Abstract
Mesothelin is a differentiation antigen present on the surface of ovarian cancers, mesotheliomas, and several other types of human cancers. Because among normal tissues, mesothelin is present only on mesothelial cells, it represents a good target for antibody-mediated delivery of cytotoxic agents. In the present study mice were immunized with an eukaryotic expression vector coding for mesothelin. When high serum antibody titers were obtained, a phage display library was made from the splenic mRNA of these mice. After three rounds of panning on recombinant mesothelin, a single-chain Fv (scFv)-displaying phage was selected that bound specifically to recombinant mesothelin and mesothelin-positive cells. The scFv was used to construct an immunotoxin by genetically fusing it with a truncated mutant of Pseudomonas exotoxin A. The purified immunotoxin binds mesothelin with high affinity (Kd 11 nm), is stable for over 40 hr at 37°C and is very cytotoxic to cells expressing mesothelin. It also produces regressions of tumors expressing mesothelin. This combination of selective cytotoxicity, high activity, and stability makes the immunotoxin a good candidate for development as a therapeutic agent. This work also shows that DNA immunization can be used to isolate and clone antibodies against epitopes present on human proteins in their native conformation.
Keywords: cancer treatment, ovarian cancer, mesothelioma
Differentiation antigens often continue to be expressed on cancer cells and have been used for the targeted therapy of cancer. This approach has been successfully carried out against hematopoietic malignancies in which CD19, CD20, CD22, and CD25 have been used as targets (1, 2). It also should be possible to target solid tumors by using differentiation antigens as the targets, provided that the tumors arise from expendable tissues such as ovary, breast, and prostate. We have identified an antigen, mesothelin, which is present on the surface of several tumors, including ovarian cancers and mesotheliomas (3–5). In the United States, an estimated 15,000 patients die of ovarian cancer each year despite therapy. Although less common, mesotheliomas are known to be resistant to all chemotherapeutic agents. Development of new therapeutic approaches to these malignancies are needed. The presence of mesothelin on the surface of these tumors make it a potential target for directed therapy. Although normal mesothelial cells also may be damaged by such an approach, they often are damaged by intraperitoneal and intrathoracic therapy without life-threatening consequences.
Mesothelin is a 40-kDa glycosylphosphatidylinositol-linked glycoprotein. It is synthesized as a precursor of molecular mass 69 kDa, which then is proteolytically processed into an N terminal secreted form of molecular mass 30 kDa and a membrane-bound form of 40 kDa (5). The 30-kDa secreted form has been termed megakaryocyte potentiating factor (6). Unlike many cell surface antigens present on cancer cells, the membrane-bound form of mesothelin cannot be detected in the blood of cancer patients and is not shed into the medium from cultured cells (3). Thus, the presence of mesothelin on the surface of cancer cells makes it a promising candidate for targeted therapies.
Our laboratory is focused on the generation of immunotoxins for cancer therapy, three of which are now in clinical trials (ref. 7 and unpublished data). We previously have tried to make an immunotoxin against cells expressing mesothelin by using mAb K1, the first antibody isolated that binds to mesothelin, by chemically conjugating a truncated form of Pseudomonas exotoxin (PE) to mAb K1. mAb K1 was isolated by immunizing mice with the ovarian cancer cell line, OVCAR3. Although in histochemical studies mAb K1 was found to bind to mesothelin-positive cells and to cancer tissues, it was not useful as an immunotoxin, probably because of its poor internalization (3). We have tried to obtain additional antibodies against mesothelin, especially antibodies that would be internalized into cells after binding to the antigen. After many unsuccessful attempts to produce anti-mesothelin antibodies by the hybridoma technology, we turned to phage display and isolated an anti-mesothelin single-chain Fv (scFv) from a phage display library made from the spleen mRNA of mice immunized with recombinant mesothelin produced in Escherichia coli (8). However, this scFv had a low affinity for mesothelin-positive cells and also was not useful for targeted therapy. One possible reason for low binding is that the antibody was made against the recombinant protein produced in E. coli. The ideal solution would be to immunize mice with the native protein made in eukaryotic cells, but purification of sufficient amounts of mesothelin from mammalian cells has not been possible (unpublished data). Therefore, we decided to immunize mice with DNA encoding mesothelin.
DNA immunization has the potential to replace protein-based immunization (9). It elicits antigen-specific immune responses after injection of nonreplicating transcription units that encode heterologous protein(s). After synthesis of the foreign protein, it is processed and presented to the immune system like other cellular proteins. Because it is heterologous an immune response is mounted against it and the peptides that are derived from it (10, 11). The technique has two significant advantages over protein-based immunization. One is that it does not require the purification of the protein, which at best is time consuming and in some cases, such as with many membrane proteins, is very difficult or impossible. The second is that because the immunogen is synthesized in the mammalian host, it is likely to undergo proper posttranslational modifications and fold into the native structure. Because this process is often not the case with mammalian proteins produced in E. coli, antibodies produced by immunizing animals with such recombinant proteins may not recognize important epitopes that are present only when the protein is in its natural conformation.
