Abstract
Takotsubo cardiomyopathy mimics acute coronary syndrome and is accompanied by reversible left ventricular apical ballooning in the absence of angiographically significant coronary artery stenosis. In Japanese, “tako-tsubo” means “fishing pot for trapping octopus,” and the left ventricle of a patient diagnosed with this condition resembles that shape. Takotsubo cardiomyopathy, which is transient and typically precipitated by acute emotional stress, is also known as “stress cardiomyopathy” or “broken-heart syndrome.”
Herein, we describe the clinical angiographic characteristics of 4 patients who exhibited this syndrome, and we review the existing literature and propose reasons to conduct prospective studies.
Key words: Cardiomyopathies/diagnosis/etiology/physiopathology; catecholamines/secretion; chest pain/etiology; coronary angiography; coronary disease/physiopathology; echocardiography; electrocardiography; heart/physiopathology; myocardial ischemia/diagnosis; stress, psychological/complications/physiopathology; takotsubo cardiomyopathy; ventricular dysfunction; left/diagnosis/etiology/physiopathology
Takotsubo cardiomyopathy mimics acute coronary syndrome. It is accompanied by reversible left ventricular (LV) apical ballooning in the absence of angiographically significant coronary artery stenosis. In Japanese, “tako-tsubo” means “fishing pot for trapping octopus,” because the LV of a patient diagnosed with this condition resembles that shape. A transient entity typically precipitated by acute emotional stress, takotsubo cardiomyopathy is also called “stress cardiomyopathy” or “broken-heart syndrome.” Several cases of this interesting cardiomyopathy have been reported in Japan,1–8 and more recently in the United States9–13 and Belgium.14 Herein, we describe the clinical and angiographic characteristics of 4 patients diagnosed with takotsubo cardiomyopathy, and we review the existing literature.
Case Reports
Patient 1
A 57-year-old black woman with a history of hypertension and dyslipidemia was hospitalized because of the sudden onset of angina-like chest pain and dyspnea. Auscultation revealed bilateral basilar crackles and an S3 gallop. Electrocardiography (ECG) showed ST-segment elevation of 1 mm in leads V1 through V3. The QT interval was prolonged. Levels of creatine kinase-MB and troponin T were mildly elevated. Echocardiography showed substantial apical dysfunction, preserved basal function, and no intraventricular pressure gradient. The patient underwent emergency cardiac catheterization, which disclosed no substantial epicardial coronary artery stenosis. Left ventriculography showed systolic ballooning of the apex and hypercontraction of the basal segment (Fig. 1). The patient was treated with aspirin, an angiotensin-converting enzyme inhibitor, a diuretic, and statins. Two weeks later, the ECG showed complete resolution of the ST-segment elevation and no Q-wave formation. Echocardiography revealed remarkable improvement of the apical wall motion abnormality and normalization of the ejection fraction. It was concluded that acute emotional stress after the death of a relative had precipitated the initial symptoms.
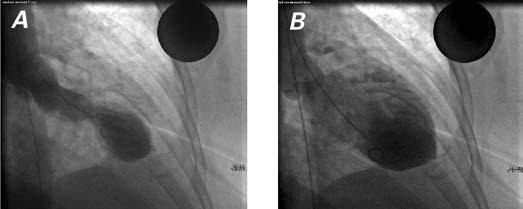
Fig. 1 Patient 1. Left ventricular angiography in systole (A) and diastole (B) shows apical ballooning and hypercontraction of the basal segments.
Patient 2
A 64-year-old black woman with a history of diabetes mellitus presented with severe angina-like chest pain. Deep negative T waves were seen on ECG. Her cardiac enzyme levels were mildly elevated. The patient was admitted with a diagnosis of acute non-ST-segment-elevation myocardial infarction. An echocardiogram showed apical dyskinesis, an ejection fraction of 0.45, and no intraventricular pressure gradient. The patient's ongoing chest pain prompted emergency coronary angiography with intra-aortic balloon pump support. The angiogram revealed apical ballooning in systole with concomitantly increased contractility in the basal segments. The overall LV systolic function (LVSF) was mildly depressed. With medical therapy, the patient's symptoms improved. Echocardiography after 1 week showed normalization of the systolic apical ballooning and the LVSF. When questioned, the patient revealed that she had recently experienced severe emotional stress due to financial instability.
