Abstract
Postoperative tricuspid valve regurgitation is moderate to severe in 15% to 20% of heart transplant recipients despite use of the bicaval surgical technique. We hypothesized that the regurgitation might be partly due to increased tension on the donor right atrium.
To study the right atrial distortion, we modified the standard bicaval anastomosis. Our technique involves augmenting the donor right atrial anterior wall with a flap of the recipient's right atrium, which is left attached in continuity with the anterior aspect of the inferior vena cava along 65% of its circumference.
We measured tricuspid regurgitation, right atrial area, and right atrioventricular diameter in 7 consecutive patients who underwent orthotopic heart transplantation with the modified anastomosis. Tricuspid regurgitation was graded as follows: 1 = trace, <10%; 2 = mild, 10%–24%; 3 = moderate, 25%–50%; and 4 = severe, >50%.
All patients were weaned from inotropic support within 1 week after transplantation with excellent ventricular function, no heart block, and 100% survival at 30 days. The median follow-up time was 173 days (44–358 days). Other median measurements included tricuspid valve regurgitation jet area, 0.30 cm2 (0–1.90 cm2); right atrial area, 15.90 cm2 (14.47–18.00 cm2); atrioventricular diameter, 2.70 cm (2.63–3.09 cm); and tricuspid regurgitation, 1.67% (0–12.42%). Mild regurgitation occurred in 1 recipient; in all others, it was trace.
The modified inferior vena caval anastomosis is simple and safe. It eliminates moderate and severe tricuspid valve regurgitation without routine annuloplasty after orthotopic heart transplantation via the bicaval technique.
Key words: Anastomosis, surgical/methods; bicaval anastomosis; echocardiography methods; heart atria/surgery; heart transplantation/adverse effects/pathology/methods/physiology; humans; tricuspid valve/surgery; tricuspid valve insufficiency/prevention & control; vena cava, inferior/surgery
Tricuspid valve regurgitation (TR) is recognized as a concern after orthotopic heart transplantation (OHT). Shown to correlate with the development of right-sided heart failure, TR has a negative influence on outcome.1,2 Although TR can be treated medically, more severe cases frequently require surgical intervention.3 Surgical techniques such as bicaval anastomosis or tricuspid valve annuloplasty at the time of OHT have been shown to minimize the incidence of TR.4–7
The technique described herein has been developed as a result of several intraoperative observations. Enlargement of the recipient's native failing heart increases all dimensions of the pericardial space, including the distance between the superior and inferior venae cavae. The traditional left atrial anastomosis affixes the posterior aspect of the right atrium (RA), which potentially limits the reach of the donor inferior vena cava (IVC) to the recipient's (Fig. 1A). This limitation can result in stress on the right-sided chambers of the donor heart. The resultant stretch on the anterior wall of the right atrium may cause tension on the tricuspid valve annulus (Fig. 1B), leading to valve incompetence. Such strain is easily corrected in the superior vena caval anastomosis, but not necessarily in that of the IVC. We hypothesized that relieving this tension could minimize distortion of the tricuspid valve, thereby reducing regurgitation. Herein, we review the results in patients who underwent our modified IVC anastomosis, which attempts to avoid RA and tricuspid valve distortion.
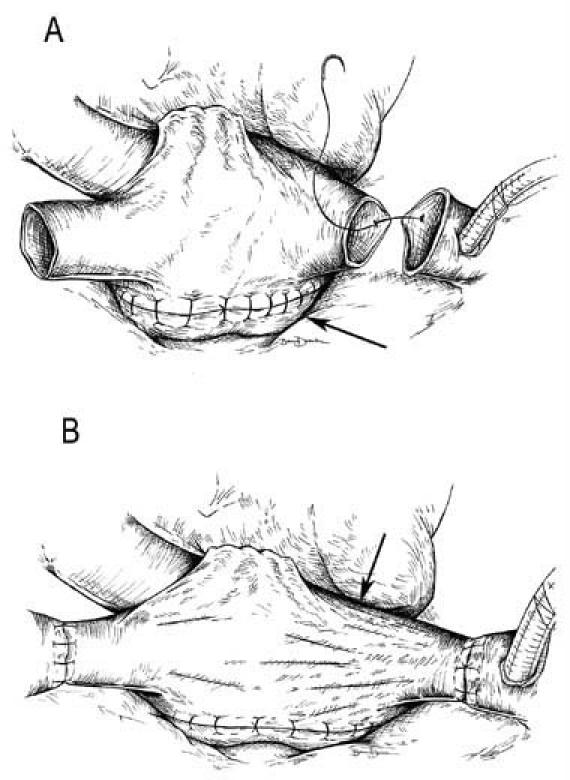
Fig. 1 Right atrial tension in the traditional bicaval anastomosis. A) The left atrial anastomosis affixes the posterior aspect of the right atrial septum (arrow). B) Potential distortion of the donor right atrium due to increased distances in the recipient pericardium results in tension on the tricuspid valve annulus (arrow).
