Abstract
Nonsteroidal antiinflammatory drugs (NSAIDs) can inhibit colorectal tumorigenesis and are among the few agents known to be useful for the chemoprevention of neoplasia. Here, we show that the tumor suppressive effects of NSAIDs are not likely to be related to a reduction in prostaglandins but rather are due to the elevation of the prostaglandin precursor arachidonic acid (AA). NSAID treatment of colon tumor cells results in a dramatic increase in AA that in turn stimulates the conversion of sphingomyelin to ceramide, a known mediator of apoptosis. These results have significant implications for understanding and improving colon cancer chemoprevention.
Because common epithelial cancers have resisted most therapeutic efforts, much hope is currently placed on chemoprevention. Chemopreventive measures are especially important in patients who are at elevated risk for neoplasia caused by genetic or environmental factors. Though research on such agents is now blossoming, only a few compounds have been shown to be useful in vivo. Among these are nonsteroidal antiinflammatory drugs (NSAIDs), which are effective in reducing colon tumors in genetically susceptible humans (reviewed in ref. 1) and rodents (2–4). Epidemiological studies have demonstrated that NSAID use in the general population is associated with a reduced risk of colon cancer death (reviewed in ref. 5).
Further progress in this area will in part depend on understanding the mechanisms through which such chemopreventive agents exert their protective effects. It is well known that NSAIDs can inhibit cyclooxygenases (COXs) (reviewed in ref. 6), and some studies have shown that NSAIDs can induce apoptosis (also called programmed cell death) (7–11). Moreover, expression of COX2 is elevated in colorectal tumors (12–14), and this elevation can protect intestinal epithelial cells from apoptosis (15). However, the biochemical mechanisms through which NSAIDs and diminished COX expression alter colonic tumorigenesis are largely unknown. In this report, we describe a potential mechanism by which NSAIDs induce apoptosis in human colorectal cancer cells.
MATERIALS AND METHODS
Apoptosis Assays.
Apoptosis was assessed by morphological criteria and by phosphatidylserine exposure. Morphological assessment of apoptosis was made after Hoechst 33258 staining as described (16). Cells with fragmented nuclei were scored as apoptotic. Phosphatidylserine exposure was assessed by merocyanine staining (5 μg/ml, Sigma) as described (17).
Arachidonic Acid (AA) Assays.
AA levels were determined by release of H3-AA as described (18). For representative time points, the identity of released material was confirmed to be AA by thin layer chromatography as described (19). The increases in AA levels were further confirmed by gas chromatographic analysis of selected time points as described (20).
Ceramide Assays.
Ceramide levels were measured by using the Escherichia coli diacylglycerol kinase assay as described (21) with the following modifications. After the initial lipid extraction, phases were broken by adding 2 ml of chloroform and 2 ml of 1 M NaCl, and lipids were washed with 1 M NaCl before drying under nitrogen (22, 23). After solubilization, samples were sonicated for 15 sec. The diacylglycerol reaction was performed for 30 min at 25°C in a 100 μl reaction volume with 3.5 μg of diacylglycerol kinase (Calbiochem) and 30 μCi [γ-32P]ATP (Dupont/NEN, 6,000 Ci/mmol, 1 Ci = 37 GBq). After extraction of the labeled lipids, the lower chloroform phase was washed with 1% HCl before drying under nitrogen. The solvent mixture for thin layer chromatography was chloroform:acetone:methanol:acetic acid:water (10:4:3:2:1). Samples were quantitated by using a PhosphorImager (Molecular Dynamics).
Controls for Apoptosis-Induced Ceramide Increases.
Three observations suggest that the ceramide increases were not the result of apoptosis. (i) Ceramide levels increased to substantial levels in sulindac sulfide (SUS)-treated cells as early as 8 hr posttreatment, when the cells are alive and lack any morphologic features of apoptosis (i.e., nuclear fragmentation and membrane blebs). (ii) Cells treated with SUS and cycloheximide (CHX) do not undergo apoptosis but still have substantial increases in ceramide levels. HCT116 and SW480 cells were treated with 125 μM and 200 μM SUS, respectively, with and without 10 μM CHX for 48 hr. Cells treated with SUS alone underwent apoptosis, whereas cells treated with SUS and CHX did not. HCT116 and SW480 cells treated with SUS alone displayed 10.0 ± 0.4-fold and 9.2 ± 0.1-fold increases in ceramide levels relative to vehicle controls, respectively. Likewise, HCT116 and SW480 cells treated with SUS in the presence of CHX exhibited 5.1 ± 0.5-fold and 7.9 ± 1.3-fold increases in ceramide levels relative to controls, respectively. (iii) Apoptosis induced by other means in colon cells (p53 transferred by adenovirus infection) was not associated with a ceramide increase. p53 was overexpressed in cells through infection with a recombinant adenovirus containing the p53 gene as previously described (24, 25). The p53-infected cells express p53 protein by 2 hr postinfection. In these cells, death begins at 24 hr, and at 48 hr, 90–100% of the cells have undergone apoptosis. Adenovirus containing the β-galactosidase gene instead of the p53 gene was used as a control. Cells were harvested at 16 hr postinfection (when p53-virus infected cells express p53 but before the onset of apoptosis) and at 40 hr postinfection (when most cells are apoptotic). Relative to ceramide levels of β-galactosidase virus-infected cells, p53 virus-infected cells at 16 hr exhibited a ceramide level of 86.4 ± 18.6%. At 40 hr postinfection, control virus-infected cells had a ceramide level of 81.4 ± 12.3%, and p53 virus-infected cells exhibited a ceramide level of 85 ± 8.3%.