Patient 3
A 44-year-old white man without a pertinent medical history underwent urgent coronary angiography because of acute chest pain and marked precordial T-wave inversions suggestive of acute myocardial ischemia. Coronary angiography showed no significant stenosis of the coronary arteries. A challenge with acetylcholine elicited no spasm of either the right or the left coronary artery. Left ventriculography showed systolic apical ballooning with mild basal hypercontraction. Levels of creatine kinase-MB and troponin T were mildly elevated. One week later, echocardiography showed complete resolution of the wall motion abnormality. Severe occupation-related emotional stress had preceded the onset of this patient's symptoms.
Patient 4
A 64-year-old black woman with a history of hypertension and hyperlipidemia presented after 1 day of severe nausea and vomiting. An ECG showed ST-segment elevation and deep T-wave inversion in the anterior leads. The QT interval was also prolonged (Fig. 2). The cardiac enzyme levels were mildly elevated. Emergency cardiac catheterization disclosed no obstructive coronary artery disease. Left ventricular angiography revealed apical ballooning in systole with mild basal hypercontraction. The patient's LVSF was moderately reduced. Two weeks later, results of echocardiography showed resolution of the apical wall motion abnormality and the LV systolic dysfunction. The patient had not experienced emotional distress before the onset of symptoms.
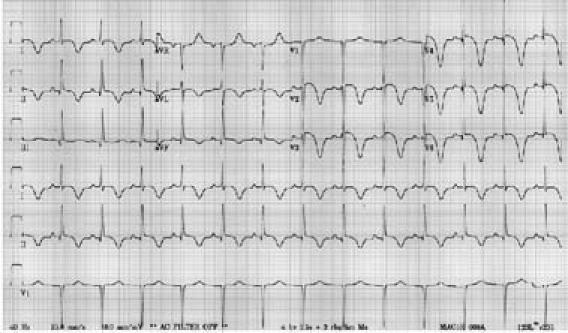
Fig. 2 Patient 4. A 12-lead electrocardiogram of a patient diagnosed with takotsubo cardiomyopathy shows ST-segment elevation and deep T-wave inversions in the anterior leads, in association with a prolonged QT interval.
Discussion
These 4 cases exhibited most of the characteristics of takotsubo cardiomyopathy that have been described in the literature:
A preponderant occurrence of the syndrome in elderly or postmenopausal females2,8–10
Onset consequent to acute emotional stress or an acute medical condition2,7,8,11
ST-segment elevation or depression, or T-wave changes1–11
A prolonged QT interval2,11
A mild increase in cardiac enzymes2,9,10
Typical akinesis of the apical and distal anterior wall together with hypercontraction of the basal wall2,8–10
The occasional presence of transient intracavitary pressure gradients in some patients2,8,10
A need for acute hemodynamic support in some cases2,8,10
Complete resolution of the apical wall motion abnormality and the depressed LVSF.2,3,8,9,11
Although the exact pathogenesis of takotsubo cardiomyopathy remains unclear, various mechanisms have been proposed. Dote and associates1 suggested coronary vasospasm as the pathogenic mechanism; however, induction of coronary vasospasm by acetylcholine or ergonovine has yielded mixed results. In some series, vasospasm in at least 1 epicardial coronary artery was present in most patients,3,8 whereas Akashi and colleagues2 found no coronary vasospasm in patients who underwent an acetylcholine challenge. Multivessel coronary spasm would be required to account for the apical wall motion abnormality seen in this syndrome. Similarly, the duration of wall motion abnormality in takotsubo cardiomyopathy typically is longer than would be expected in conventional cases of coronary vasospasm.
The possibility of myocardial injury due to microvascular spasm has also been suggested.3 Ako and coworkers,15 by the use of an intracoronary Doppler wire technique, demonstrated microcirculation impairments in instances of transient LV hypocontraction. Although this is an interesting explanation, several factors challenge its causative potential. First, microscopic findings in some patients who had LV apical ballooning were different from those in patients who had myocardial ischemia. The most common pathologic finding in takotsubo cardiomyopathy, focal myocytolysis, is not typically seen in patients with myocardial infarction.2 Second, in several cases, coronary angiography failed to reveal the slow-flow phenomenon, even in the presence of ST-segment elevation. Finally, impaired microcirculation during the acute phase is not direct evidence of causation, because microcirculatory impairment can result from a primary myocardial injury.