Eliciting an efficient immune response is just the first step in the process of obtaining a good mAb. The second step, which may be very difficult, is to find a clone with high affinity in the whole population of antibody-producing cells. Finding the best binder is dictated by the efficiency of the selection process and depends on the number of clones that can be screened and the stringency with which it is done. Phage antibody display allows one to meet both these requirements, because the DNA encoding the antigen-binding portion of each antibody in the form of a single-chain Fv (scFv), a disulfide stabilized Fv (dsFv), or a Fab is fused in-frame with gene III (gIII), which encodes the minor surface protein gIIIp of the filamentous phage (12–14).
In the current study, we have successfully used a mesothelin expression plasmid to immunize mice and by using splenic RNA have isolated an scFv (SS scFv) that binds with high affinity to mesothelin. We have constructed an immunotoxin containing this Fv, which selectively kills mesothelin-expressing cells and produce regressions of mesothelin-containing tumors.
MATERIALS AND METHODS
Immunization of Mice.
Four-month-old female BALB/c mice were immunized intradermally with 15 μg of pcD3CAK1–9 encoding full-length mesothelin (5) in 0.15 M sodium chloride. Three weeks later four more intradermal injections of 15 μg pcD3CAK1–9 were given at intervals of 3–5 weeks. The spacing between the injections were done by following the ELISA titer in the blood weekly. The titer is defined as the reciprocal of the highest dilution of antiserum, which gave an ELISA signal 2- to 3-fold higher than the background. Each injection was given when the titer in the blood declined from a peak level except for the last injection when the titer remained at a plateau for 5 weeks. Ten days after the last injection, when the titer was more than 100,000, the mice were sacrificed and the spleens were collected.
RNA Extraction and Initial Construction of the scFv Library.
These procedures were performed as described earlier (8) with the following modifications. About 150 ng of insert was used for ligation into 125 ng of cut vector and the library was made in XL-1 blue MRF′ cells instead of XL-2 blue MRF′. The library contained about 9 × 105 independent clones. To the library, which was in 18 ml SOC (20 g bactotryptone/5 g bacto-yeast extract/0.5 g NaCl per liter in water containing 2.5 mM potassium chloride, 10 mM magnesium chloride, and 20 mM glucose) medium, glucose was added to a final concentration of 2% (wt/vol). After incubation at 37°C for 90 min, ampicillin and tetracycline were added to final concentrations of 100 and 10 μg/ml, respectively. Incubation was continued at 37°C for another 90 min and the culture stored as glycerol stocks.
Transfer of the Library from E. coli XL-1 to TG-1 Strain.
Because deletions occurred when the antibody phagemid library was propagated in XL-1, it was transferred to the TG-1 strain where this did not occur. First, the polyethylene glycol-precipitated recombinant phage were rescued from a pool of glycerol stocks of the library in XL-1. TG-1 cells (50 ml) at an OD600 nm of 0.55 in 2 × YT (16 g bacto-tryptone/10 g bacto-yeast extract/5 g NaCl per liter in water) was infected with the rescued phage at a multiplicity of infection of 5. Incubation was at 37°C for 30 min at 110 rpm and then for 30 min at 250 rpm. Ampicillin (100 μg/ml) was added, and an additional incubation for 30 min at 37°C, 250 rpm was carried out. Recombinant phagemid particles were rescued with M13KO7 as described (8) except that rescue was at 37°C and 30°C in separate cultures. The phage were polyethylene glycol-precipitated three times (15) before being used for panning on recombinant mesothelin (amino acids 291–606).
Panning of the Library.
Recombinant mesothelin was prepared as described (8). Panning was done by two different methods.
Method 1 (direct panning).
Thirty-five-millimeter diameter wells of tissue culture grade plates were coated with 1.5 ml of recombinant mesothelin at 5 μg/ml (125 nM) in 50 mM bicarbonate buffer, pH 9.4 and blocked with 3% nonfat milk in PBS containing 0.05% Tween 20 (TPBS). In the first round of panning 2 × 1011 colony-forming units were allowed to bind to the plates for 2 hr at 37°C, and after 20 rounds of washing with TPBS and PBS the bound phage were eluted with 1 ml of 50 mM HCl, pH 1.3, for 10 min at 37°C. After neutralization with Tris an aliquot of the eluate was used for titration on Luria–Bertani plates containing 100 μg/ml of ampicillin and 2% glucose. The rest was used to infect 10 ml of E. coli TG-1 cells grown to a OD600 of 0.3 in 2 × YT. During this infection phase, glucose was added to the culture to a final concentration of 2%. The culture was incubated at 37°C for 30 min at 110 rpm and then for 30 min at 250 rpm. Ampicillin then was added to a final concentration of 100 μg/ml, and the culture was incubated for an additional 30 min at 37°C shaking at 250 rpm followed by the addition of M13KO7 at a multiplicity of infection of 15. The culture was incubated for 60 min at 37°C shaking at 110 rpm for the first 30 min and then at 250 rpm. The cells then were pelleted and resuspended in 20 ml 2 × YT containing 100 μg/ml of ampicillin and 50 μg/ml of kanamycin. The culture was incubated at 37°C with shaking at 250 rpm overnight. The rescued phage in the culture medium were polyethylene glycol-precipitated, titrated, and used for the next round of panning. After the third round of panning, 10 clones were selected randomly and minipreps were analyzed for BstN1 fingerprinting and nucleotide sequence analysis.