Sphingomyelin Assays.
HCT116 and SW480 cells were incubated with [3H]choline chloride (0.5 mCi ml−1, 80 Ci mmol−1, NEN) for 48 hr. After labeling, cells were washed with Hanks’ balanced salt solution (GIBCO) and treated with SUS or indomethacin (INDO). Sphingomyelin measurements were performed by using the bacterial sphingomyelinase method as described (18).
Neutral Sphingomyelinase Assays.
Neutral sphingomyelinase activity was assayed as described (18). After cell lysis, protein concentrations were determined by using the Bradford method (Bio-Rad), and 200 mg of cellular protein was used in each assay. Neutral sphingomyelinase activity was determined by measuring the rate of hydrolysis of 14C-labeled sphingomyelin (Amersham). SW480 and HCT116 cell lines were treated with a SUS (50, 200, and 600 μM) for 6–24 hr. None of these treatments enhanced neutral sphingomyelinase activity in cell lysates. In a typical experiment, treatment of HCT116 with 200 μM of SUS resulted in a neutral sphingomyelinase activity of 90% after 6 hr and 92% after 16 hr relative to untreated controls.
RESULTS
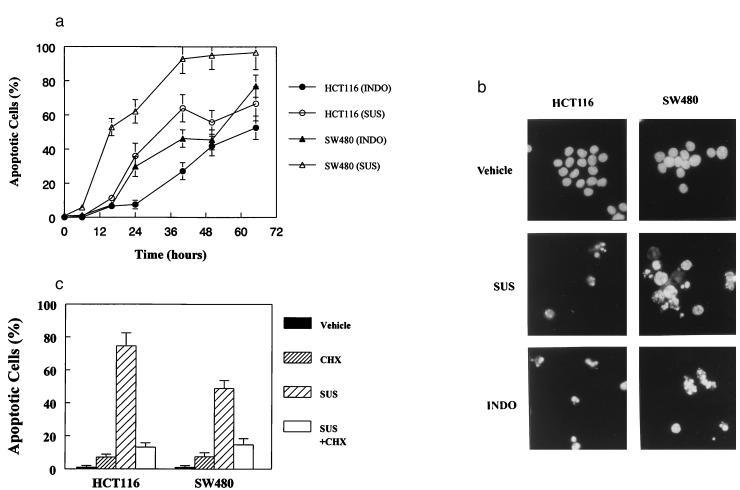
The most extensively investigated NSAID for chemoprevention is sulindac, an agent that can reduce the size and number of colorectal tumors in familial adenomatous polyposis patients (reviewed in ref. 1) as well as in mouse models of familial adenomatous polyposis (2–4). First, we attempted to determine whether the active metabolite of sulindac, SUS, could induce the death of commonly used colorectal cancer cell lines (HCT116 and SW480) at physiologically relevant drug concentrations. SUS resulted in death of both lines in a dose- and time-dependent fashion, consistent with previous studies (7–11). At concentrations of 125 μM and 200 μM SUS in HCT116 and SW480 cells, respectively, the majority of cells died within 48 hr (Fig. 1A). This death appeared to be apoptotic, as it was accompanied by nuclear condensation and fragmentation and exposure of phosphatidylserine groups on the cell surface (Fig. 1B), two hallmarks of apoptosis (26–28).
Figure 1.
