Abstract
We prospectively investigated the effects of adding carvedilol to the standard treatment of ischemic and nonischemic dilated cardiomyopathy (DCM), by measuring the plasma levels of pro-inflammatory cytokines. Sixty patients with DCM (35 ischemic and 25 nonischemic) were divided into 2 subgroups: patients on standard therapy alone (digoxin, angiotensin-converting enzyme inhibitors, and diuretics) and patients on standard therapy plus carvedilol. Study participants' serum levels of tumor necrosis factor-α (TNF-α), interleukin-2 (IL-2), and interleukin-6 (IL-6) were measured at the beginning and again at the end of the study. Left ventricular ejection fraction and left ventricular diastolic function were evaluated by means of radionuclide ventriculography.
In ischemic patients on carvedilol, levels of IL-6 and TNF-α dropped significantly (P= 0.028 and P=0.034, respectively). In ischemic patients on standard treatment, plasma IL-2 levels were elevated after treatment (P=0.047). No significant differences occurred in IL-6 levels, while TNF-α levels were elevated (P=0.008). In nonischemic patients on carvedilol, IL-6 and TNF-α levels dropped significantly (P=0.018 and P=0.004, respectively). The left ventricular ejection fraction increased significantly (P=0.006). In nonischemic patients on standard treatment, no significant change occurred in any value. Carvedilol suppressed the plasma levels of TNF-α and IL-6 in both ischemic and nonischemic patients. The carvedilol effect was more pronounced in patients with nonischemic dilated cardiomyopathy than in those with ischemic disease.
Key words: Biological markers/blood; cardiomyopathy, dilated; carbazoles/therapeutic use; carvedilol; cytokines/blood; heart failure, congestive; interleukin-2; interleukin-6; tumor necrosis factor-alpha; ventricular function, left/drug effects
Chronic heart failure is a progressive syndrome that is characterized by heart dysfunctions and increases in neurohormonal activity. During the past half century, as various physiopathologic mechanisms have been proposed for chronic heart failure, subsequent changes have occurred in its treatment. Prolonged activation of the sympathetic system is one of the proposed mechanisms.
Pro-inflammatory cytokines have been shown to contribute, by various mechanisms, to the alteration of cardiovascular functions such as left ventricular (LV) remodeling, contractile dysfunctions, and reduction of the myocardial β-adrenergic receptors.1 In heart failure (HF) patients, plasma levels of pro-inflammatory cytokines are higher and are positively correlated with the mortality rate.2 Pro-inflammatory cytokines are influential pleotropic endogenic peptides, which are produced by several cell types. Tumor necrosis factor-α (TNF-α), interleukin-2 (IL-2), and interleukin-6 (IL-6) are classified as pro-inflammatory cytokines.3
β-blockers, which were thought to be contraindicated for LV dysfunction, are now the 1st step in HF treatment. It has been shown that the addition of β-blockers—with or without angiotensin-converting enzyme (ACE) inhibitors, diuretics, and digoxin—to standard HF treatment reduced the mortality rate. This useful effect of β-blockers is thought to be due to their suppression of the sympathetic nervous system.4,5
Carvedilol is a nonselective β-blocker (β1/β2 rate, 7.3) with α-blocking, vasodilatory, and antioxidant effects.6 Recent reports have indicated that carvedilol use in dilated cardiomyopathy (DCM) patients reduced the severity of the ventricular dysfunction, increased the LV ejection fraction (EF), and consequently reduced the morbidity and mortality rates.6 It is not well known whether the useful effect of carvedilol includes the suppression of pro-inflammatory cytokines in addition to the suppression of the increased sympathetic activity. There are no data concerning the effect of carvedilol when it is added to standard therapy in patients with ischemic DCM and nonischemic DCM. In this study, we aimed to investigate the effects of carvedilol on LV functions and on the levels of pro-inflammatory cytokines in patients with ischemic and nonischemic DCM.