Another putative mechanism is neurogenic stunned myocardium. This condition is also observed during acute cerebrovascular accidents16,17 and during the catecholamine-induced cardiomyopathy in patients with pheochromocytoma.18–20 Enhanced sympathetic activity appears to play a very important role in the pathophysiology of takotsubo cardiomyopathy. Triggering factors, such as intense emotional stress, are frequently seen in patients with this syndrome. Excessive levels of catecholamines have been observed in patients with takotsubo cardiomyopathy.2 Catecholamines have been shown to induce myocardial damage,21,22 and excessive stimulation of cardiac adrenergic receptors has led to transient LV hypocontraction in animal models.23 A 2005 case series12 showed a strong relationship between stress-induced cardiomyopathy and increased plasma catecholamine levels, suggesting that exaggerated sympathetic activation may be important in the development of the cardiomyopathy.
The possibility that myocarditis leads to transient LV dysfunction has also been suggested, but results of biopsies and paired serum tests for viral serology have been negative in the patients studied.2
Another intriguing question surrounding takotsubo cardiomyopathy is that of why the apical wall is affected but the base is spared. Several explanations have been proposed.8 The apex is structurally vulnerable because it does not have a 3-layered myocardial configuration, it has a limited elasticity reserve, it can easily become ischemic as a consequence of its relatively limited coronary circulation, and it is more responsive to adrenergic stimulation.24 All of these factors might make the apex more sensitive to the catecholamine-induced surge frequently observed in takotsubo cardiomyopathy.
Transient intraventricular pressure gradients have also been detected in some patients diagnosed with takotsubo cardiomyopathy.2,8,10 However, the absence of significant LV hypertrophy in takotsubo cardiomyopathy, along with the distinctive histologic features, rules out the possibility of an acute midventricular obstruction, as seen in patients who have hypertrophic cardiomyopathy.
Although short-term outcomes are excellent, with complete resolution in all reported cases,2,8,9,11 there are no data in the literature regarding long-term outcome in patients who have experienced takotsubo cardiomyopathy.
Takotsubo cardiomyopathy has important implications, because its clinical presentation mimics that of an acute coronary syndrome. Increased awareness of takotsubo cardiomyopathy will likely result in its being diagnosed more frequently. Prospective studies are needed in order to determine more accurately the incidence of takotsubo cardiomyopathy and to ascertain the long-term outcomes. Studies are also needed to elucidate the specific pathophysiologic mechanisms responsible for this cardiomyopathy.
Footnotes
Address for reprints: Salim S. Virani, MD, Division of Cardiology, Texas Heart Institute at St. Luke's Episcopal Hospital, 6720 Bertner, P-332, Houston, TX 77030. E-mail: virani@bcm.tmc.edu
References
- 1.Dote K, Sato H, Tateishi H, Uchida T, Ishihara M. Myocardial stunning due to simultaneous multivessel coronary spasms: a review of 5 cases [in Japanese]. J Cardiol 1991;21:203–14. [PubMed]
- 2.Akashi YJ, Nakazawa K, Sakakibara M, Miyake F, Koike H, Sasaka K. The clinical features of takotsubo cardiomyopathy. QJM 2003;96:563–73. [DOI] [PubMed]
- 3.Kurisu S, Sato H, Kawagoe T, Ishihara M, Shimatani Y, Nishioka K, et al. Tako-tsubo-like left ventricular dysfunction with ST-segment elevation: a novel cardiac syndrome mimicking acute myocardial infarction. Am Heart J 2002;143:448–55. [DOI] [PubMed]
- 4.Shimizu M, Takahashi H, Fukatsu Y, Tatsumi K, Shima T, Miwa Y, et al. Reversible left ventricular dysfunction manifesting as hyperkinesis of the basal and the apical areas with akinesis of the mid portion: a case report [in Japanese]. J Cardiol 2003;41:285–90. [PubMed]
- 5.Abe Y, Kondo M, Matsuoka R, Araki M, Dohyama K, Tanio H. Assessment of clinical features in transient left ventricular apical ballooning. J Am Coll Cardiol 2003;41:737–42. [DOI] [PubMed]