NSAIDs induce apoptosis in human colorectal cancer cells. (A) HCT116 and SW480 cells were treated with INDO (300 μM) or SUS (125 μM for HCT116 and 200 μM for SW480 cells). Cells were collected at the indicated times and scored for morphological evidence of apoptosis as described in Materials and Methods. (B) HCT116 and SW480 cells were treated with SUS or INDO for 60 hr, harvested, and assessed for morphological signs of apoptosis. Cells treated with SUS or INDO show the hallmarks of apoptosis. Independent evidence of apoptosis was obtained by examining phosphatidylserine exposure. SUS treatment of HCT116 and SW480 cells resulted in substantial phosphatidylserine exposure indicative of membrane unpacking and apoptosis. Forty-eight hours after treatment with 125 μM SUS, 44% of HCT116 were apoptotic as judged by phosphatidylserine exposure vs. 4% in the vehicle-treated control cells. Likewise, 74% of SW480 cells were apoptotic 50 hr after treatment with 200 μM SUS vs. 1.5% of the vehicle-treated control cells. (C) CHX inhibits SUS-induced apoptosis in HCT116 and SW480 cells. Cells were treated with SUS as above in the presence or absence of CHX (10 μM). Apoptotic cells were evaluated as described above.
Though the morphology of SUS-treated cells was consistent with apoptotic cell death, other cell death processes can mimic these changes. To more definitively demonstrate that SUS can actually induce apoptosis, we employed the criteria originally used to distinguish apoptosis from other death processes, namely, the requirement for macromolecular synthesis (reviewed in ref. 26). We found that inhibition of protein synthesis by CHX (Fig. 1C), or of RNA synthesis by actinomycin D (data not shown), could completely inhibit apoptosis at the concentrations of SUS used in Fig. 1A. Interestingly, CHX could not inhibit SW480 cell death at higher concentrations of SUS (not shown). This finding suggests that SUS can kill cells by two mechanisms: apoptosis at relatively low concentrations and nonspecific toxic effects at higher concentrations. Such nonspecific toxicity at high concentrations of pharmaceutical compounds is not unusual (29) and may have confounded some previous studies of NSAID action. Therefore, all subsequent analyses were performed with SUS concentrations that resulted in bona fide apoptosis.
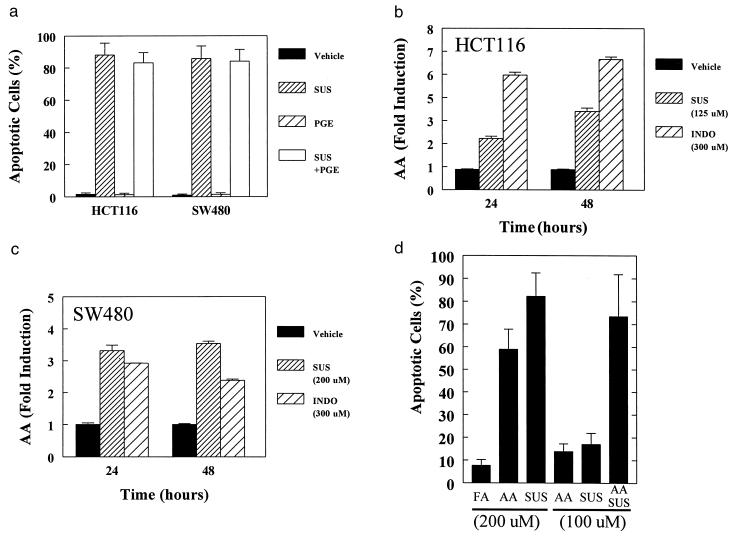
To investigate the mechanisms by which SUS induces apoptosis, we first considered the previously suggested possibility that sulindac functions through inhibiting the production of prostaglandins. To test this idea, we treated cells with both SUS and prostaglandin E2 (PGE2), the major COX product produced by colonic tumors (30), and no inhibition of apoptosis was observed (Fig. 2A). Similarly, PGA2, PGJ2, 15-deoxy-Δ12,14-PGJ2 and Δ12-PGJ2 failed to protect against NSAID-induced apoptosis and in some instances potentiated apoptosis (data not shown). This result is consistent with previous studies that suggested that prostaglandins do not affect NSAID-induced death (31, 32).
Figure 2.
AA mediates NSAID-induced apoptosis. (A) Prostaglandin E2 does not inhibit SUS-induced apoptosis. Cells were treated with the indicated combination of SUS (same concentrations as in Fig. 1) and prostaglandin E2 (PGE, 10 μM). (B, C) Increased AA levels after NSAID treatment. HCT116 and SW480 cells were treated with the indicated concentrations of SUS or INDO. AA levels were determined by measuring release of 3H-AA into the media as described in the Methods. Data are indicated as the mean of three replicates. Error bars = SD. Independent confirmation of AA increases were obtained by gas chromatographic evaluation of AA levels. Treatment of HCT116 and SW480 cells with SUS for 16 hr as described in Fig. 1 resulted in a 2.3- and 3.1-fold increase in AA levels. (D) AA can mimic the effects of SUS treatment and act synergistically with SUS to induce apoptosis. SW480 cells were treated with AA, SUS, or behenic acid as a control fatty acid (FA) as indicated. After 48 hr of treatment, apoptosis was assessed and expressed as percent apoptotic cells − percent apoptotic cells in untreated cells (5.6% ± 2.4). Data are indicated as the mean of duplicates. Error bars = SD.