Patients and Methods
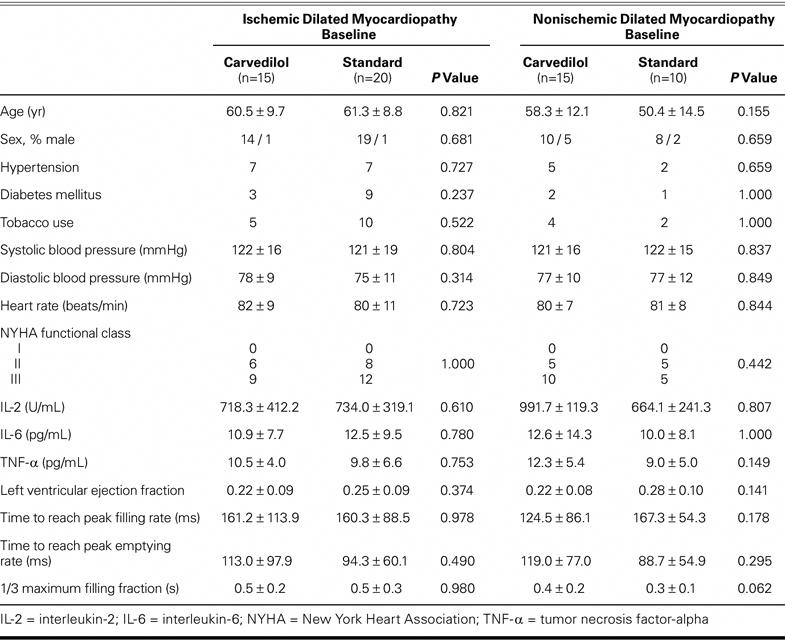
In this prospective study, 60 patients with ischemic or nonischemic DCM were treated in our department of cardiology as either inpatients or outpatients from February 2001 through November 2001. After approval of the study by our hospital's ethics committee, we obtained signed consent from all patients. Baseline characteristics of the study population at randomization are shown in Table I. There were no significant baseline differences between the groups. Entry criteria for this study included age between 30 and 70 years, an EF of 0.40 or lower, and symptomatic HF as a consequence of ischemic or nonischemic DCM. Exclusion criteria were chronic obstructive pulmonary disease, significant valvular heart disease, thyrotoxicosis, hypothyroidism, chronic kidney and liver diseases, malignancy, anemia (hemoglobin, <9 g/dL), systolic blood pressure lower than 90 mmHg, heart rate lower than 50 beats/min, and 1st- or 2nd-level heart block. Patients on antiarrhythmic drugs or β-blockers, with a permanent pacemaker, and with psychiatric problems were also excluded from the study. The ischemic DCM (n=35) and nonischemic DCM (n=25) groups were each divided into 2 subgroups: ischemic DCM on standard HF treatment (n=20), ischemic DCM on standard HF treatment plus carvedilol (n=15), nonischemic DCM on standard HF treatment (n=10), and nonischemic DCM on standard HF treatment plus carvedilol (n=15). For all patients, we recorded age; sex; presence of coronary artery disease, hypertension, diabetes mellitus, and hyperlipidemia; history of smoking; drugs in use now or in the past; New York Heart Association (NYHA) functional class; blood pressure; and heart rate. A standard HF treatment protocol was applied to all patients in the ischemic and nonischemic DCM groups. Carvedilol at an initial oral dose of 3.125 mg daily was added to the standard treatment in the subgroups of ischemic and nonischemic DCM patients who were assigned to receive carvedilol.
TABLE I. Baseline Characteristics of the Study Population
All patients (on carvedilol or not) were checked in our polyclinics every 2 weeks. The carvedilol dosage (in patients so assigned) could be doubled every 2 weeks, if the dosage was tolerated by the patients. Carvedilol treatment was monitored for the study period of 4 months. Functional class was considered to have improved if the patient's functional status increased by one or more grades of the NYHA classification. It was considered to have deteriorated if the functional class decreased by one or more grades, or if the patient died. Because all deaths were due to progression of HF, all patients who died were classified in NYHA functional class IV at the final follow-up.