Method 2 (off-rate panning).
The procedure described above was followed except that after washing the unbound phage particles the plates were incubated at 37°C in PBS for an additional 2 hr before eluting the bound phage.
Binding Assays.
Binding of phage to mesothelin was assayed by ELISA. Wells of Immunolon-4 plates were coated with 200 ng of mesothelin (amino acids 291–606), BSA, p55, or streptavidin in 100 μl of bicarbonate buffer, pH 9.4 overnight at 4°C. The wells were blocked with 3% nonfat dry milk in TPBS for 60 min. One hundred microliters of blocking solution containing varying numbers of phage was applied to each well and incubated for 60 min at 37°C. After washing with TPBS, bound phage were detected with anti-M13 antibody conjugated to horseradish peroxidase for 60 min at 37°C. After washing with TPBS and PBS the ELISA wells were developed with 100 μl of BM-Blue substrate (Boehringer) for horseradish peroxidase. Color development was stopped after 2 min with 100 μl of 2N H2SO4, and the OD readings were taken at 450 nm.
Competition ELISA.
Competition ELISA was done on immobilized recombinant mesothelin as described above except that 100 μl of various dilutions of TG-1 culture supernatant containing the selected scFv (SS scFv) or K1 scFv recombinant phagemid particles was used directly for ELISA either with or without 1.0 μg K1 IgG.
Surface plasmon resonance assays.
Binding of the immunotoxin to mesothelin was determined by surface plasmon resonance (BIACore) as described earlier (19).
Cell Lines.
NIH 3T3 is a Swiss mouse fibroblast cell line. NIH 3T3K20 is stably transfected with an eukaryotic expression vector (pcDNA3) containing the full-length mesothelin gene (pcD3CAK1–9). A431 is a cervical epidermoid carcinoma, and A431-K5 is stably transfected with pcD3CAK1–9. Other cell lines were previously described (3, 16).
Immunofluorescence Examination of Live Cells.
Phage selected by panning on recombinant mesothelin (amino acids 291–606) were checked for binding to mesothelin-positive cells by immunofluorescence on NIH 3T3 and NIH 3T3K20 cells. The cells were grown in 35-mm tissue culture dishes. The cells were washed with Dulbecco’s PBS (DPBS) containing Ca2+ and Mg2+ (DPBS++) and cooled over ice. They then were blocked with 0.2% BSA in DPBS++ at 4°C and exposed to 1011 phage particles selected by panning or to anti-Tac phage, which displays an scFv to p55 subunit of the interleukin 2 receptor for 60 min at 4°C. Bound phage were detected with a mouse mAb to gVIIIp of M13 phage as the first antibody followed by goat anti-mouse IgG coupled to rhodamine. After washing, the cells were fixed in 3.7% formaldehyde and mounted in situ under coverslips in buffered glycerol.
Construction of Plasmid for Expression and Purification of Immunotoxin SS(scFv) PE38.
The scFv from the phagemid vector was PCR-amplified by using primer pairs New G2 NdeI 5′-TAAGAAGGAGATATACATATGCAGGTACAACTGCAGCAGTCTGGG-3′ as the back primer and New G2 HindIII 5′-GCCCTCGGGACCTCCGGAAGCTTTTATTTCCAACTTTGTCCC-3′ as the forward primer which introduced a NdeI and a HindIII site at the 5′ and 3′ ends of SS scFv. After purification the PCR product was digested with NdeI and HindIII and ligated into the 4,180-bp fragment of pULI7 (17) obtained by cutting with the same enzymes. The resulting plasmid, pPSC 7–2, had the SS scFv fused in-frame with domain II and III of PE A. Recombinant proteins were produced from inclusion bodies (17).
Cytotoxicity Assays.
Specific cytotoxic activity of immunotoxin, SS(scFv)-PE38 was determined by inhibition of protein synthesis (18). The IC50 is the amount of immunotoxin required to inhibit protein synthesis by 50%.
Stability Assays.
Stability assays were performed by incubating aliquots of a 10 μg/ml stock of the immunotoxin in 0.2% human serum albumin in DPBS++ for varying times at 37°C. At the end of incubation the samples were frozen at −80°C and tested for their cytotoxic activity on A431 K5 cells as a group.
Anti-Tumor Activity of SS scFv-PE38.