Because mouse models had demonstrated critical roles for COX2 (33) and sPLA2 (an enzyme that produces AA) (34–36) in intestinal tumorigensis, we next considered the possibility that NSAIDs’ tumor suppressive effects might be due to increased levels of the prostaglandin precursor AA resulting from inhibition of COX. To test this hypothesis, we first determined whether SUS stimulated AA accumulation. In both SW480 and HCT 116 cells, apoptosis-inducing doses of SUS increased AA by 2- to 3-fold at 24 hr and by ≈5-fold at 48 hr (Fig. 2 B and C). The AA increase after SUS treatment was as great as that following treatment with mellitin, a known activator of phospholipase A2 (data not shown). Furthermore, doses of SUS slightly lower than that required to induce apoptosis failed to induce AA release. For example, treatment of HCT116 cells with 50 μM SUS did not induce apoptosis and did not increase AA levels (0.90, 1.05, and 1.14 relative to control after 8, 16, and 24 hr of treatment, respectively).
The elevated levels of AA is not simply the result of apoptosis. Treatment with 200 μM SUS for 16 hr resulted in a 5.1- and 3.4-fold increase in AA in HCT116 and SW480 cells, respectively. At this time point, the majority of cells had not yet undergone apoptosis. In contrast, treatment with 100 μM ceramide for 16 hr resulted in nearly complete (>90%) induction of apoptosis but only modest (1.5- and 1.9-fold) increases in AA in HCT116 and SW480 cells, respectively.
If increased AA is the true mediator of SUS activity then AA should mimic its effects. Accordingly, we found that 200 μM AA was a potent inducer of apoptosis (Fig. 2D). In contrast, 200 μM behenic acid, a control fatty acid that is not a substrate for COX, had virtually no effect on apoptosis. Importantly, the effects of suboptimal SUS and AA treatment were synergistic in their ability to induce apoptosis (Fig. 2D). These results solidly support the role of AA in SUS chemoprevention and provide strong evidence against the possibility that SUS functions predominantly by the classic prostaglandin-dependent model in which an antagonistic rather than a synergistic response would be expected for AA.
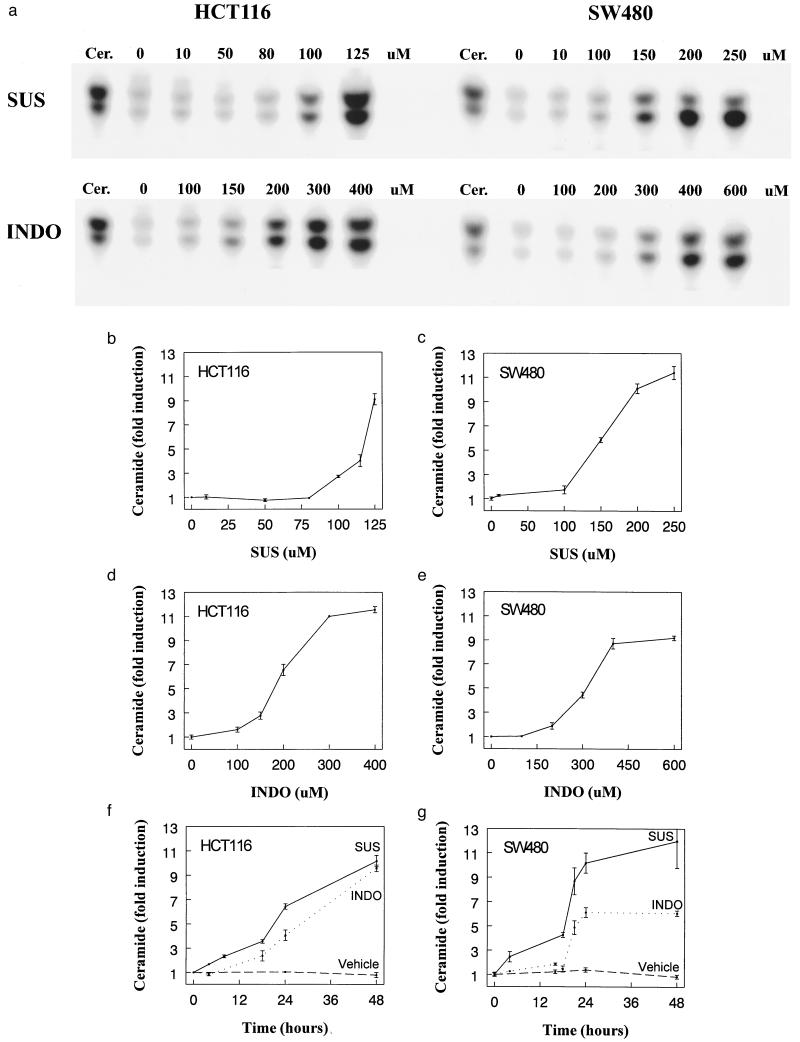
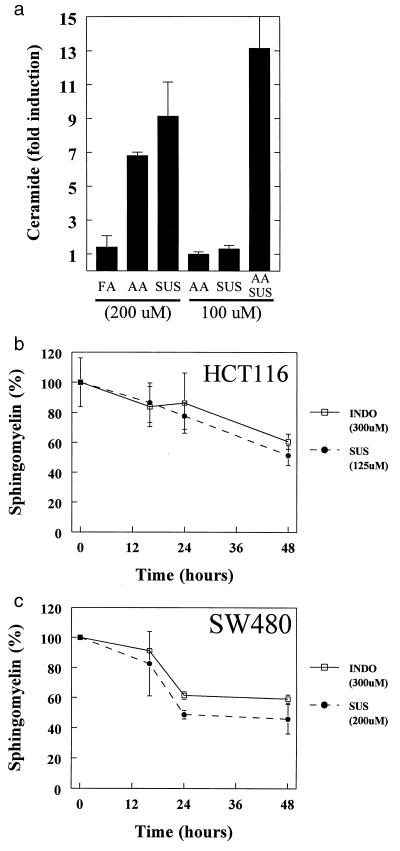
How could SUS induce apoptosis though elevation of AA levels? AA is known to stimulate neutral sphingomyelinase (18, 37), which catalyzes the conversion of sphingomyelin to ceramide, a known inducer of apoptosis. Consistent with this possibility, we found that SUS dramatically stimulated the production of ceramide (Fig. 3 A–C), with maximum induction at 24–48 hr (Fig. 3 F and G). The magnitude of the ceramide increase was especially impressive (10-fold), as the induction of ceramide in other apoptotic systems is often much less. Control