Tumor Necrosis Factor-α, Interleukin-2, and Interleukin-6 Levels
To measure TNF-α, IL-2, and IL-6 concentrations, 15 mL of blood was drawn from an antecubital vein and collected into prechilled evacuated tubes containing ethylenediaminetetraacetic acid. Plasma was separated by centrifugation at 2,500 rpm for 12 minutes within 15 minutes of collection. Samples were stored at –70°C. Measurements of TNF-α, IL-2, and IL-6 were performed in undiluted plasma with a commercially available, enzyme-linked agent for immunoassay (Immulite, Diagnostic Products Corporation; Los Angeles, Calif). Three of these measurements were then averaged to obtain the plasma level. Determinations of TNF-α, IL-2, and IL-6 plasma levels were performed at baseline and then repeated 4 months after the random assignment of patients to groups and subgroups.
Radionuclide Study
We used a multiple-gated equilibrium cardiac-blood-pool scintigraphic technique (Siemens; Erlangen, Germany) to measure LVEF. Imaging was performed in the left anterior oblique projection, which provided the best septal separation of the ventricles with a 0° to 10° caudal tilt. Calculations of LV performance were made as described elsewhere, by means of the automatic edge-detection algorithm for the determination of LV borders.7 All studies were interpreted by a single observer blinded to the treatment assigned. We calculated the LVEF, the maximum emptying and filling velocities, the time to reach these velocities, the one-third maximum filling fraction, and the regional wall EF rates. Radionuclide studies were performed at baseline and then repeated 4 months after the randomization of patients.
Statistical Analysis
Continuous data were shown as mean ± SD. The suitability of continuous variables with the normal distribution was examined in the 1st study using the Kolmogorov-Smirnov test. The independent-sample t test and the paired-sample test were used in the analysis of the changes that were suitable with the normal distribution. The Mann-Whitney U and Wilcoxon tests were used to analyze changes that were not suitable with the normal distribution, and the χ2 or Fisher's exact test was used in analyzing categoric data. Data were analyzed using Minitab (Minitab Inc.; State College, Pa). Significance was assumed at a 2-tailed value of P <0.05.
Results
None of the 60 patients required discontinuation of the study medication. All patients during the study period received digoxin, 0.25 mg daily; furosemide, 80 mg daily; and enalapril, 10 mg twice daily. Coronary angiography showed normal coronary arteries in nonischemic DCM and abnormal coronary arteries in ischemic DCM patients. Of the ischemic DCM patients who received carvedilol, 1 patient died, and 1 patient developed permanent atrioventricular fibrillation (AF) during the study. Of the ischemic DCM patients who received standard treatment, on the other hand, 7 patients died and 3 developed permanent AF. A patient in the nonischemic DCM-with-standard-treatment group quit attending regular follow-up sessions, and permanent AF developed in 3 patients. Upon exclusion of the patients who either died or developed permanent AF, the study was completed in 13 ischemic DCM patients on carvedilol, 10 ischemic DCM patients on standard treatment, 15 nonischemic DCM patients on carvedilol, and 6 nonischemic DCM patients on standard treatment.
The results of all the subgroups at baseline and at the end of the study are presented in Table II. Carvedilol was received at an average dose of 28.3 ± 10.0 mg daily by the ischemic DCM patients. In ischemic DCM patients on carvedilol treatment, systolic blood pressure, diastolic blood pressure, and heart rate were found to be suppressed at the end of the study (P = 0.004, P = 0.010, and P = 0.001, respectively). Before carvedilol use, 6 patients were in NYHA functional class II and 7 patients were in class III; at the end of the study, 8 patients were in functional class I and 5 patients were in class II (P = 0.226). The initial LVEF (0.22 ± 0.08) improved to 0.27 ± 0.10, which, however, was not significant (P = 0.117). Similarly, there were no significant changes in LV diastolic functions. Although no significant changes occurred in IL-2 level, both IL-6 and TNF-α levels fell significantly (9.7 ± 7.2 vs 5.1 ± 0.4 pg/mL, P = 0.028; and 10.5 ± 4.0 vs 6.0 ± 4.9 pg/mL, P = 0.034, respectively).