SS scFv-PE38 was assayed for its ability to inhibit the growth or cause regression of subcutaneous tumor xenografts in nude mice. This assay was done by injecting 1.5 × 106 A431 K5 cells subcutaneously into 4- to 6-week-old nude mice on day 0. Treatment was started on day 5 when tumors measured about 50 mm3. Animals were treated intravenously with three doses on days 5, 7, and 9. The control group received either anti-Tac(scFv)-PE38, which is not cytotoxic to A431 K5 cells, or just the carrier (0.2% human serum albumin in DPBS). Each group consisted of five animals.
RESULTS
Immunization, Library Construction, and Panning.
Mice were immunized intradermally with pcD3CAK1–9, which expresses mesothelin under the control of a cytomegalovirus promoter (5). BALB/c mice were used because intradermal DNA immunization has been shown to induce strong humoral immune response in this strain (20). Immunized mice had high antibody titers in the serum; after the fourth immunization, the serum titer was found to be more than 1/100,000 by ELISA using recombinant mesothelin. These antisera also reacted strongly and specifically with mesothelin-transfected cells at a 1/500 dilution (data not shown). When the titer remained constant without a significant decline for nearly 5 weeks the mice were boosted with one more injection and the spleen was collected after 10 days. A scFv phagemid library was made in XL-1; the library contained 9 × 105 independent clones. Phagemid rescue and panning were done at 37°C. During the panning and reamplification process in XL-1 we noticed frequent deletion of the scFv sequences from the phagemid (unpublished data). The library therefore was transferred to the TG-1 strain for panning. We used 37°C for panning to select for an scFv that would be stable at this temperature. After each round of panning elution was done either immediately after the extensive washing procedure to obtain all the binding phage or after an additional 2-hr incubation in PBS to select for phage that would have a slower off rate. Titration of eluted phage showed that there was a 1,000- to 2,000-fold enrichment in the number of eluted phage between the first and third rounds of panning (Table 1). After each round of panning, recombinant phage were rescued from 48 randomly picked individual phagemid colonies and checked for binding to recombinant mesothelin (amino acids 291–606) by ELISA. As shown in Table 1 there was no detectable mesothelin-positive clone among colonies picked randomly from the unpanned library and in the eluate after the first round of panning. In the second eluate there were 1–2 mesothelin-positive clones of 48 tested. In the third eluate the number increased to about 30/48. BstN1 fingerprinting of six positive clones from each group showed that the restriction pattern was identical for all the clones (data not shown). DNA sequencing of six clones from each group after three rounds of panning revealed that the positive clones obtained were identical in their nucleotide sequence. We also carried out a similar selection by using phage grown and selected at 30°C and obtained the same clone. Thus, the procedure selected for a single scFv capable of binding to mesothelin, which was termed SS scFv. The nucleotide sequence has been deposited in the GenBank database. According to Kabat’s classification, the VH belongs to subgroup IIA and family V and the VL belongs to subgroup VI and family XI.
Table 1.
Enrichment of anti-mesothelin phage over three rounds of panning at 37°C by using direct or off-rate selection
| Panning round (selection) | Input no. of phage | No. of phage binding | No. of mesothelin positive phage/ no. tested |
|---|---|---|---|
| 1 Direct | 2 × 1011 | 7 × 104 | 0/48 |
| Off-rate | 2 × 1011 | 2 × 104 | 0/48 |
| 2 Direct | 7 × 1010 | 8 × 104 | 2/48 |
| Off-rate | 3 × 1010 | 8 × 103 | 1/48 |
| 3 Direct | 1 × 1010 | 6 × 106 | 32/48 |
| Off-rate | 3 × 109 | 4 × 105 | 30/48 |
Characterization of the Selected Clone.
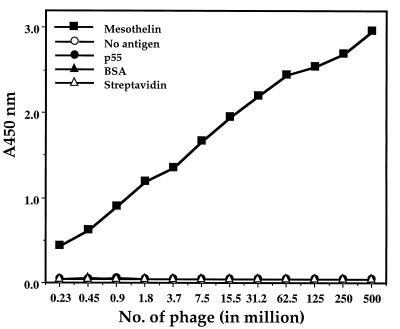
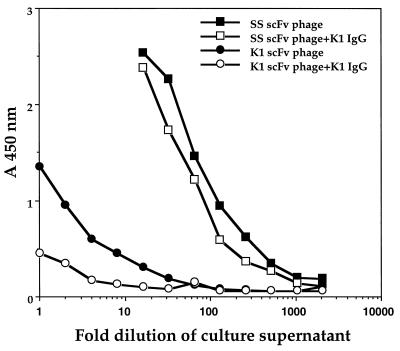
Recombinant phage were rescued by superinfection with M13KO7 and tested for binding to recombinant mesothelin (amino acids 291–606). Fig. 1 shows they bound to mesothelin in a concentration-dependent manner and did not show any binding to ELISA wells that were uncoated or were coated with various control proteins. This finding indicates that the SS scFv is specific for mesothelin. To determine whether the SS scFv bound to a different epitope than mAb K1, a competition ELISA was performed. As a positive control inhibition of binding of a K1 phage by the same IgG also was tested in parallel. As shown in Fig. 2, the binding of the SS scFv phage was only slightly inhibited by K1 IgG and there was no concentration dependence whereas binding of the K1 phage was greatly reduced by K1 IgG. This finding indicates that the epitope for SS scFv is different from that of K1.
