Abstract
Chylopericardium after an intrapericardial procedure is rare, and satisfactory explanations of its possible causes are lacking.
Herein, we present 4 cases of chylopericardium that developed after intrapericardial surgery, and we review the literature.
Our literature review revealed 29 cases of chylopericardium that complicated intrapericardial operations, to which we added our 4 cases for analysis. The 33 surgical procedures involved repair for congenital heart disease (n=21), valve surgery (n=5), coronary artery bypass grafting (n=6), and other (n=1). Causes were verified in 7 patients: small lymphatic injury in 3 and high venous pressure or venous thrombosis in 4. Of the 26 patients with chylopericardium of unknown origin, 15 had congenital heart disease. Ten of these 15 had chromosomal abnormalities, especially trisomy 21 (Down syndrome); these patients typically had increased lymphatic permeability, which raised the likelihood of chylopericardium. Five revascularizations for coronary artery disease required harvesting of the left internal thoracic artery for reconstruction, incurring a risk of damage to the drainage site of the right efferent lymphatic trunk. In addition, all 26 patients with chylopericardium of unknown origin underwent dissection of the ascending aorta and the main pulmonary artery, near the right efferent lymphatic trunk. Inadvertent injury to the trunk during the dissection would have increased the risk of chylopericardium. Accordingly, even though the overall incidence of chylopericardium during intrapericardial procedures is low, we recommend a meticulous dissection of the ascending aorta from the main pulmonary artery.
Key words: Cardiac surgical procedures/adverse effects; chylopericardium/etiology/pathology; chyle; chylous ascites/complications/diagnosis/etiology/surgery; drainage/methods; heart defects, congenital/surgery; lymphatic system/injuries; pericardial effusion/diagnosis/etiology/therapy; pericardium/injuries; postoperative complications; thoracic duct
Chylopericardium is rare after an intrapericardial procedure, because the sites of surgical dissection are usually anatomically remote from the thoracic duct. Even though some procedures may impede thoracic duct drainage to the subclavian vein, with the resultant elevation in venous pressure, the usual manifestation is chylothorax rather than chylopericardium. A 2002 cadaver study of the intrathoracic tributaries of the thoracic duct revealed that tributaries from the heart arrive at the thoracic duct via 2 routes.1 Injury to these tributaries along their course within the pericardium may account for postoperative chylopericardium. Nevertheless, chylopericardium arising from post-intrapericardial procedures is described in few reports in the medical literature.2–20 Herein, we present our experience with 4 patients, review the relevant literature, and refer to the cadaver study as we speculate on probable injury sites.
Case Reports
Patient 1
A 3-year-old boy was admitted with a diagnosis of a secundum-type atrial septal defect. To facilitate accurate cross-clamping of the aorta during the operation, we performed limited dissection of the ascending aorta (AAo) from the main pulmonary artery (MPA), separating the connecting soft tissues for clamping purposes. Forty-eight hours postoperatively, the mediastinal fluid drainage increased and became opalescent. The cholesterol and triglyceride levels of the effusion were 53 and 1,515 mg/dL, respectively. A diet of milk rich in mid-chain fatty acids was prescribed. By day 6, the chylous fluid turned transparent, and its total daily volume decreased substantially. On the basis of these observations, the mediastinal tube was removed on day 7. No recurrence of the fluid accumulation was noted during regular echocardiographic follow-up over the next 5 years.
Patient 2
A 2-year-old boy was admitted for corrective surgery for tetralogy of Fallot. The AAo was dissected from the MPA extensively because of the expectation that a transannular patch would be required. The ventricular septal defect was repaired, and a transannular patch was used for right ventricular outflow tract reconstruction. The persistence of a moderate amount of serosanguineous pericardial effusion (>400 cc/day) was noted immediately after the operation. The effusion turned milky in color on day 14, and the levels of cholesterol and triglycerides therein were 42 and 202 mg/dL, respectively. A diet of mid-chain fatty-acid milk was administered to the patient until the effusion decreased; the mediastinal tubes were removed on day 20.