TABLE II. Results at Baseline and at 4 Months for the Patients Who Completed the Study Period
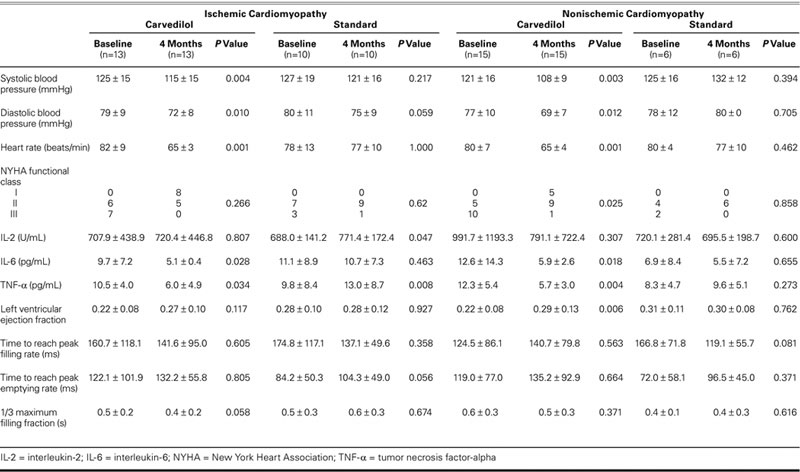
In ischemic DCM patients on standard treatment, no significant changes occurred in systolic and diastolic blood pressures, heart rate, and functional capacity at the end of the study. The plasma IL-2 level (688.0 ± 141.2 U/mL) was elevated to 771.4 ± 172.4 U/mL (P = 0.047). Although no significant changes occurred in the IL-6 level, the TNF-α level (9.8 ± 8.4 pg/mL) was elevated to 13.0 ± 8.7 pg/mL (P = 0.008). No significant changes occurred in the LVEF, the time to reach peak filling velocity, the time to reach peak emptying rate, and the 1/3 filling fraction. The comparisons of measurements obtained before and after carvedilol treatment in ischemic DCM patients are presented in Table II.
In the nonischemic DCM group, there were no significant differences between the standard-treatment and carvedilol subgroups in regard to age, sex, hypertension, diabetes mellitus, cigarette use, systolic and diastolic blood pressures, heart rate, pro-inflammatory cytokine levels, and LV systolic and diastolic functions as evaluated by means of radionuclide ventriculography (Table I). The nonischemic DCM patients were given carvedilol at an average dose of 28.4 ± 12.9 mg. The systolic blood pressure, diastolic pressure, and heart rate were found to be suppressed in nonischemic DCM patients on carvedilol treatment (P = 0.003, P = 0.012, and P = 0.001, respectively). At the beginning of the study, of the 15 nonischemic DCM patients, 10 were in NYHA functional class III and 5 were in class II; at the end of the study, 1 was in class III, 5 were in class II, and 9 were in class I. Although no changes occurred in IL-2 level, levels of IL-6 and TNF-α dropped significantly (from 12.6 ± 14.3 pg/mL to 5.9 ± 2.6 pg/mL, P = 0.018; and from 12.3 ± 5.4 pg/mL to 5.7 ± 3.0 pg/mL, P = 0.004, respectively). The LVEF rate improved from 0.22 ± 0.08 to 0.29 ± 0.13 (P = 0.006), but no changes occurred in numbers indicative of diastolic function. The comparisons of results obtained before and after carvedilol treatment in nonischemic DCM patients are presented in Table II.
In nonischemic DCM patients on standard treatment, no significant changes occurred in hemodynamic results, pro-inflammatory cytokine levels, and radionuclide ventriculography measurements at the end of the study (Table II). The changes in cytokine levels before and after treatment with carvedilol in ischemic and nonischemic DCM patients are shown in Figures 1–4.
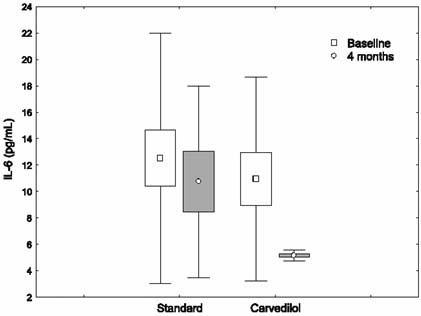
Fig. 1 The changes in interleukin-6 (IL-6) levels before and after treatment with carvedilol, in ischemic dilated cardiomyopathy patients.