Figure 1.
Phage displaying SS scFv bind specifically to mesothelin (amino acids 291–606) coated ELISA wells in a dose-dependent manner. Phage were exposed to wells coated with mesothelin, p55, BSA, and streptavidin or to uncoated wells. Bound phage was detected as described in Materials and Methods.
Figure 2.
Epitopes for SS scFv and K1 are different. Mesothelin-coated wells were incubated with various dilutions of SS scFv or K1 scFv phage in the presence or absence of mAb K1 at 1 μg/ml. Bound phage was detected as mentioned in Materials and Methods.
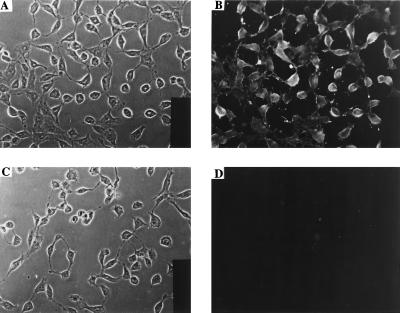
To see whether the antibody selected by panning on recombinant mesothelin (amino acids 291–606) can bind to mesothelin displayed on the mammalian cell surface, an immunofluorescence experiment was performed with mesothelin-positive cells. Fig. 3 shows that phage that display SS scFv do not bind to mesothelin-negative NIH 3T3 cells but bind strongly to NIH 3T3 K20 cells that have been transfected with a mesothelin expression plasmid and express mesothelin. A control phage that displays anti-Tac scFv that bind to p55 did not bind to either of these cell lines (results not shown). This result indicated that the SS scFv specifically bound to cell surface mesothelin.
Figure 3.
Binding of SS scFv phage to mesothelin positive and negative cells as detected by immunofluorescence. (A and B) NIH 3T3 K20 cells. (C and D) NIH 3T3 cells. A and C are phase contrast, and B and D are immunofluorescence pictures. All cells were exposed to SS scFv phage as described in Materials and Methods. Binding of anti-Tac phage to both the cell lines was tested and found to be negative (data not shown).
SS scFv Immunotoxin Expression and Purification.
To determine whether the SS scFv could target a cytotoxic agent to mesothelin-positive cells, we constructed a recombinant immunotoxin by fusing the Fv to PE38, a truncated form of PE A. In this construction, the Fv replaces the binding domain of PE and targets the toxin to cells to which the Fv binds. The recombinant protein was produced in E. coli and after renaturation from inclusion bodies purified by ion exchange and size exclusion chromatography. The recombinant immunotoxin eluted as a monomer in TSK gel filtration chromatography and migrated as a single band of about 67 kDa in SDS/PAGE. Calculating from total inclusion body proteins subjected to refolding, the yield was about 12%.
Binding Characteristics of the Immunotoxin.
The binding characteristics of the immunotoxin was determined by surface plasmon resonance. Recombinant mesothelin (amino acids 291–606) was coupled to BIACore sensor chip CM5 and a 25 μg/ml solution of the immunotoxin was run over the chip. The kon was found to be 1.68 × 105 M−1 sec−1. The koff was found to be 1.83 × 10−3 sec−1. The Kd calculated from these data is 11 nM.
Cytotoxicity of the Immunotoxin in Different Cell Lines.
The ability of the SS(Fv)-PE38 to inhibit protein synthesis was used as a measure of its cytotoxic effect. To determine whether the Fv could be internalized so that the toxin could kill the target cells, we exposed antigen-positive and antigen-negative cells to the immunotoxin for 20 hr and measured 3H-leucine incorporation. On A431 K5 cells, which is the transfected cell line expressing mesothelin, the IC50 was 0.5 ng/ml (Table 2), whereas on ATAC4− cells, which are A431-transfected with the α-subunit of the interleukin 2 receptor, the IC50 was 400 ng/ml. Furthermore, on nontransfected A431 cells the IC50 was 450 ng/ml. Thus, the cytotoxic activity of SS scFv-PE38 is highly specific. The recombinant immunotoxin also was tested on several antigen-negative cell lines (HUT102, Raji, and JD38) and showed very little cytotoxic activity (IC50 > 1,000 ng/ml). However, on the mesothelin-positive ovarian cancer cell line A1847, the IC50 is 16 ng/ml. Also, two gastric carcinoma cell lines, AGS and N87, which express mesothelin, were sensitive to SS scFv-PE38 with IC50 of 6 ng/ml. These studies indicate that sufficient amounts of SS scFv-PE38 are internalized to kill antigen-positive cell lines.
Table 2.