- 6.Iga K, Hori K, Kitaguchi K, Matsumura T, Gen H, Tomonaga G, Tamamura T. Transient segmental asynergy of the left ventricle of patients with various clinical manifestations possibly unrelated to the coronary artery disease. Jpn Circ J 1991;55:1061–7. [DOI] [PubMed]
- 7.Akashi YJ, Sakakibara M, Miyake F. Reversible left ventricular dysfunction “takotsubo” cardiomyopathy associated with pneumothorax. Heart 2002;87:E1. [DOI] [PMC free article] [PubMed]
- 8.Tsuchihashi K, Ueshima K, Uchida T, Oh-mura N, Kimura K, Owa M, et al. Transient left ventricular apical ballooning without coronary artery stenosis: a novel heart syndrome mimicking acute myocardial infarction. Angina Pectoris-Myocardial Infarction Investigations in Japan. J Am Coll Cardiol 2001;38:11–8. [DOI] [PubMed]
- 9.Seth PS, Aurigemma GP, Krasnow JM, Tighe DA, Untereker WJ, Meyer TE. A syndrome of transient left ventricular apical wall motion abnormality in the absence of coronary disease: a perspective from the United States. Cardiology 2003; 100:61–6. [DOI] [PubMed]
- 10.Bybee KA, Prasad A, Barsness GW, Lerman A, Jaffe AS, Murphy JG, et al. Clinical characteristics and thrombolysis in myocardial infarction frame counts in women with transient left ventricular apical ballooning syndrome. Am J Cardiol 2004;94:343–6. [DOI] [PubMed]
- 11.Bybee KA, Kara T, Prasad A, Lerman A, Barsness GW, Wright RS, Rihal CS. Systematic review: transient left ventricular apical ballooning: a syndrome that mimics ST-segment elevation myocardial infarction. Ann Intern Med 2004;141:858–65. [DOI] [PubMed]
- 12.Wittstein IS, Thiemann DR, Lima JA, Baughman KL, Schulman SP, Gerstenblith G, et al. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med 2005;352:539–48. [DOI] [PubMed]
- 13.Sharkey SW, Lesser JR, Zenovich AG, Maron MS, Lindberg J, Longe TF, Maron BJ. Acute and reversible cardiomyopathy provoked by stress in women from the United States. Circulation 2005;111:472–9. [DOI] [PubMed]
- 14.Desmet WJ, Adriaenssens BF, Dens JA. Apical ballooning of the left ventricle: first series in white patients. Heart 2003; 89:1027–31. [DOI] [PMC free article] [PubMed]
- 15.Ako J, Takenaka K, Uno K, Nakamura F, Shoji T, Iijima K, et al. Reversible left ventricular systolic dysfunction–reversibility of coronary microvascular abnormality. Jpn Heart J 2001;42:355–63. [DOI] [PubMed]
- 16.Pollick C, Cujec B, Parker S, Tator C. Left ventricular wall motion abnormalities in subarachnoid hemorrhage: an echocardiographic study. J Am Coll Cardiol 1988;12:600–5. [DOI] [PubMed]
- 17.Sakamoto H, Nishimura H, Imataka K, Ieki K, Horie T, Fujii J. Abnormal Q wave, ST-segment elevation, T-wave inversion, and widespread focal myocytolysis associated with subarachnoid hemorrhage. Jpn Circ J 1996;60:254–7. [DOI] [PubMed]
- 18.Yamanaka O, Yasumasa F, Nakamura T, Ohno A, Endo Y, Yoshimi K, et al. “Myocardial stunning”-like phenomenon during a crisis of pheochromocytoma. Jpn Circ J 1994;58: 737–42. [DOI] [PubMed]
- 19.Salathe M, Weiss P, Ritz R. Rapid reversal of heart failure in a patient with phaeochromocytoma and catecholamine-induced cardiomyopathy who was treated with captopril. Br Heart J 1992;68:527–8. [DOI] [PMC free article] [PubMed]
- 20.Scott IU, Gutterman DD. Pheochromocytoma with reversible focal cardiac dysfunction. Am Heart J 1995;130:909–11. [DOI] [PubMed]
- 21.Mann DL, Kent RL, Parsons B, Cooper G 4th. Adrenergic effects on the biology of the adult mammalian cardiocyte. Circulation 1992;85:790–804. [DOI] [PubMed]
- 22.White M, Wiechmann RJ, Roden RL, Hagan MB, Wollmering MM, Port JD, et al. Cardiac beta-adrenergic neuroeffector systems in acute myocardial dysfunction related to brain injury. Evidence for catecholamine-mediated myocardial damage. Circulation 1995;92:2183–9. [DOI] [PubMed]
- 23.Ueyama T, Kasamatsu K, Hano T, Yamamoto K, Tsuruo Y, Nishio I. Emotional stress induces transient left ventricular hypocontraction in the rat via activation of cardiac adrenoceptors: a possible animal model of ‘tako-tsubo’ cardiomyopathy. Circ J 2002;66:712–3. [DOI] [PubMed]
- 24.Mori H, Ishikawa S, Kojima S, Hayashi J, Watanabe Y, Hoffman JI, Okino H. Increased responsiveness of left ventricular apical myocardium to adrenergic stimuli. Cardiovasc Res 1993;27:192–8. [DOI] [PubMed]