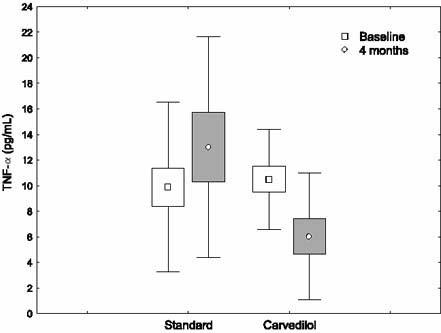
Fig. 2 The changes in tumor necrosis factor-α (TNF-α) levels before and after treatment with carvedilol, in ischemic dilated cardiomyopathy patients.
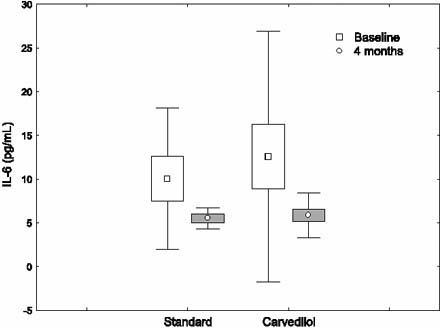
Fig. 3 The changes in interleukin-6 (IL-6) levels before and after treatment with carvedilol, in nonischemic dilated cardiomyopathy patients.
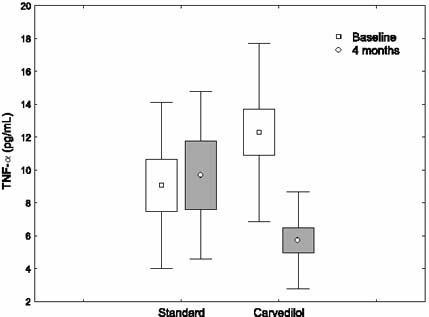
Fig. 4 The changes in tumor necrosis factor-α (TNF-α) levels before and after treatment with carvedilol, in nonischemic dilated cardiomyopathy patients.
During the course of the study, no patient in the nonischemic DCM groups died; however, 8 patients died in the ischemic DCM groups (P = 0.016). Atrioventricular fibrillation developed in 4 ischemic DCM patients and in 3 nonischemic DCM patients (P = 0.625). Of the 8 deaths that occurred among ischemic DCM patients, 7 were in the standard-treatment group and 1 was in the carvedilol group. All deaths were due to the progression of HF. In the group of ischemic DCM patients on standard treatment, the incidence of death was higher than it was among ischemic DCM patients on carvedilol treatment, but this difference was not statistically significant (P = 0.101).
Discussion
Carvedilol improves the functional capacity of HF patients by increasing the LVEF and cardiac output and by decreasing the heart rate and pulmonary capillary wedge pressure.6 Metra and coworkers8 reported in a clinical study of 40 patients who had NYHA class II to IV HF that the addition of carvedilol to the standard HF treatment (ACE inhibitors, diuretics, and digoxin) improved the effort capacity of patients and decreased the severity of HF.8 In our study, before carvedilol use by 13 ischemic DCM patients, 6 were in NYHA functional class II and 7 were in class III. After 4 months of carvedilol treatment, 8 patients were in class I and 5 were in class II (P = 0.266). Of the 15 nonischemic DCM patients on carvedilol, 5 were in class II and 10 were in class III before carvedilol use. After 4 months of carvedilol treatment, functional capacity significantly improved: 5 patients were in class I, 9 patients were in class II, and 1 patient was in class III (P = 0.025). In both ischemic and nonischemic DCM patients on standard treatment, no significant changes were observed in functional capacity. The probable reason for the less pronounced effects of carvedilol on ischemic DCM patients is that necrotic areas, which have no function as β-adrenergic receptors, are relatively larger in ischemic DCM patients than in nonischemic DCM patients.