Patient 3
A 1-year-old boy with known Down syndrome and severe pulmonary hypertension (Qp/Qs=5.3) was admitted for surgical correction of an atrioventricular septal defect (complete form), and a small patent ductus arteriosus. The routine dissection of the AAo from the MPA was extended to encircle the patent ductus arteriosus before cardiopulmonary bypass was instituted. The atrioventricular septal defect was repaired by means of a 2-patch technique, with the coronary sinus draining into the left atrium. On postoperative day 10, massive pericardial effusion was observed on echocardiography. Subxiphoid pericardial drainage was begun immediately. The cholesterol and triglyceride levels of the milky fluid were 33 and 278 mg/dL, respectively. Chylopericardium was diagnosed, and a diet rich in mid-chain fatty acids was promptly implemented. After 18 days of dietary control, the drainage volume decreased, with no recurrent fluid accumulation noted during follow-up.
Patient 4
A full-term newborn boy was transferred to our hospital due to an imperforate anus, and an urgent colostomy was performed. On the basis of the infant's facial dysmorphism, Down syndrome was suspected, and trisomy 21 was confirmed upon subsequent chromosomal examination. Echocardiography and cardiac catheterization revealed a large ventricular septal defect, severe tricuspid valve regurgitation, and pulmonary artery hypertension. At the age of 4 months, the infant underwent surgical correction of the ventricular septal defect. The AAo was dissected from the MPA in standard fashion. Although the postoperative course was smooth, the pericardial discharge turned milky-yellow on day 3. The levels of cholesterol and triglycerides in the effusion were 28 and 185 mg/dL, respectively. A diet rich in mid-chain fatty acids was prescribed. Seven days later, the drainage volume decreased, and the pericardial tubes were removed.
Review
We reviewed the English-language medical literature, searching for the key word “chylopericardium.” Studies that provided insufficient individual patient data were excluded; we considered only the 29 cases from 19 reports that exclusively dealt with isolated chylopericardium that had developed after intrapericardial procedures. Including our 4 patients, our review encompassed 33 cases.
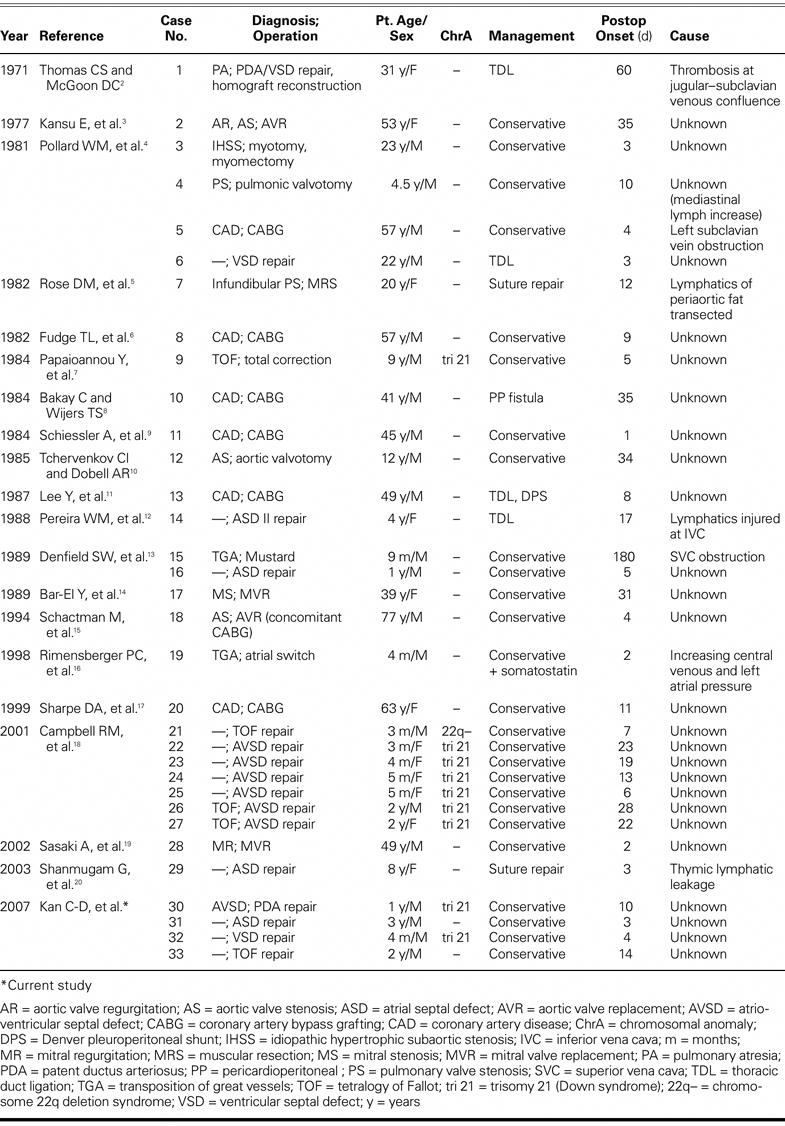
