci. 2004;24:7821–7828. doi: 10.1523/JNEUROSCI.1697-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Monyer H, Sprengel R, Schoepfer R, Herb A, Higuchi M, Lomeli H, Burnashev N, Sakmann B, Seeburg PH. Heteromeric NMDA receptors: Molecular and functional distinction of subtypes. Science. 1992;256:1217–1221. doi: 10.1126/science.256.5060.1217. [DOI] [PubMed] [Google Scholar]
- Morikawa E, Mori H, Kiyama Y, Mishina M, Asano T, Kirino T. Attenuation of focal ischemic brain injury in mice deficient in the epsilon1 (NR2A) subunit of NMDA receptor. J Neurosci. 1998;18:9727–9732. doi: 10.1523/JNEUROSCI.18-23-09727.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishita W, Lu W, Smith GB, Nicoll RA, Bear MF, Malenka RC. Activation of NR2B-containing NMDA receptors is not required for NMDA receptor-dependent long-term depression. Neuropharmacology. 2006 doi: 10.1016/j.neuropharm.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Nase G, Weishaupt J, Stern P, Singer W, Monyer H. Genetic and epigenetic regulation of NMDA receptor expression in the rat visual cortex. Eur J Neurosci. 1999;11:4320–4326. doi: 10.1046/j.1460-9568.1999.00859.x. [DOI] [PubMed] [Google Scholar]
- Neyton J, Paoletti P. Relating NMDA receptor function to receptor subunit composition: limitations of the pharmacological approach. J Neurosci. 2006;26:1331–1333. doi: 10.1523/JNEUROSCI.5242-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Otano I, Schulteis CT, Contractor A, Lipton SA, Trimmer JS, Sucher NJ, Heinemann SF. Assembly with the NR1 subunit is required for surface expression of NR3A-containing NMDA receptors. J Neurosci. 2001;21:1228–1237. doi: 10.1523/JNEUROSCI.21-04-01228.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philpot BD, Espinosa JS, Bear MF. Evidence for altered NMDA receptor function as a basis for metaplasticity in visual cortex. J Neurosci. 2003;23:5583–5588. doi: 10.1523/JNEUROSCI.23-13-05583.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philpot BD, Sekhar AK, Shouval HZ, Bear MF. Visual experience and deprivation bidirectionally modify the composition and function of NMDA receptors in visual cortex. Neuron. 2001a;29:157–169. doi: 10.1016/s0896-6273(01)00187-8. [DOI] [PubMed] [Google Scholar]
- Philpot BD, Weisberg MP, Ramos MS, Sawtell NB, Tang YP, Tsien JZ, Bear MF. Effect of transgenic overexpression of NR2B on NMDA receptor function and synaptic plasticity in visual cortex. Neuropharmacology. 2001b;41:762–770. doi: 10.1016/s0028-3908(01)00136-8. [DOI] [PubMed] [Google Scholar]
- Priestley T, Laughton P, Myers J, Le Bourdelles B, Kerby J, Whiting PJ. Pharmacological properties of recombinant human N-methyl-D-aspartate receptors comprising NR1a/NR2A and NR1a/NR2B subunit assemblies expressed in permanently transfected mouse fibroblast cells. Mol Pharmacol. 1995;48:841–848. [PubMed] [Google Scholar]
- Prybylowski K, Rumbaugh G, Wolfe BB, Vicini S. Increased exon 5 expression alters extrasynaptic NMDA receptors in cerebellar neurons. J Neurochem. 2000;75:1140–1146. doi: 10.1046/j.1471-4159.2000.0751140.x. [DOI] [PubMed] [Google Scholar]
- Quinlan EM, Olstein DH, Bear MF. Bidirectional, experience-dependent regulation of N-methyl-D-aspartate receptor subunit composition in the rat visual cortex during postnatal development. Proc Natl Acad Sci USA. 1999a;96:12876–12880. doi: 10.1073/pnas.96.22.12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan EM, Philpot BD, Huganir RL, Bear MF. Rapid, experience-dependent expression of synaptic NMDA receptors in visual cortex in vivo. Nat Neurosci. 1999b;2:352–357. doi: 10.1038/7263. [DOI] [PubMed] [Google Scholar]
- Rumbaugh G, Prybylowski K, Wang JF, Vicini S. Exon 5 and spermine regulate deactivation of NMDA receptor subtypes. J Neurophysiol. 2000;83:1300–1306. doi: 10.1152/jn.2000.83.3.1300. [DOI] [PubMed] [Google Scholar]