Patients and Methods
The lead author (DM) has been using this modified IVC anastomosis since 1997.
This retrospective review includes 7 consecutive recipients at Thomas Jefferson University Hospital during the first 13 months of its heart transplantation program (November 2004–November 2005). Institutional Review Board approval to review the records was obtained. Only the records of routine studies were analyzed for the purposes of this study.
Patients' Demographics
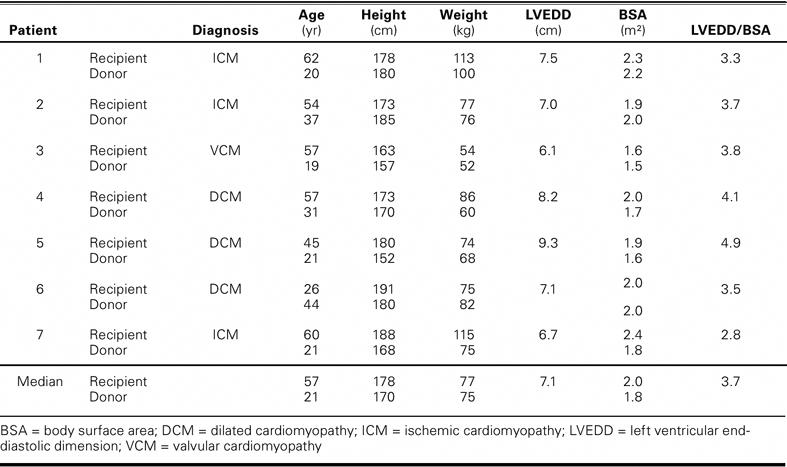
All OHT recipients were men. Their median age at the time of transplantation was 57 years (range, 26–62 yr). The indications for transplantation were dilated cardiomyopathy (3 patients), ischemic cardiomyopathy (3), and valvular cardiomyopathy (1). The characteristics of the recipients and donors are summarized in Table I.
TABLE I. Recipient and Donor Characteristics
Organ Preservation, Surgical Technique, and Postoperative Care
Donor hearts were preserved, transported, and transplanted as described by Marelli and colleagues.8 The donor hearts were pretreated with thyroid hormone. Then, University of Wisconsin solution (a total of 10–12 cc per kg of the donor's weight) was infused into each heart at a pressure of 80 mmHg, until arrest occurred. After arrest, infusion was maintained for 8 min at 50 mmHg. For transport, the donor hearts were immersed in University of Wisconsin solution and packed in ice at 4°C. When the hearts reached their destination, cold Plasma-Lyte® A solution (Baxter Healthcare Corporation; Deerfield, Ill) was infused into the left ventricles to aid in topical cooling and the removal of air during implantation. Reperfusion was performed with leukocyte-depleted, aspartate-glutamate–enriched Buckberg cardioplegic solution for 3 min at 150 to 225 cc/min. Before the aortic clamps were released, the cardioplegic solution was followed with leukocyte-depleted warm blood for 10 min at a flow rate similar to that used during reperfusion.
The standard bicaval surgical technique was modified as follows: the IVC anastomosis was modified by the attachment of a triangular flap of the recipient's RA tissue to the anterior aspect of the native IVC remnant. The length of the attached edge of the flap was at least 65% of the circumference of the recipient's native IVC. Following the line of a conventional RA incision, we incised the donor RA from the corresponding point on the IVC anteriorly toward the RA appendage, away from the sinoatrial node. The flap was then incorporated into the continuous suture line to augment the donor RA (Fig. 2).