experiments demonstrated that these increases in ceramide were not simply the result of apoptosis (see Materials and Methods). Also, as with apoptosis and AA induction, there was a remarkable threshold in the SUS doses required for ceramide increases. At a concentration of 125 μM, SUS induced both cell death and ceramide in HCT116 cells (Fig. 3B and data not shown), but at 50 μM, SUS induced neither. Similarly, SUS at 200 μM, but not at 100 μM, induced both ceramide and cell death in SW480 cells (Fig. 3C and data not shown). Finally, as with apoptosis, the induction of ceramide could be achieved by directly adding AA and strong synergism between suboptimal doses of AA and SUS was observed (Fig. 4A). For example, doses of AA and SUS (100 μM each) that had little effect on ceramide levels separately resulted in a >10-fold increase in ceramide together.
Figure 3.
NSAID treatment induces ceramide generation. (A) Ceramide increases in a dose-dependent fashion in colon cancer cells treated with SUS or INDO. HCT116 and SW480 cells were treated with the indicated concentration of SUS and INDO for 48 hr. Cells were harvested and ceramide was quantified as described in Materials and Methods. The first lane of each panel corresponds to a ceramide standard (ceramide type III, Sigma). (B–E) Dose response of ceramide induction. HCT116 and SW480 cells were treated with SUS or INDO as indicated. Ceramide levels were determined as described in the Methods. Data shows mean of at least two experiments. Error bars = SD. Dimethyl sulfoxide vehicle controls (using equivalent volumes) were performed in parallel with the drug treatments; no significant ceramide induction resulted (not shown). (F, G) Time course of ceramide induction. HCT116 and SW480 cells were treated with SUS (125 μM for HCT116 and 200 μM for SW480) or INDO (300 μM). Control cells were treated with dimethyl sulfoxide vehicle only. Cells were harvested at the indicated times following treatment and ceramide levels were determined as described in Materials and Methods. The data are plotted as the mean of at least two experiments. Error bars = SD.
Figure 4.
Ceramide production in NSAID-mediated apoptosis. (A) AA can mimic the effects of SUS treatment and act synergistically with SUS to induce ceramide. SW480 cells were treated with AA and SUS as indicated. After 48 hr of treatment ceramide increases were assessed and are expressed as fold increases relative to untreated cells. Data are indicated as the mean of duplicates. Error bars = SD. (B, C) Induction of sphingomyelin turnover in response to NSAID treatment. Sphingomyelin levels at each time point are plotted as a percentage of that in untreated control cells. For each point, the mean of at least two independent experiments is presented. Error bars = SD.