- Sakimura K, Kutsuwada T, Ito I, Manabe T, Takayama C, Kushiya E, Yagi T, Aizawa S, Inoue Y, Sugiyama H, et al. Reduced hippocampal LTP and spatial learning in mice lacking NMDA receptor epsilon 1 subunit. Nature. 1995;373:151–155. doi: 10.1038/373151a0. [DOI] [PubMed] [Google Scholar]
- Sans N, Petralia RS, Wang YX, Blahos J. n., Hell JW, Wenthold RJ. A developmental change in NMDA receptor-associated proteins at hippocampal synapses. J Neurosci. 2000;20:1260–1271. doi: 10.1523/JNEUROSCI.20-03-01260.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M, Cummings J, Roldan LA, Jan YN, Jan LY. Changing subunit composition of heteromeric NMDA receptors during development of rat cortex. Nature. 1994;368:144–147. doi: 10.1038/368144a0. [DOI] [PubMed] [Google Scholar]
- Shi J, Townsend M, Constantine-Paton M. Activity-dependent induction of tonic calcineurin activity mediates a rapid developmental downregulation of NMDA receptor currents. Neuron. 2000;28:103–114. doi: 10.1016/s0896-6273(00)00089-1. [DOI] [PubMed] [Google Scholar]
- Sobczyk A, Scheuss V, Svoboda K. NMDA receptor subunit-dependent [Ca2+] signaling in individual hippocampal dendritic spines. J Neurosci. 2005;25:6037–6. doi: 10.1523/JNEUROSCI.1221-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele PM, Mauk MD. Inhibitory Control of LTP and LTD: Stability of Synapse Strength. J Neurophysiol. 1999;81:1559–1566. doi: 10.1152/jn.1999.81.4.1559. [DOI] [PubMed] [Google Scholar]
- Steigerwald F, Schulz TW, Schenker LT, Kennedy MB, Seeburg PH, Kohr G. C-Terminal truncation of NR2A subunits impairs synaptic but not extrasynaptic localization of NMDA receptors. J Neurosci. 2000;20:4573–4581. doi: 10.1523/JNEUROSCI.20-12-04573.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocca G, Vicini S. Increased contribution of NR2A subunit to synaptic NMDA receptors in developing rat cortical neurons. J Physiol (Lond) 1998;507:13–24. doi: 10.1111/j.1469-7793.1998.013bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsumoto T, Hagihara K, Sato H, Hata Y. NMDA receptors in the visual cortex of young kittens are more effective than those of adult cats. Nature. 1987;327:513–514. doi: 10.1038/327513a0. [DOI] [PubMed] [Google Scholar]
- Vicini S, Wang JF, Li JH, Zhu WJ, Wang YH, Luo JH, Wolfe BB, Grayson DR. Functional and pharmacological differences between recombinant N-methyl-Daspartate receptors. J Neurophysiol. 1998;79:555–566. doi: 10.1152/jn.1998.79.2.555. [DOI] [PubMed] [Google Scholar]
- Vissel B, Krupp JJ, Heinemann SF, Westbrook GL. Intracellular domains of NR2 alter calcium-dependent inactivation of N-methyl-D-aspartate receptors. Mol Pharmacol. 2002;61:595–605. doi: 10.1124/mol.61.3.595. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Inoue Y, Sakimura K, Mishina M. Distinct spatio-temporal distributions of the NMDA receptor channel subunit mRNAs in the brain. Ann N Y Acad Sci. 1993;707:463–466. doi: 10.1111/j.1749-6632.1993.tb38099.x. [DOI] [PubMed] [Google Scholar]
- Weitlauf C, Honse Y, Auberson YP, Mishina M, Lovinger DM, Winder DG. Activation of NR2A-containing NMDA receptors is not obligatory for NMDA receptor-dependent long-term potentiation. J Neurosci. 2005;25:8386–8390. doi: 10.1523/JNEUROSCI.2388-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshii A, Sheng MH, Constantine-Paton M. Eye opening induces a rapid dendritic localization of PSD-95 in central visual neurons. Proc Natl Acad Sci U S A. 2003;100:1334–1339. doi: 10.1073/pnas.0335785100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng H, Chattarji S, Barbarosie M, Rondi-Reig L, Philpot BD, Miyakawa T, Bear MF, Tonegawa S. Forebrain-specific calcineurin knockout selectively impairs bidirectional synaptic plasticity and working/episodic-like memory. Cell. 2001;107:617–629. doi: 10.1016/s0092-8674(01)00585-2. [DOI] [PubMed] [Google Scholar]
- Zhao MG, Toyoda H, Lee YS, Wu LJ, Ko SW, Zhang XH, Jia Y, Shum F, Xu H, Li BM, et al. Roles of NMDA NR2B Subtype Receptor in Prefrontal Long-Term Potentiation and Contextual Fear Memory. Neuron. 2005;47:859–872. doi: 10.1016/j.neuron.2005.08.014. [DOI] [PubMed] [Google Scholar]