Cytotoxicity of SS scFv-PE38 on human cancer cell lines
| Cell line | Origin | K1 reactivity by IF | IC50 ng/ml |
|---|---|---|---|
| A431 | Epidermoid carcinoma | − | 450 |
| A431 K5 | Mesothelin-transfected A431 | ++++ | 0.5 |
| ATAC-4 | p55-transfected A431 | − | 400 |
| AGS | Gastric adenocarcinoma | ++ | 6 |
| N87 | Gastric adenocarcinoma | ++ | 6 |
| A1847 | Ovarian adenocarcinoma | ++/het | 16 |
| JD38 | Burkitt’s lymphoma | − | >1,000 |
| Raji | Burkitt’s lymphoma | − | >1,000 |
| HUT 102 | T cell leukemia | − | >1,000 |
IF, immunofluorescence.
Stability of the Immunotoxin.
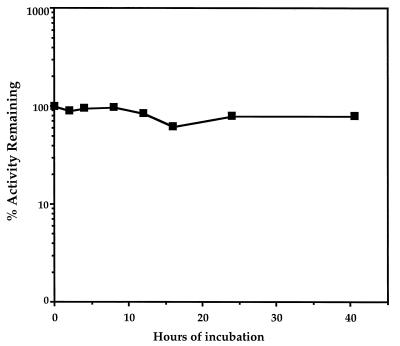
To be useful in targeted therapy, an Fv must be stable for many hours at 37°C while it penetrates the interior of tumors (21). The stability of the immunotoxin was analyzed by measuring its cytotoxic activity after incubation at 37°C for varying periods of time. Fig. 4 shows that after incubation at 37°C for up to 40 hr, there is barely any change in the cytotoxic activity of the immunotoxin, indicating that the molecule is very stable at physiological temperature.
Figure 4.
Stability of SS scFv-PE38 at 37°C. SS scFv-PE38 (10 μg/ml) was incubated at 37°C for up to 40.5 hr, and then its cytotoxic activity was measured. The % of initial activity remaining after various periods of incubation is shown.
Tumor Studies.
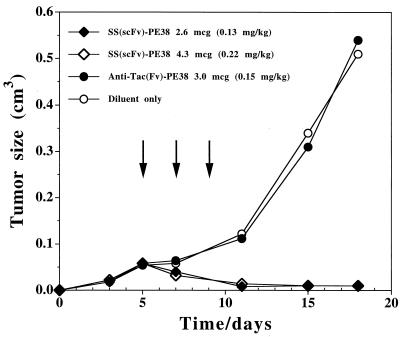
To justify development as an anti-tumor agent, an agent must be capable of causing tumor regressions in an animal model. To produce tumors, A431 K5 cells were injected subcutaneously into immunodeficient mice. When the tumors reached about 50 mm3, the animals were injected i.v. with 2.6 or 4.3 μg of SS scFv-PE38 every other day × 3. In both groups of mice tumor regressions were observed (Fig. 5). Control mice were injected with 3 μg × 3 of anti-Tac(Fv)-PE38, an immunotoxin directed at the α-subunit of the interleukin 2 receptor, which is not present on A431 K5 cells. No responses were noted with this treatment. Anti(Tac)-PE38 previously has been shown to produce complete regression of tumors expressing the interleukin 2 receptor (26).
Figure 5.
Anti-tumor effect of SS scFv-PE38 in nude mice. Groups of five animals were injected with 1.5 × 106 A431 K5 cells on day 0. On day 5, tumors reached a size of 50 mm3. Animals were treated intravenously on days 5, 7, and 9 with 2.6 μg (⧫) or 4.3 μg (□) of SS scFv-PE38 in DPBS containing 0.2% human serum albumin. Control groups received either the carrier alone (○) or 3 μg anti-Tac(scFv)-PE38 (•). No death or toxicity was observed at these doses.
DISCUSSION
Mesothelin is a differentiation antigen that is expressed on many ovarian cancers, mesotheliomas, and a number of other human cancers (3, 4). Because of its limited expression on normal cells, it could be a useful target for immunotherapy. Previously only one mAb to mesothelin, mAb K1, was available. It was not active as an immunotoxin when a recombinant form of PE that lacks the cell binding domain of PE was coupled to it (3). Attempts to isolate high-affinity antibodies to mesothelin by injection of mice with recombinant mesothelin produced in E. coli produced only a low-affinity antibody. We report here the isolation by phage display of a stable Fv with a relative high affinity for mesothelin by using animals immunized with DNA. The binding affinity of 11 nM for the SS scFv immunotoxin is similar to several other scFv immunotoxins that we have made previously from mAbs obtained from hybridomas (24). We report the isolation of a high-affinity Fv antibody after DNA immunization using phage antibody display.
Several features of the immunization scheme are worth emphasizing. One is that the plasmid used for immunization encoded a full-length mesothelin cDNA under the control of cytomegalovirus promoter. As a result, the mesothelin cDNA was transcribed in mouse cells and the translated protein underwent proper posttranslational modifications. Because the encoded protein was a membrane protein and not a secreted protein, antibody production could be monitored in serum without interference from blocking antigens. Also, the plasmid carried an ampicillin resistance gene that is known to have immunostimulatory sequences for Th1 responses that is necessary for good antibody production (22).