Olsen and coworkers9 reported in a clinical study of 60 DCM patients (ischemic and nonischemic) who were given carvedilol twice daily—at a starting dose of 3.125 mg with gradual increases up to 25 to 50 mg during 4 months of treatment—that the LVEF and stroke volume index were significantly increased and that pulmonary capillary wedge pressure was reduced, with decreases in the severity of HF. Similarly, in our study, the LVEF was found to be significantly increased in nonischemic DCM patients (P = 0.006). The LVEF in ischemic DCM patients also improved, but not significantly so (P = 0.117). In ischemic and nonischemic DCM patients on standard HF treatment, no changes occurred in the LVEF.
Plasma levels of TNF-α, IL-1, IL-2, IL-6, and IL-10 are elevated in chronic HF patients. Levine and colleagues10 evaluated the plasma TNF-α levels in patients with NYHA class III and IV HF. In that study, plasma TNF-α levels of 33 HF patients and 33 healthy age-paired individuals were measured. The TNF-α level of the HF patients (115 ± 25 U/mL) was significantly higher (P <0.001) than that of healthy individuals (9 ± 3 U/mL). Moreover, the TNF-α level was much higher in patients with the most severe HF. A search of the Left Ventricular Dysfunction Studies database revealed that TNF-α levels in HF patients with NYHA class I, II, and III HF were 1.95 ± 0.54 pg/mL, 2.60 ± 0.48 pg/mL, and 6.4 ± 1.9 pg/mL, respectively, or 0.75 ± 0.05 pg/mL higher than those of age-paired healthy individuals.11 The IL-6 levels in patients with NYHA class I, II, and III HF were 3.3 ± 0.5 pg/mL, 6.2 ± 0.48 pg/mL, and 5.2 ± 0.9 pg/mL, respectively, or 1.8 ± 0.5 pg/mL higher than those of age-paired healthy individuals. Cytokine levels were found to be lower in 14 patients with idiopathic DCM who were being treated with a mechanical device implanted on the LV.12 When cytokine analyses were done 24 hours before implantation, 11 of the 14 patients (79%) had elevated levels of IL-6, 10 (71%) had elevated levels of IL-8, and 2 (14%) had elevated levels of TNF-α. Thirty days after the mechanical-aid implantation, the LVEF was improved and there were decreases in IL-6 and IL-8 levels; however, no significant changes occurred in TNF-α levels. Matsumura and associates13 reported that the addition of carvedilol to the standard treatment of 9 patients with idiopathic DCM (in NYHA class II) caused a significant drop in IL-6 level, while TNF-α level was not affected. Othsuka and colleagues14 showed that serum levels of IL-10, TNF-α, and sTNF-R2 significantly decreased during β-blocker therapy.
Our study included enough larger numbers of ischemic and nonischemic DCM patients that the addition of carvedilol to standard HF treatment significantly lowered the plasma levels of IL-6 and TNF-α in both of our ischemic and nonischemic groups.
In a study conducted by Sliwa and associates15 on 28 patients, 14 patients received a 400-mg daily or twice-daily dose of pentoxifylline, a xanthine derivative, while the other 14 received a placebo. After a follow-up period of 6 months, the TNF-α level was suppressed and functional capacity and LVEF were improved in the pentoxifylline group. In another study, Skudicky and coworkers16 used ACE inhibitors, digoxin, and carvedilol for at least 3 months in treating 39 patients with idiopathic DCM and LVEF lower than 0.40. One group (n=20) was given pentoxifylline and the other group (n=19) was given a placebo. At the end of 6 months, the LV end-systolic diameter was decreased in the pentoxifylline group and the LVEF was increased, while the LV end-diastolic diameter was not changed. The TNF-α level was not significantly (P >0.05) changed, either; the initial TNF-α level, 2.5 ± 1.8 pg/mL, dropped slightly to 2.3 ± 1.8 pg/mL. We could find no study that demonstrated definitively the effects of carvedilol on the suppression of cytokines in patients with ischemic or nonischemic DCM; however, we can make inferences from related studies. In Sliwa's study,15 pentoxifylline improved LV functions and suppressed cytokine levels in DCM patients; however, in Skudicky's study,16 with a similar experimental design, pentoxifylline did not affect cytokine levels. In this latter study, patients had been receiving carvedilol longer than 3 months before pentoxifylline use. Therefore, carvedilol might already have suppressed the cytokine levels, before pentoxifylline administration. We designed our study to determine whether the useful effects of carvedilol are also related to the suppression of cytokines in patients with ischemic DCM, versus nonischemic DCM. Subsequently, we found that TNF-α and IL-6 levels declined significantly (P = 0.001) in both groups after 4 months of carvedilol treatment. Moreover, in patients with ischemic DCM who were on standard treatment, plasma TNF-α and IL-2 levels were significantly elevated (P = 0.008 and P = 0.047, respectively).