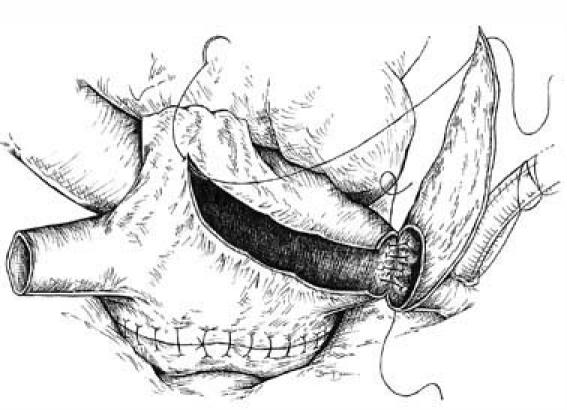
Fig. 2 Modified inferior vena caval anastomosis. A flap of the recipient's right atrium, left attached to the native inferior vena cava, is used to augment the donor right atrium. The flap is then closed by extending the continuous suture line.
After transplantation, all patients were placed on inotropic support for at least 48 hours. They received standard triple immunosuppression (cyclosporine, mycophenolic acid, and prednisone) and prophylactic antibiotics per standard protocol. All patients underwent routine right heart catheterization and biopsy weekly for the first 4 to 6 postoperative weeks.
Study Design and Echocardiographic Follow-Up
A single experienced practitioner performed the echocardiography at least 30 days after transplantation. Short and long axes in 2-dimensional mode, M-mode, and color-flow Doppler echocardiography were used to calculate jet area, RA area, and right atrioventricular annulus diameter. The tricuspid regurgitation percentage (TR%) was calculated as (jet area/RA area) × 100. The TR% was graded as <10% = trace (grade 1), 10% to 24% = mild (grade 2), 25% to 50% = moderate (grade 3), and >50% = severe (grade 4).9 All studies were interpreted by a single observer (DZ). Echocardiographic measurements were done in triplicate at each time point.
Statistical Analysis
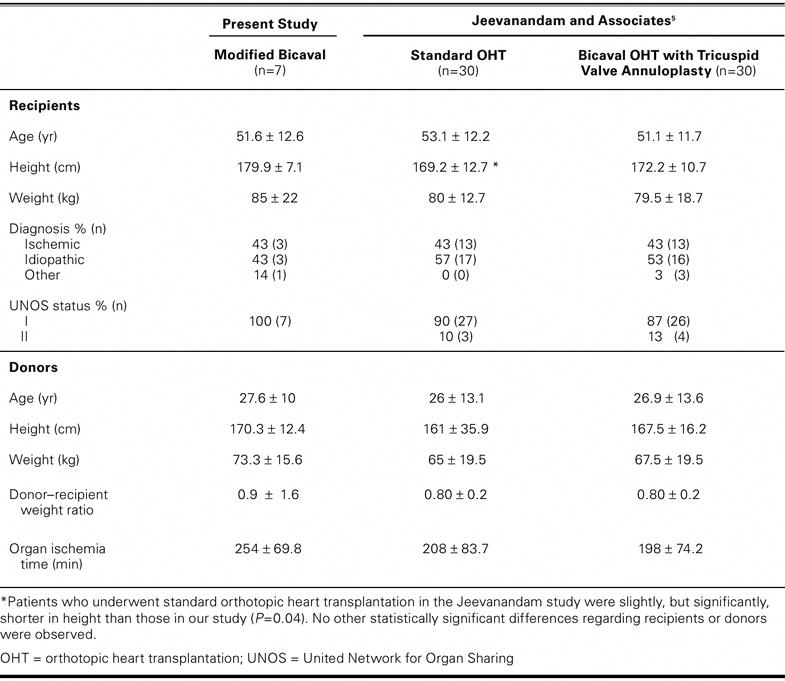
The surgical team's usual technique formed the basis of this observational study. The outcomes were compared with those of previously published reports. This comparison is justified by the similarity between the patients presented herein and those studied previously (Table II). Patient populations were compared using Student's t-test. Univariate linear regression analysis was performed by means of STATA statistical software, release 6.0 (StataCorp LP; College Station, Tex) to determine possible correlations between degree of TR and recipients' height, weight, and body surface area (BSA); the corresponding donor–recipient ratios; tricuspid valve annulus diameter; donors' age; ischemia time; and recipients' left ventricular end-diastolic dimension indices. A P value of ≥0.05 was considered significant. The BSA values were calculated by use of the Dubois equation.10
TABLE II. Characteristics of Patients Enrolled in Present Study Compared with Those Reported by Jeevanandam and Associates5
Results
The 30-day survival rate was 100%. There was no incidence of heart block. One patient died on postoperative day 51 of sepsis after small-bowel resection for a previously undiagnosed bleeding stromal tumor. No episodes of hemodynamically significant rejection occurred. Overall, no statistically significant preoperative demographic or clinical difference was observed between the patients described in previous studies5 and those presented herein (Table II).