To test whether the increased ceramide was associated with evidence of augmented sphingomyelinase activity, we measured total sphingomyelin levels after SUS treatment. Sphingomyelin levels decreased by 40–50% at 48 hr after SUS treatment and, as with the other responses evaluated, suboptimal doses of SUS were inadequate to decrease sphingomyelin levels (Fig. 4 B and C). We also measured neutral sphingomyelinase activity in lysates from the treated cells, and no differences were found (see Materials and Methods). This was consistent with the idea that it was not an increased concentration of enzyme, but rather an AA-mediated stimulation of activity, that was responsible for the decrease in sphingomyelin and the increase in ceramide. The interaction between AA and neutral sphingomyelinase would not have been predicted to be preserved during preparation of cellular lysates (18).
If our model for sulindac action is valid, the biochemical events described above would be predicted to be induced by any inhibitor of COX, not simply SUS. To test this prediction, we treated the cells with INDO, an NSAID structurally distinct from sulindac but that also displays tumor suppressive activity (reviewed in ref. 38). INDO was found to induce apoptosis, increase AA and ceramide concentrations, and activate sphingomyelin hydrolysis to similar degrees as SUS (Figs. 1 A and B; 2 B and C, 3 A, D–G; 4 B and C). For example, in SW480 cells, INDO induced a 3- to 4-fold increase in AA, a 6-fold increase in ceramide, and resulted in 94% of the cells undergoing apoptosis in a CHX-sensitive manner.
DISCUSSION
The results described above suggest a mechanism for the chemopreventive activity of NSAIDs (Fig. 5). NSAIDs such as sulindac and INDO appear to affect tumor growth by inhibiting COX activity, causing a build-up of the COX substrate AA (Fig. 2 B–D), and activating sphingomyelinase activity (Fig. 4 B and C) leading to production of the powerful apoptosis-inducer ceramide (Fig. 3). This ability of SUS is not limited to colon cancer cells; primary fibroblasts and immortalized keratinocytes also increase ceramide and undergo apoptosis in response to SUS (data not shown). This is consistent with NSAIDs’ ability to reduce tumor formation in a variety of tissues but does not explain the apparent greater sensitivity of tumor cells. The in vivo effect will likely depend on the local concentration of the NSAID in the incipient tumor microenvironment as well as the target cell’s “lipid biostat” (39). The fact that the concentration of SUS is higher in the intestines than in other organs, due to enterohepatic circulation and to the metabolic activation of sulindac to SUS by gut flora (40), may be responsible for its particular efficacy in intestinal tumorigenesis.
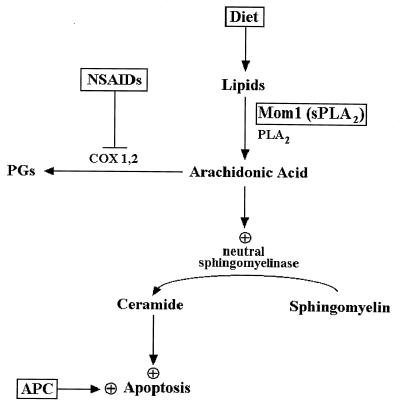
Figure 5.
Model relating AA and ceramide to colorectal cancer chemoprevention. Mutation of the APC gene initiates colorectal tumorigenesis and results in abnormally decreased apoptosis (16). Perturbation of lipid metabolism by pharmacological, dietary, or genetic means can partially correct the deficits. AA is generated by cytosolic and secreted phospholipase A2, which hydrolyzes plasma membrane lipids or lipids derived from the diet. AA is normally used as a substrate by the COXs to produce eicosanoids such as prostaglandins. NSAIDs inhibit the activity of the COXs, which increases the cellular pool of AA. AA stimulates neutral sphingomyelinase activity (18, 37), which catalyzes the hydrolysis of sphingomyelin to generate ceramide. Ceramide acts as second messenger that activates the cellular apoptotic machinery. The Mom1 gene is a modifier of polyp formation in the Min mouse, a model for APC mutation-induced colon tumors. The Mom1 gene encodes a secreted phospholipase A2, an enzyme predicted to increase the level of free AA. Inactivating mutations in Mom1 predispose Min mice to developing intestinal polyps and would be expected to result in reduced levels of AA (34–36). This in turn could result in decreased production of ceramide and therefore a relative resistance to programmed cell death and increased tumor susceptibility. The lipid compositions of diets are known to affect colon cancer risk (41, 42). Diets rich in unsaturated fatty acids such as AA are associated with a decreased incidence of colon cancer (43). This effect could be due to increased levels of AA and subsequent increased susceptibility to apoptosis as described above.