Table I summarizes the demographic data. Twenty patients (61%) were male, and 19 were children (58%). Surgical procedures comprised repair for congenital heart disease (n=21), valve surgery (n=5), coronary artery bypass surgery (n=6), and correction of idiopathic hypertrophic subaortic stenosis (plus a myomectomy) (n=1). After we excluded 7 patients for whom no clinical symptoms were mentioned, the most prevalent pre-sentations were cardiac tamponade (10/26) and persistent or increased drainage volume with the fluid becoming opalescent (9/26) after postoperative food intake. Twenty-seven patients were diagnosed with chylopericardium within 30 days of surgery; the late-onset average for the other 6 patients was 62.5 days. Eighteen patients were prescribed dietary regimens involving foods rich in medium-chain fatty acids, low-fat intake, or nothing by mouth. Five patients underwent low thoracic duct ligation or additional pericardioperitoneal shunt surgery, 2 underwent re-exploration for suture repair, and 1 received conservative therapy with additional somatostatin treatment. The causes of the chylopericardium were determined to be small lymphatic injury (n=3; cases 7, 14, and 29) and high venous pressure or venous thrombosis (n=4; cases 1, 5, 15, and 19). The remaining 26 cases were of unknown origin.
TABLE I. Summary of Cases of Chylopericardium after Intrapericardial Surgery
We explored the possible causes of chylopericardium in these 26 patients. Ten of the 15 congenital heart disease patients (cases 9, 21–27, 30, and 32) had chromosomal abnormalities; 9 of these patients had trisomy 21. Increased lymphatic permeability due to congenital lymphatic dysplasia probably led to the postoperative chylopericardium that was reported in the Down syndrome patients and in the other patient with a chromosomal anomaly. Five of the 6 patients who underwent coronary artery bypass had the left internal thoracic artery harvested as a conduit (cases 8, 10, 11, 13, and 20), and these dissections were extended to the 1st intercostal space near the drainage site of the right efferent lymphatic trunk (RET)—a possible site of injury that would cause chylopericardium. Of the remaining 6 patients, cases 2, 3, 12, and 18 underwent an aortotomy, and cases 17 and 28 underwent mitral valve replacement. No single procedure appeared to jeopardize the lymphatic trunk in these 6 patients. However, all 26 patients underwent varying degrees of dissection of the AAo from the MPA, in proximity to the RET. An inadvertent injury to the RET during blunt dissection would engender chylopericardium.
Discussion
Chylopericardium that complicates intrapericardial surgery is extremely rare. Thomas and McGoon2 first reported this problem in 1971 after procedures involving cardiopulmonary bypass. Chylopericardium has been reported after valve replacement, myocardial revascularization, and corrections of congenital heart anomalies2–20; the overall incidence in the literature is less than 0.22%.4,7,18,21 Upon retrospective review of all of the surgical patients in our hospital from September 1988 through February 2004, we calculated an incidence of 0.12% (4 of 3,332).