The last follow-up transthoracic echocardiograms of the 7 consecutive recipients who underwent the modified bicaval OHT during the enrollment period were reviewed (Table III). The median follow-up time at echocardiographic observation was 173 days (range, 44–358 days). The median TR% was 1.67% (range, 0–12.42%). One recipient had mild TR; all others had trace TR. The median TR grade was 1, indicating trace TR. Four of the recipients were analyzed at 2 time points after transplantation. Two of these patients presented with trace TR at both examinations, and the other 2 improved from mild to trace TR. Except for the patient who died at 51 days, all recipients had trace regurgitation of less than 6% (Table III) at the end of the follow-up period.
TABLE III. Tricuspid Valve Regurgitation
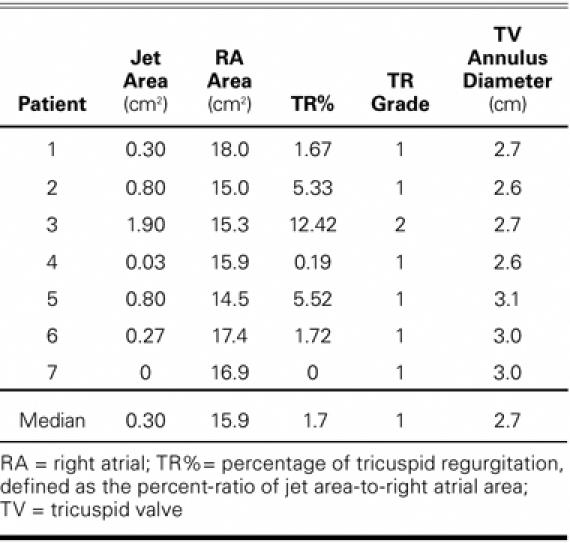
The median number of biopsies was 12 (range, 1–17). No correlation was found between the number of biopsies and TR. To determine whether a recipient and donor size-mismatch could explain the progression of TR, single linear regression was used to examine the relationship between TR and pertinent donor–recipient ratios. The median donor–recipient height ratios were 0.94 (range, 0.84–1.07) and weight ratios were 0.92 (range, 0.65–1.08). The median BSA ratio was 0.96 (range, 0.77–1.18). No significant correlations were found between TR% and recipient height, weight, or left ventricular end-diastolic dimension; donor–recipient weight, BSA, or height ratios; tricuspid valve annulus diameter; donor age; or ischemia time. Linear regression analysis showed that the TR% was possibly related to the annulus size/recipient weight index with a regression coefficient of 3.28 (95% confidence interval; range, 0.69–6.85; P = 0.026; R2 = 0.55).
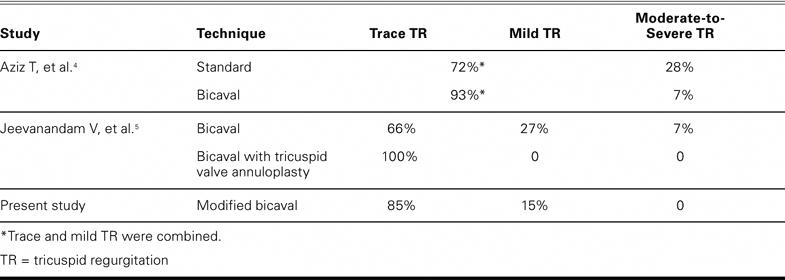
Table IV compares the results of this technique with those of other published techniques. In the present study, recipients did not exhibit any moderate or severe TR.