The model depicted in Fig. 5 links together several apparently disparate observations. Patients genetically predisposed to colorectal tumorigenesis have a defect in a gene (adenomatous polyposis coli, or APC) that can induce apoptosis in neoplastic colorectal epithelium (16). The efficacy of sulindac in such patients may be attributable to its functional substitution for APC in such cells. It has also been shown that modification of lipid metabolism, through inherited mutation of genes encoding a secreted phospholipase, can dramatically alter tumor incidence in mice with germline mutations of APC (34–36), and that certain dietary lipids are correlated with colorectal cancer incidence in human populations (41, 42). Finally, recent studies with COX-2 null mice strongly implicate inhibition of COX activity as a critical effector of NSAID chemoprevention (33). All of these observations are consistent with the idea, summarized in Fig. 5, that lipids, in particular ceramide and AA, play pivotal roles in protecting humans and mice from colorectal tumorigenesis through the control of apoptosis. Accordingly, manipulation of the lipids, by dietary or pharmaceutical agents, could lead to improved methods for the prevention or treatment of colorectal tumors.
Acknowledgments
We thank Drs. Supriya Jayadev, Subroto Chatterjee, and Jay C. Strum for helpful discussions and technical advice. We thank Dr. Kornelia Polyak for providing reagents and expertise for the p53 adenovirus studies, Dr. Walter Hubbard for performing the gas chromatographic analysis of AA, and Dr. Robert Coffey for suggesting specific prostaglandins to test. Genzyme Molecular Oncology provided research support to K.W.K. The University and researchers (K.W.K. and B.V.) have a financial interest in Genzyme Molecular Oncology, the arrangements for which are managed by the University in accordance with its conflict of interest policies. This work was supported by the Clayton Fund and by National institutes of Health Grants CA57345 and CA62924. B.V. is an Investigator of the Howard Hughes Medical Institute.
ABBREVIATIONS
- AA
arachidonic acid
- APC
adenomatous polyposis coli
- CHX
cycloheximide
- COX
cyclooxygenase
- NSAID
nonsteroidal antiinflammatory drug
- SUS
sulindac sulfide
- INDO
indomethacin
References
- 1.Giardiello F M. Gastroenterol Clin North Am. 1996;25:349–362. doi: 10.1016/s0889-8553(05)70251-x. [DOI] [PubMed] [Google Scholar]
- 2.Beazer-Barclay Y, Levy D B, Moser A M, Dove W F, Hamilton S R, Vogelstein B, Kinzler K W. Carcinogenesis. 1996;17:1757–1760. doi: 10.1093/carcin/17.8.1757. [DOI] [PubMed] [Google Scholar]
- 3.Boolbol S K, Dannenberg A J, Chadurn A, Martucci C, Guo X, Ramonetti J T, Abreu-Goris M, Newmark H L, Lipkin M L, DeCosse J J, Bertagnolli M M. Cancer Res. 1996;56:2556–2560. [PubMed] [Google Scholar]
- 4.Jacoby R F, Marshall D J, Newton M A, Novakovic K, Tutsch K, Cole C E, Lubet R A, Kelloff G J, Verma A, Moser A R, Dove W F. Cancer Res. 1996;56:710–714. [PubMed] [Google Scholar]
- 5.Thun M J. Gastroenterol Clin North Am. 1996;25:333–48. doi: 10.1016/s0889-8553(05)70250-8. [DOI] [PubMed] [Google Scholar]
- 6.Vane J R. Br J Rheumatol. 1996;35:1–3. doi: 10.1093/rheumatology/35.suppl_1.1. [DOI] [PubMed] [Google Scholar]
- 7.Piazza G A, Rahm A L K, Krutzsch M, Sperl G, Paranka N S, Gross P H, Brendel K, Burt R W, Alberts D S, Pamukcu R, Ahnen D J. Cancer Res. 1995;55:3110–3116. [PubMed] [Google Scholar]