Normally, the lymphatic system drains chylous fluid, which is filtered by the capillaries from the interstitial spaces into the thoracic duct. The thoracic duct passes through the chest near the aorta and azygos vein and then terminates at or near the junction of the left subclavian artery and the jugular veins, into which the chyle drains. It is generally accepted that direct injury to the thoracic duct results in chylothorax rather than chylopericardium because the thoracic duct is located in the region of the descending aorta, and direct injury to the thoracic duct during a purely intrapericardial procedure is unlikely. Identifying the mechanism responsible for the development of chylopericardium is of interest to surgeons; however, due to the paucity of clinical experience with chylopericardium, few satisfactory explanations have been forthcoming to account for its occurrence.
Causes for chylopericardium could be established in only 7 of the patients who underwent intrapericardial procedures. Injury to the lymphatic system near the inferior vena cava during re-exploration was diagnosed in case 14. Small lymphatic leaks in the thymic tissues or periaortic fat were noted during reoperation in cases 7 and 29; both were treated by direct suture ligation. In cases 1, 5, 15, and 19, the chylopericardium was related to obstruction of normal thoracic ductal flow, as confirmed by venography, actual symptoms, or pressure tracing. Both direct obstruction of the systemic venous return (as seen in cases 1, 5, and 15) and abnormally elevated systemic venous pressure transmitted into the lymphatic channels (case 19) may increase the pressure within the thoracic duct; the same is true when patients undergo a Fontan procedure for tricuspid atresia and develop chylothorax, which results in pericardial chyle accumulation. Whereas in adults the thymus is nearly devoid of lymphatic tissue, in children the thymus is rich in lymphatic tissues. This may explain why chylopericardium develops more often after pediatric surgery, and why most such instances involve the anterior mediastinum of the thymic tissue.
Although many patients in our literature review were surveyed for possible pathogenesis, in most it remained undetermined. Previous researchers18,22 have concluded that an important cause of postoperative chylopericardium is the increased lymphatic permeability due to congenital lymphatic dysplasia that contributes to the chylous effusions present in Down, Noonan, and Turner syndromes. Ten patients in our survey were found to have a chromosomal anomaly, mainly trisomy 21. However, the pathogenesis was unclear in the other 16 patients. We analyzed the general clinical manifestations in this subgroup. All 16 patients had undergone either some degree of dissection of the AAo from the MPA (to facilitate accurate aortic cross-clamping) or harvesting of the left internal thoracic artery (for coronary artery bypass).
In a study of the intrathoracic tributaries of the thoracic duct in 530 cadavers, 8.9% of lymphatic vessels were found to arise from the heart and connect with the thoracic duct. 1 There are 2 routes for these tributaries—the RET and the left efferent lymphatic trunk (LET). The RET, which often drains lymph from the right ventricle, ascends cephalad between the AAo and the MPA and joins the upper part of the left anterior mediastinal node chain, which is located at the level of origin of the internal thoracic artery. The RET then travels to the left of the thymus gland and drains into the arch of the thoracic duct (Fig. 1). The LET, which drains lymph from the left ventricle, ascends behind the MPA, joins the right paratracheal node, and drains into the thoracic duct at the mediastinum or arch. This confirms the observation of Pollard and colleagues,4 who demonstrated the phenomenon of increased numbers of superior mediastinal lymphatics in 1 patient with chylopericardium (case 4).