TABLE IV. Comparison of the Incidence of Tricuspid Regurgitation among Various Studies Using Different Heart Transplantation Techniques
Discussion
Development of TR after OHT is associated with morbidity and death. Even mild intraoperative TR (≥ grade 2) decreases long-term survival after OHT.1 Long-term moderate-to-severe TR leads to right-sided heart failure and potential kidney and liver failure.2,11 Determining the origin of TR after OHT is complex. Proposed mechanisms include atrial dysfunction, asynchronous contraction of the donor and recipient RA remnants, and distortion of the RA geometry.12,13 The bicaval anastomosis ameliorates TR by decreasing the RA distortion that can occur with the standard biatrial technique. The bicaval technique reduces the incidence of moderate-to-severe TR after OHT from 35% to 17% of patients.4 Some studies have shown a possible link between the number of endomyocardial biopsies and the development of TR, leading some researchers to suggest limiting the number of endomyocardial biopsies, if possible.14,15
Recently, prophylactic tricuspid valve annuloplasty has been used to decrease TR in both the standard biatrial and the bicaval anastomosis.5,16 In a 1-year follow-up of tricuspid valve annuloplasty after bicaval OHT, all patients had ≥ grade 1 TR.5,16 Although those results are promising, annuloplasty adds to the complexity of the operation. Prophylactic annuloplasty introduces these risks: addition of a foreign body, which increases the possibility of infection; additional manipulation at the time of OHT; and possible iatrogenic injury to the atrioventricular node. Ideally, such hazards could be avoided by applying annuloplasty selectively. There is no accepted way to reliably make this prediction at present.
Our modified IVC anastomosis has been used extensively by author Daniel Marelli since 1997, but it was not formally studied until now. The modified procedure avoids possible stretching of the donor RA during OHT when the donor and recipient superior vena cava and IVC are joined; such stretching can occur with the standard bicaval technique. Augmentation of the implanted RA with a patch of recipient RA restores a tension-free geometry to the right-sided atrioventricular junction of the donor heart in an often-dilated pericardial space. The donor RA is allowed to relax toward the atrioventricular groove that marks the location of the tricuspid valve, enabling improved RA function and decreased TR. Because the incision is made toward the right atrial appendage, away from the sinoatrial node, none of our patients required permanent pacing. In addition, the donor RA incision can facilitate access to the tricuspid valve if annuloplasty is needed to complement the modified IVC anastomosis.
Dandel and associates17 described an augmentation similar to ours, for use with the standard biatrial cuff anastomosis; their technique reduced the incidence of moderate or severe TR to 12.7%, compared with the previously published 35% after use of the biatrial technique. Our procedure results in significantly less TR than does the standard bicaval anastomosis for OHT, which has a low, but not absent, 17% incidence of moderate or severe TR (Table IV).4 None of our patients had moderate or severe TR, and 85% of our patients had only trace TR, indicating that our technique compares favorably with annuloplasty in limiting post-transplantation TR. Mild TR was observed in the recipient with the shortest follow-up time, and he also had valvular cardiomyopathy. One can speculate that such recipients have a tendency toward higher or reactive pulmonary vascular resistance and would therefore benefit from selective use of annuloplasty. Of note, 2 recipients showed improvement from mild to trace TR over time.
We predict that our results will remain consistent when our technique is applied to larger numbers of patients. The patients enrolled in our study had baseline characteristics similar to those in studies with larger sample sizes. Although the small numbers in our study did not permit definitive prediction of TR, the patients presented herein had a narrow range of tricuspid annulus size (between 26 and 30 mm). There was a possible correlation found between TR% and the annulus–recipient weight index. Additional prospective studies with larger numbers of patients will better reveal whether there is a consistent way to predict TR after bicaval anastomosis, which could suggest an endpoint by which to measure tricuspid valve distortion among the various right atrial connection techniques.
Acknowledgment
We would like to thank Brandice Durkan for drawing the figures presented herein.
Footnotes
Address for reprints: Scott C. Silvestry, MD, College Building, Suite 607, 1025 Walnut St., Philadelphia, PA 19107. E-mail: scott.silvestry@jefferson.edu
Mr. Dempsey is a National Institutes of Health, Heart, Blood, and Lung Medical Student Research Fellow (NIH-T35-HL07845-06A1).