Bristow and colleagues17 administered 6.25 to 25 mg of carvedilol to their DCM patients, who were followed up for 6 months. Of 49 patients with ischemic DCM on standard treatment, 9 died, whereas 7 patients died among 133 with ischemic DCM in the carvedilol group (P = 0.003). Of 38 patients with nonischemic DCM on standard treatment, 4 died, while 5 of 128 patients with nonischemic DCM on carvedilol died (P = 0.11). In our study, all of the deaths (8 patients) occurred in the ischemic DCM groups (P = 0.016). No patient died in the nonischemic DCM groups. Of the 8 deaths, only 1 patient (7%) was in the carvedilol group and 7 patients (35%) were in the standard-treatment group. Thus, the mortality rate was lower in the carvedilol group, but not significantly so (P = 0.101). The reason for our not observing a significant difference in the mortality rate might be the low number of patients in our study or the short period of study.
Krum and associates18 showed that the severity of ventricular tachycardia and ventricular fibrillation were remarkably reduced in HF patients who received carvedilol. Regardless of whether they had AF or sinus rhythm, DCM patients on carvedilol showed improvements in LV functions and in functional capacity.19 However, we have found no report that carvedilol prevents the development of AF in DCM patients who are in sinus rhythm. In our study, AF developed in 4 ischemic and 3 nonischemic DCM patients (P = 0.625). In ischemic DCM patients on carvedilol, AF developed in just 1 patient (7%); in ischemic DCM patients on standard treatment, AF developed in 3 patients (15%). However, the incidence of AF was not statistically different between the 2 groups (P = 0.619). A major limitation of this study was the small number of patients.
Conclusion
Carvedilol suppresses the plasma levels of TNF-α and IL-6 in both ischemic and nonischemic DCM patients. It also improves the functional capacity and the LVEF significantly in nonischemic DCM patients and insignificantly in ischemic DCM patients. Overall, carvedilol seems to be more useful in nonischemic DCM patients than in ischemic DCM patients. In our study, carvedilol appeared to reduce the mortality rate in ischemic DCM patients, but by an insignificant amount. Our results need to be confirmed in a larger series of patients.
Footnotes
Address for reprints: Turhan Kurum, MD, Department of Cardiology, Trakya University School of Medicine, Gullapoglu Yerleskesi, 22030 Edirne, Turkey. E-mail: atkurum@trakya.edu.tr
References
- 1.Pagani FD, Baker LS, I C, Knox M, Fink MP, Visner MS. Left ventricular systolic and diastolic dysfunction after infusion of tumor necrosis factor-alpha in conscious dogs. J Clin Invest 1992;90:389–98. [DOI] [PMC free article] [PubMed]
- 2.Deswal A, Petersen NJ, Feldman AM, Young JB, White BG, Mann DL. Cytokines and cytokine receptors in advanced heart failure: an analysis of the cytokine database from the Vesnarinone trial (VEST). Circulation 2001;103:2055–9. [DOI] [PubMed]
- 3.Mann DL, Young JB. Basic mechanisms in congestive heart failure. Recognizing the role of proinflammatory cytokines. Chest 1994;105:897–904. [DOI] [PubMed]
- 4.Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet 1999;353:2001–7. [PubMed]
- 5.Packer M, Bristow MR, Cohn JN, Colucci WS, Fowler MB, Gilbert EM, Shusterman NH. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. U.S. Carvedilol Heart Failure Study Group. N Engl J Med 1996;334:1349–55. [DOI] [PubMed]