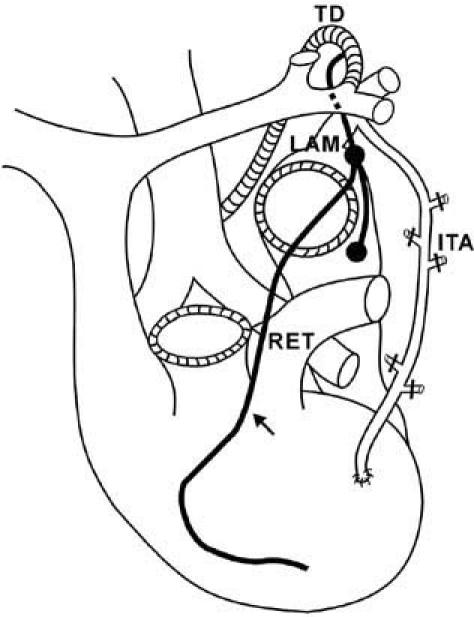
Fig. 1 The right efferent lymphatic trunk (RET), which drains the lymph from the right ventricle, arises between the ascending aorta and the main pulmonary artery (arrow), joins the upper part of the left anterior mediastinal node chain (LAM) located at the origin of the internal thoracic artery (ITA), and empties into the arch of the thoracic duct (TD).
Riquet and associates1 have suggested that injury to an incompetent RET along its course within the pericardium is a possible cause of isolated postoperative chylopericardium.1 (Note: We frequently dissect the AAo from the MPA in order to cross-clamp the AAo accurately after cardiopulmonary bypass. Inadvertent injury to the RET might be unavoidable during this dissection or the subsequent clamping; however, backflow from the thoracic duct actually occurs only if the integrity of the RET is compromised and results in leakage of chyle. This may explain why the incidence of isolated chylopericardium is so low even though dissection of the AAo from the MPA is a routine procedure.)
In patients who have undergone coronary artery bypass grafting, harvesting of the left internal thoracic artery above the left subclavian vein with ligation of the upper intercostal branch may incur an additional risk of chylopericardium or chylothorax if the pleural membrane is opened. The risk may be increased because the anterior mediastinal node chain is easily jeopardized during proximal dissection of the internal thoracic artery, and chylous leakage may ensue. This complication is under-reported and shows the danger of high dissection of the internal mammary artery. The intrapericardial collection of pericardial effusion after cardiac surgery is primarily related to not opening the pleural membrane.
Two research groups7,23 showed improvements in patients with chyluria and chylothorax who were prescribed a medium-chain triglyceride diet. This regimen has since proved effective in many cases, under varied conditions. Therefore, we recommend treating chylothorax and chylopericardium with 2 weeks of conservative therapy: decompression of the thoracic lymphatics by parenteral hyperalimentation or oral medium-chain triglycerides, together with decompression of the pericardial space by closed chest-tube suction. To avoid prolonged hospitalization, we recommend outpatient drainage of a chylous effusion with the use of a soft silastic catheter (Pleurx; Denver Biomedical, Inc.; Golden, Colo) that is tunneled to prevent infection. More than half of the patients whose cases we reviewed were successfully treated by use of this method.
Control of chyle output with somatostatin has also been advocated.25,26 Surgical reintervention was required in only 6 of 34 patients so treated. Generally, the indications for surgery are drainage of more than 1,000 mL/day in adults, or 100 mL × years of age in children, for the first 7 days, or persistent chylous drainage for longer than 2 weeks.26–29 Ligating the thoracic duct at the diaphragmatic level confers the advantage of stopping flow from accessory ducts that may be unrecognized30,31; however, sometimes the thoracic duct cannot be found intraoperatively, or ligation fails to control the leak.28,32 Other surgical options include pleurodesis, pleurectomy, low ligation of the thoracic duct by video-assisted thoracoscopic surgery, and pericardial–peritoneal shunting,28,30,33,34 but the results are not always satisfactory.24
Summary
Chylopericardium after simple intrapericardial surgery is a rare entity, but one that is associated with potentially serious problems. Chylopericardium can result from injury to the lymphatic branches of the thymus, increased lymphatic permeability associated with the lymphatic dysplasia in patients with Down syndrome, or thrombosis of the great venous system. An inadvertent injury to the RET during dissection can also lead to chylopericardium. We advise caution when dissecting the tissues between the AAo and the MPA, and we discourage harvesting the internal mammary artery as far as the upper intercostal branches. Although surgical reintervention may be required for refractory chylopericardium, a conservative treatment strategy is still the 1st choice.