References
- 1.Anderson CA, Shernan SK, Leacche M, Rawn JD, Paul S, Mihaljevic T, et al. Severity of intraoperative tricuspid regurgitation predicts poor late survival following cardiac transplantation. Ann Thorac Surg 2004;78:1635–42. [DOI] [PubMed]
- 2.Aziz TM, Saad RA, Burgess MI, Campbell CS, Yonan NA. Clinical significance of tricuspid valve dysfunction after orthotopic heart transplantation. J Heart Lung Transplant 2002; 21:1101–8. [DOI] [PubMed]
- 3.Filsoufi F, Salzberg SP, Anderson CA, Couper GS, Cohn LH, Adams DH. Optimal surgical management of severe tricuspid regurgitation in cardiac transplant patients. J Heart Lung Transplant 2006;25:289–93. [DOI] [PubMed]
- 4.Aziz T, Burgess M, Khafagy R, Wynn Hann A, Campbell C, Rahman A, et al. Bicaval and standard techniques in orthotopic heart transplantation: medium-term experience in cardiac performance and survival. J Thorac Cardiovasc Surg 1999;118:115–22. [DOI] [PubMed]
- 5.Jeevanandam V, Russell H, Mather P, Furukawa S, Anderson A, Grzywacz F, Raman J. A one-year comparison of prophylactic donor tricuspid annuloplasty in heart transplantation. Ann Thorac Surg 2004;78:759–66. [DOI] [PubMed]
- 6.Forni A, Faggian G, Chiominto B, Perini G, Bertolini P, Zanini M, Mazzucco A. Avoidance of atrioventricular valve incompetence following orthotopic heart transplantation using direct bicaval anastomosis. Transplant Proc 1995;27:3478–82. [PubMed]
- 7.Traversi E, Pozzoli M, Grande A, Forni G, Assandri J, Vigano M, Tavazzi L. The bicaval anastomosis technique for orthotopic heart transplantation yields better atrial function than the standard technique: an echocardiographic automatic boundary detection study. J Heart Lung Transplant 1998; 17:1065–74. [PubMed]
- 8.Marelli D, Laks H, Kobashigawa JA, Bresson J, Ardehali A, Esmailian F, et al. Seventeen-year experience with 1,083 heart transplants at a single institution. Ann Thorac Surg 2002; 74:1558–67. [DOI] [PubMed]
- 9.Mugge A, Daniel WG, Herrmann G, Simon R, Lichtlen PR. Quantification of tricuspid regurgitation by Doppler color flow mapping after cardiac transplantation. Am J Cardiol 1990;66:884–7. [DOI] [PubMed]
- 10.Du Bois D, Du Bois EF. A formula to estimate the approximate surface area if height and weight be known. 1916. Nutrition 1989;5:303–13. [PubMed]
- 11.Chan MC, Giannetti N, Kato T, Kornbluth M, Oyer P, Valantine HA, et al. Severe tricuspid regurgitation after heart transplantation. J Heart Lung Transplant 2001;20:709–17. [DOI] [PubMed]
- 12.Chen JM, Sinha P, Rajasinghe HA, Suratwala SJ, McCue JD, McCarty MJ, et al. Do donor characteristics really matter? Short- and long-term impact of donor characteristics on recipient survival, 1995–1999. J Heart Lung Transplant 2002; 21:608–10. [DOI] [PubMed]
- 13.Angermann CE, Spes CH, Tammen A, Stempfle HU, Schutz A, Kemkes BM, Theisen K. Anatomic characteristics and valvular function of the transplanted heart: transthoracic versus transesophageal echocardiographic findings. J Heart Transplant 1990;9:331–8. [PubMed]
- 14.Nguyen V, Cantarovich M, Cecere R, Giannetti N. Tricuspid regurgitation after cardiac transplantation: how many biopsies are too many? J Heart Lung Transplant 2005;24(7 Suppl):S227–31. [DOI] [PubMed]
- 15.Hausen B, Albes JM, Rohde R, Demertzis S, Mugge A, Schafers HJ. Tricuspid valve regurgitation attributable to endomyocardial biopsies and rejection in heart transplantation. Ann Thorac Surg 1995;59:1134–40. [DOI] [PubMed]
- 16.Brown NE, Muehlebach GF, Jones P, Gorton ME, Stuart RS, Borkon AM. Tricuspid annuloplasty significantly reduces early tricuspid regurgitation after biatrial heart transplantation. J Heart Lung Transplant 2004;23:1160–2. [DOI] [PubMed]
- 17.Dandel M, Hummel M, Loebe M, Weng Y, Muller J, Buz S, et al. Right atrial geometry and tricuspid regurgitation after orthotopic heart transplantation: benefits of a modified biatrial surgical technique. J Heart Lung Transplant 2001;20: 246–7. [DOI] [PubMed]