After immunization we made a phage antibody display library to select a good scFv. After three rounds of panning we had enriched mesothelin binding phage by about 2,000-fold and found that all the phage examined encoded the same antimesothelin scFv (SS scFv). This finding indicates that the panning conditions were efficient in selecting for the best binder in the library or there was only a single binder present. We have not examined the sera of the immunized mice to determine whether antibodies to other epitopes were present but not represented in the phage library. The scFv isolated by panning on recombinant mesothelin (amino acids 291–606), also bound to mammalian cells transfected with mesothelin. The epitope for this SS scFv is different from that of mAb K1 because mAb K1 do not significantly inhibit the binding of scFv displaying phage particles to mesothelin.
An immunotoxin was made by fusing the DNA encoding SS scFv to PE38 in a T7 expression vector. The protein was expressed at a very high level upon isopropyl β-d-thiogalactopyranoside induction. Purification from the inclusion bodies by ion exchange and gel filtration gave a yield of about 12%, which is much above the average yield of other single-chain immunotoxins that we have produced. This result indicates that we have selected for a single-chain Fv that folds well because the Fv portion of the immunotoxin is limiting in the refolding reaction (23).
Cytotoxicity studies reveal that SS scFv immunotoxin has an IC50 of about 0.5 ng/ml and is very cytotoxic to a cell line (A431K5) transfected with the mesothelin expression plasmid. SS sc(Fv)-PE38 also produces regressions of mesothelin containing subcutaneous tumors (Fig. 5). The immunotoxin inhibits protein synthesis in other mesothelin-positive cancer cell lines (AGS, N87 and A1847), although cytotoxicity in these cell lines is less than that in A431-K5. This decrease in the sensitivity of untransfected cancer cell lines toward the immunotoxin is probably because of less antigen on the cell surface. We have noted that mesothelin expression is high in specimens of human tumors but is usually greatly decreased when the cells are placed in culture (4). Expression in the A431-K5 cell line is close to that of cancer cells in patients (unpublished data).
For an immunotoxin to be useful as a therapeutic agent it must be stable at 37°C. We have found that SS scFv-PE38 remains fully active for up to 40 hr at 37°C. This finding is in striking contrast to several scFv immunotoxins that we have made previously from hybridoma-derived mAbs (25–27). One important feature contributing to the selection of a stable scFv is that the production of recombinant phage particles involved incubation of the bacterial culture at 37°C for 12–16 hr before they were panned for binding to an antigen. Also, panning is performed at 37°C. Therefore, there is a strong inherent bias toward the selection of stable scFvs. Also, the phage display system is biased to select for stable scFvs because it involves secretion of the scFv-gIIIp fusion protein across the bacterial inner membrane. scFvs that are very unstable tend to aggregate in the cytoplasm and therefore cannot be secreted or assembled in the periplasm for incorporation on the surface of phage (unpublished data).
In conclusion, we have isolated a scFv (SS scFv) by phage display technology from a library made from spleen mRNA of DNA-immunized mice. The SS scFv is stable, binds with high affinity to mesothelin and an immunotoxin made by fusing the SS scFv to a truncated mutant of PE A kills mesothelin-positive cancer cell lines and causes regression of a mesothelin-positive tumor. The SS scFv also could be used to target radioisotopes or other cytotoxic drugs to mesothelin-positive cancer cells.
Acknowledgments
We thank Dr. Vijay Chaudhary, University of Delhi, New Delhi, India, for providing us with M13 anti-gVIIIp mAb. Drs. U. Brinkmann, M. Essand, A. Hafkemeyer, and B. Jwang for reviewing the manuscript and J. Evans and A. Jackson for editorial assistance.
ABBREVIATIONS
- scFv
single-chain Fv
- PE
Pseudomonas exotoxin
- TPBS
PBS containing 0.05% Tween 20
- DPBS
Dulbecco’s PBS
Footnotes
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AF035617).