Footnotes
Address for reprints: Yu-Jen Yang, MD, Department of Surgery, National Cheng Kung University Hospital, 138 Sheng-Li Road, Tainan, Taiwan 704, ROC. E-mail: kcd56@mail.ncku.edu.tw
References
- 1.Riquet M, Le Pimpec Barthes F, Souilamas R, Hidden G. Thoracic duct tributaries from intrathoracic organs. Ann Thorac Surg 2002;73:892–9. [DOI] [PubMed]
- 2.Thomas CS Jr, McGoon DC. Isolated massive chylopericardium following cardiopulmonary bypass. J Thorac Cardiovasc Surg 1971;61:945–8. [PubMed]
- 3.Kansu E, Fraimow W, Smullens SN. Isolated massive chylopericardium. Complication of open heart surgery for aortic valve replacement. Chest 1977;71:408–10. [DOI] [PubMed]
- 4.Pollard WM, Schuchmann GF, Bowen TE. Isolated chylopericardium after cardiac operations. J Thorac Cardiovasc Surg 1981;81:943–6. [PubMed]
- 5.Rose DM, Colvin SB, Danilowicz D, Isom OW. Cardiac tamponade secondary to chylopericardium following cardiac surgery: case report and review of the literature. Ann Thorac Surg 1982;34:333–6. [DOI] [PubMed]
- 6.Fudge TL, Daniels CB, Harrington OB, Crosby VG, Wolf RY, Painter MW, Burke LD. Chylopericardial tamponade following myocardial revascularization. J La State Med Soc 1982;134:11–3. [PubMed]
- 7.Papaioannou Y, Vomvoyannis A, Andritsakis G. Combined chylopericardium and chylothorax after total correction of Fallot's tetralogy. Thorac Cardiovasc Surg 1984;32:115–6. [DOI] [PubMed]
- 8.Bakay C, Wijers TS. Treatment of cardiac tamponade due to isolated chylopericardium following open heart surgery. J Cardiovasc Surg (Torino) 1984;25:249–51. [PubMed]
- 9.Schiessler A, John A, Pallua N, Bucherl ES. Chylopericardium following aorto-coronary bypass-procedure. Thorac Cardiovasc Surg 1984;32:112–4. [DOI] [PubMed]
- 10.Tchervenkov CI, Dobell AR. Chylopericardium following cardiac surgery. Can J Surg 1985;28:542–3. [PubMed]
- 11.Lee Y, Lee WK, Doromal N, Ganepola GA, Hutchinson J 3rd. Cardiac tamponade resulting from massive chylopericardium after an aorta-coronary bypass operation. J Thorac Cardiovasc Surg 1987;94:449–50. [PubMed]
- 12.Pereira WM, Kalil RA, Prates PR, Nesralla IA. Cardiac tamponade due to chylopericardium after cardiac surgery. Ann Thorac Surg 1988;46:572–3. [DOI] [PubMed]
- 13.Denfield SW, Rodriguez A, Miller-Hance WC, Stein F, Ott DA, Jefferson LS, Bricker JT. Management of postoperative chylopericardium in childhood. Am J Cardiol 1989;63:1416–8. [DOI] [PubMed]
- 14.Bar-El Y, Smolinsky A, Yellin A. Chylopericardium as a complication of mitral valve replacement. Thorax 1989;44:74–5. [DOI] [PMC free article] [PubMed]
- 15.Schactman M, Scott C, Glibbery-Fiesel DR, Murello M, Kerr P. Chylopericardium following aortic valve replacement and coronary artery bypass surgery: a case report and discussion. Am J Crit Care 1994;3:313–5. [PubMed]
- 16.Rimensberger PC, Muller-Schenker B, Kalangos A, Beghetti M. Treatment of a persistent postoperative chylothorax with somatostatin. Ann Thorac Surg 1998;66:253–4. [DOI] [PubMed]
- 17.Sharpe DA, Pullen MD, McGoldrick JP. A minimally invasive approach to chylopericardium after coronary artery surgery. Ann Thorac Surg 1999;68:1062–3. [DOI] [PubMed]
- 18.Campbell RM, Benson LN, Williams WW, Adatia I. Chylopericardium after cardiac operations in children. Ann Thorac Surg 2001;72:193–6. [DOI] [PubMed]
- 19.Sasaki A, Watanabe Y, Tokunaga C. Chylopericardium following mitral valve replacement. Jpn J Thorac Cardiovasc Surg 2002;50:518–9. [DOI] [PubMed]
- 20.Shanmugam G, Sundar P, Shukla V, Korula RJ. Chylopericardial tamponade following atrial septal defect repair: an usual entity. IJTCVS 2003;19:124–5.