- 6.Gilbert EM, Abraham WT, Olsen S, Hattler B, White M, Mealy P, et al. Comparative hemodynamic, left ventricular functional, and antiadrenergic effects of chronic treatment with metoprolol versus carvedilol in the failing heart. Circulation 1996;94:2817–25. [DOI] [PubMed]
- 7.Reiber JH. Quantitative analysis of left ventricular function from equilibrium gated blood pool scintigrams: an overview of computer methods. Eur J Nucl Med 1985;10:97–110. [DOI] [PubMed]
- 8.Metra M, Nardi M, Giubbini R, Dei Cas L. Effects of short- and long-term carvedilol administration on rest and exercise hemodynamic variables, exercise capacity and clinical conditions in patients with idiopathic dilated cardiomyopathy. J Am Coll Cardiol 1994;24:1678–87. [DOI] [PubMed]
- 9.Olsen SL, Gilbert EM, Renlund DG, Taylor DO, Yanowitz FD, Bristow MR. Carvedilol improves left ventricular function and symptoms in chronic heart failure: a double-blind randomized study. J Am Coll Cardiol 1995;25:1225–31. [DOI] [PubMed]
- 10.Levine B, Kalman J, Mayer L, Fillit HM, Packer M. Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. N Engl J Med 1990;323:236–41. [DOI] [PubMed]
- 11.Torre-Amione G, Kapadia S, Benedict C, Oral H, Young JB, Mann DL. Proinflammatory cytokine levels in patients with depressed left ventricular ejection fraction: a report from the Studies of Left Ventricular Dysfunction (SOLVD). J Am Coll Cardiol 1996;27:1201–6. [DOI] [PubMed]
- 12.Goldstein DJ, Moazami N, Seldomridge JA, Laio H, Ashton RC Jr, Naka Y, et al. Circulatory resuscitation with left ventricular assist device support reduces interleukins 6 and 8 levels. Ann Thorac Surg 1997;63:971–4. [DOI] [PubMed]
- 13.Matsumura T, Tsushima K, Ohtaki E, Misu K, Tohbaru T, Asano R, et al. Effects of carvedilol on plasma levels of interleukin-6 and tumor necrosis factor-alpha in nine patients with dilated cardiomyopathy. J Cardiol 2002;39:253–7. [PubMed]
- 14.Ohtsuka T, Hamada M, Hiasa G, Sasaki O, Suzuki M, Hara Y, et al. Effect of β-blockers on circulating levels of inflammatory and anti-inflammatory cytokines in patients with dilated cardiomyopathy. J Am Coll Cardiol 2001;37:412–7. [DOI] [PubMed]
- 15.Sliwa K, Skudicky D, Candy G, Wisenbaugh T, Sareli P. Randomised investigation of effects of pentoxifylline on left-ventricular performance in idiopathic dilated cardiomyopathy. Lancet 1998;351:1091–3. [DOI] [PubMed]
- 16.Skudicky D, Bergemann A, Sliwa K, Candy G, Sareli P. Beneficial effects of pentoxifylline in patients with idiopathic dilated cardiomyopathy treated with angiotensin-converting enzyme inhibitors and carvedilol: results of a randomized study. Circulation 2001;103:1083–8. [DOI] [PubMed]
- 17.Bristow MR, Gilbert EM, Abraham WT, Adams KF, Fowler MB, Hershberger RE, et al. Carvedilol produces dose-related improvements in left ventricular function and survival in subjects with chronic heart failure. MOCHA Investigators. Circulation 1996;94:2807–16. [DOI] [PubMed]
- 18.Krum H, Sackner-Bernstein JD, Goldsmith RL, Kukin ML, Schwartz B, Penn J, et al. Double-blind, placebo-controlled study of the long-term efficacy of carvedilol in patients with severe chronic heart failure. Circulation 1995;92:1499–506. [DOI] [PubMed]
- 19.Fung JW, Chan SK, Yeung LY, Sanderson JE. Is β-blockade useful in heart failure patients with atrial fibrillation? An analysis of data from two previously completed prospective trials. Eur J Heart Fail 2002;4:489–94. [DOI] [PubMed]