References
- 1.Press O W, Eary J F, Appelbaum F R, Martin P J, Badger C C, Nelp W B, Glenn S, Butchko G, Fisher D, Porter B. N Eng J Med. 1993;329:1219–1224. doi: 10.1056/NEJM199310213291702. [DOI] [PubMed] [Google Scholar]
- 2.Osterborg A, Dyer M J, Bunjes D, Pangalis G A, Bastion Y, Catovsky D, Mellstedt H. J Clin Oncol. 1997;15:1567–1574. doi: 10.1200/JCO.1997.15.4.1567. [DOI] [PubMed] [Google Scholar]
- 3.Chang K, Pai L H, Batra J K, Pastan I, Willingham M C. Cancer Res. 1992;52:181–186. [PubMed] [Google Scholar]
- 4.Chang K, Pai L H, Pass H, Pogrebriak H W, Tsao M-S, Pastan I, Willingham M C. Am J Surg Pathol. 1992;16:259–268. doi: 10.1097/00000478-199203000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Chang K, Pastan I. Proc Natl Acad Sci USA. 1996;93:136–140. doi: 10.1073/pnas.93.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamaguchi N, Hattori K, Oh-eda M, Kojima T, Imai N, Ochi N. J Biol Chem. 1994;269:805–808. [PubMed] [Google Scholar]
- 7.Pai L H, Wittes R, Setser A, Willingham M C, Pastan I. Nat Med. 1996;2:350–353. doi: 10.1038/nm0396-350. [DOI] [PubMed] [Google Scholar]
- 8.Chowdhury P S, Chang K, Pastan I. Mol Immunol. 1997;34:9–20. doi: 10.1016/s0161-5890(97)00011-4. [DOI] [PubMed] [Google Scholar]
- 9.Tang D C, DeVit M, Johnston S A. Nature (London) 1992;356:152–154. doi: 10.1038/356152a0. [DOI] [PubMed] [Google Scholar]
- 10.Donnelly J J, Ulmer J B, Liu M A. J Immunol Methods. 1994;176:145–152. doi: 10.1016/0022-1759(94)90308-5. [DOI] [PubMed] [Google Scholar]
- 11.Boyer J D, Wang B, Ugen K E, Agadjanyan M, Javadian A, Frost P, Dang K, Carrano R A, Ciccarelli R, Coney L, Williams W V, Weiner D B. J Med Primatol. 1996;25:242–250. doi: 10.1111/j.1600-0684.1996.tb00022.x. [DOI] [PubMed] [Google Scholar]
- 12.Marks J D, Hoogenboom H R, Griffiths A D, Winter G. J Biol Chem. 1992;267:16007–16010. [PubMed] [Google Scholar]
- 13.Marks J D, Winter G. Behring Inst Mitt. 1992;91:6–12. [PubMed] [Google Scholar]
- 14.Brinkmann U, Chowdhury P S, Roscoe D M, Pastan I. J Immunol Methods. 1995;182:41–50. doi: 10.1016/0022-1759(95)00016-4. [DOI] [PubMed] [Google Scholar]
- 15.Lin T-C, Webster R E, Konigsberg W. J Biol Chem. 1980;255:10331–10337. [PubMed] [Google Scholar]
- 16.Reiter Y, Brinkmann U, Kreitman R, Jung S-H, Lee B, Pastan I. Biochemistry. 1994;33:5451–5459. doi: 10.1021/bi00184a014. [DOI] [PubMed] [Google Scholar]
- 17.Brinkmann U, Pai L H, FitzGerald D J, Pastan I. Proc Natl Acad Sci USA. 1991;88:8616–8620. doi: 10.1073/pnas.88.19.8616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chaudhary V K, Queen C, Junghans R P, Waldmann T A, FitzGerald D J, Pastan I. Nature (London) 1989;339:394–397. doi: 10.1038/339394a0. [DOI] [PubMed] [Google Scholar]
- 19.Brinkmann U, Webber K, Di Carlo A, Beers R, Chowdhury P, Chang K, Chaudhary V, Gallo M, Pastan I. Int J Cancer. 1997;71:638–644. doi: 10.1002/(sici)1097-0215(19970516)71:4<638::aid-ijc21>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 20.Raz E, Tighe H, Sato Y, Corr M, Dudler J A, Roman M, Swain S L, Spiegelberg H L, Carson D A. Proc Natl Acad Sci USA. 1996;93:5141–5145. doi: 10.1073/pnas.93.10.5141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brinkmann U, Pastan I. Biochem Biophys Acta. 1994;1198:27–45. doi: 10.1016/0304-419x(94)90004-3. [DOI] [PubMed] [Google Scholar]
- 22.Sato Y, Roman M, Tighe H, Lee D, Corr M, Nguyen M D, Silverman G J, Lotz M, Carson D A, Raz E. Science. 1996;273:352–354. doi: 10.1126/science.273.5273.352. [DOI] [PubMed] [Google Scholar]
- 23.Buchner J, Pastan I, Brinkmann U. Anal Biochem. 1992;205:263–270. doi: 10.1016/0003-2697(92)90433-8. [DOI] [PubMed] [Google Scholar]
- 24.Reiter Y, Brinkmann U, Lee B, Pastan I. Nat Biotechnol. 1996;14:1239–1245. doi: 10.1038/nbt1096-1239. [DOI] [PubMed] [Google Scholar]
- 25.Reiter Y, Pai L H, Brinkmann U, Wang Q-C, Pastan I. Cancer Res. 1994;54:2714–2718. [PubMed] [Google Scholar]
- 26.Reiter Y, Kreitman R J, Brinkmann U, Pastan I. Int J Cancer. 1994;58:142–149. doi: 10.1002/ijc.2910580123. [DOI] [PubMed] [Google Scholar]
- 27.Reiter Y, Brinkmann U, Jung S-H, Lee B, King C R, Pastan I. J Biol Chem. 1994;269:18327–18331. [PubMed] [Google Scholar]