- 21.Ossiani MH, McCauley RG, Patel HT. Primary idiopathic chylopericardium. Pediatr Radiol 2003;33:357–9. [DOI] [PubMed]
- 22.Lanning P, Simila S, Suramo I, Paavilainen T. Lymphatic abnormalities in Noonan's syndrome. Pediatr Radiol 1978;7: 106–9. [DOI] [PubMed]
- 23.Hashim SA, Roholt HB, Babayan VK, Vanitallie TB. Treatment of chyluria and chylothorax with medium-chain triglyceride. N Engl J Med 1964;270:756–61. [DOI] [PubMed]
- 24.Akamatsu H, Amano J, Sakamoto T, Suzuki A. Primary chylopericardium. Ann Thorac Surg 1994;58:262–6. [DOI] [PubMed]
- 25.Ulibarri JI, Sanz Y, Fuentes C, Mancha A, Aramendia M, Sanchez S. Reduction of lymphorrhagia from ruptured thoracic duct by somatostatin. Lancet 1990;336(8709):258. [DOI] [PubMed]
- 26.Kelly RF, Shumway SJ. Conservative management of postoperative chylothorax using somatostatin. Ann Thorac Surg 2000;69:1944–5. [DOI] [PubMed]
- 27.Hargus EP, Carson SD, McGrath RL, Wolfe RR, Clarke DR. Chylothorax and chylopericardial tamponade following Blalock-Taussig anastomosis. J Thorac Cardiovasc Surg 1978;75:642–5. [PubMed]
- 28.Pego-Fernandes PM, Jatene FB, Tokunaga CC, Simao DT, Beirutty R, Iwahashi ER, de Oliveira SA. Ligation of the thoracic duct for the treatment of chylothorax in heart diseases [in Portuguese]. Arq Bras Cardiol 2003;81:309–17. [PubMed]
- 29.Chan BB, Murphy MC, Rodgers BM. Management of chylopericardium. J Pediatr Surg 1990;25:1185–9. [DOI] [PubMed]
- 30.Cerfolio RJ, Allen MS, Deschamps C, Trastek VF, Pairolero PC. Postoperative chylothorax. J Thorac Cardiovasc Surg 1996; 112:1361–6. [DOI] [PubMed]
- 31.Murphy TO, Piper CA. Surgical management of chylothorax. Am Surg 1977;43:715–8. [PubMed]
- 32.Merrigan BA, Winter DC, O'Sullivan GC. Chylothorax. Br J Surg 1997;84:15–20. [PubMed]
- 33.Skarsgard ED, Filler RM, Superina RA. Postpericardiotomy syndrome and chylopericardium: two unusual complications after aortopexy for tracheomalacia. J Pediatr Surg 1994; 29:1534–6. [DOI] [PubMed]
- 34.Furrer M, Hopf M, Ris HB. Isolated primary chylopericardium: treatment by thoracoscopic thoracic duct ligation and pericardial fenestration. J Thorac Cardiovasc Surg 1996;112: 1120–1. [DOI] [PubMed]