- 8.Lu X, Xie W, Reed D, Bradshaw W S, Simmons D L. Proc Natl Acad Sci USA. 1995;92:7961–7965. doi: 10.1073/pnas.92.17.7961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pasricha P J, Bedi A, K, O C, Rashid A, Akhtar A J, Zahurak M L, Piantadosi S, Hamilton S R, Giardiello F M. Gastroenterology. 1995;109:994–998. doi: 10.1016/0016-5085(95)90411-5. [DOI] [PubMed] [Google Scholar]
- 10.Shiff S J, Qiao L, Tsai L L, Rigas B. J Clin Invest. 1995;96:491–503. doi: 10.1172/JCI118060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shiff S J, Koutsos M I, Qiao L, Rigas B. Exp Cell Res. 1996;222:179–188. doi: 10.1006/excr.1996.0023. [DOI] [PubMed] [Google Scholar]
- 12.Eberhart C E, Coffey R J, Radhika A, Giardiello F M, Ferrenbach S, DuBois R N. Gastroenterology. 1994;107:1183–1188. doi: 10.1016/0016-5085(94)90246-1. [DOI] [PubMed] [Google Scholar]
- 13.Sano H, Kawahito Y, Wilder R L, Hashiramoto A, Mukai S, Asai K, Kimura S, Kato H, Kondo M, Hla T. Cancer Res. 1995;55:3785–3789. [PubMed] [Google Scholar]
- 14.Kutchera W, Jones D A, Matsunami N, Groden J, McIntyre T M, Zimmerman G A, White R L, Prescott S M. Proc Natl Acad Sci USA. 1996;93:4816–4820. doi: 10.1073/pnas.93.10.4816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsujii M, Dubois R N. Cell. 1995;83:493–501. doi: 10.1016/0092-8674(95)90127-2. [DOI] [PubMed] [Google Scholar]
- 16.Morin P J, Vogelstein B, Kinzler K W. Proc Natl Acad Sci USA. 1996;93:7950–7954. doi: 10.1073/pnas.93.15.7950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ashman R F, Peckham D, Alhasan S, Stunz L L. Immunol Lett. 1995;48:159–166. doi: 10.1016/0165-2478(95)02471-9. [DOI] [PubMed] [Google Scholar]
- 18.Jayadev S, Linardic C M, Hannun Y A. J Biol Chem. 1994;269:5757–5763. [PubMed] [Google Scholar]
- 19.Lands W E, Samuelsson B. Biochim Biophys Acta. 1968;164:426–429. doi: 10.1016/0005-2760(68)90168-9. [DOI] [PubMed] [Google Scholar]
- 20.MacGlashan D W, Jr, Hubbard W C. J Immunol. 1993;151:6358–6369. [PubMed] [Google Scholar]
- 21.Preiss J, Loomis C R, Bishop W R, Stein R, Niedel J E, Bell R M. J Biol Chem. 1986;261:8597–8600. [PubMed] [Google Scholar]
- 22.Van Veldhoven P P, Bell R M. Biochim Biophys Acta. 1988;959:185–196. doi: 10.1016/0005-2760(88)90030-6. [DOI] [PubMed] [Google Scholar]
- 23.Van Veldhoven P P, Matthews T J, Bolognesi D P, Bell R M. Biochem Biophys Res Commun. 1992;187:209–216. doi: 10.1016/s0006-291x(05)81480-9. [DOI] [PubMed] [Google Scholar]
- 24.Polyak K, Waldman T, He T-C, Kinzler K W, Vogelstein B. Genes Dev. 1996;10:1945–1952. doi: 10.1101/gad.10.15.1945. [DOI] [PubMed] [Google Scholar]
- 25.El-Deiry W S, Tokino T, Velculescu V E, Levy D B, Parsons R, Trent J M, Lin D, Mercer W E, Kinzler K W, Vogelstein B. Cell. 1993;75:817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- 26.Wyllie A H. Curr Opin Genet Dev. 1995;5:97–104. doi: 10.1016/s0959-437x(95)90060-8. [DOI] [PubMed] [Google Scholar]
- 27.Fadok, V. A., Voelker, D. R., Campbell, P. A., Bratton, D. L., Cohen, J. J., Noble, P. W., Riches, D. W. & Henson, P. M. (1993) Chest 103, Suppl., 102S. [DOI] [PubMed]
- 28.Martin S J, Reutelingsperger C P, McGahon A J, Rader J A, van Schie R C, LaFace D M, Green D R. J Exp Med. 1995;182:1545–1556. doi: 10.1084/jem.182.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lennon S V, Martin S J, Cotter T G. Cell Prolif. 1991;24:203–214. doi: 10.1111/j.1365-2184.1991.tb01150.x. [DOI] [PubMed] [Google Scholar]
- 30.Rigas B, Goldman I S, Levine L. J Lab Clin Med. 1993;122:518–523. [PubMed] [Google Scholar]
- 31.Hanif R, Pittas A, Feng Y, Koutsos M I, Qiao L, Staiano-Coico L, Shiff S I, Rigas B. Biochem Pharmacol. 1996;52:237–245. doi: 10.1016/0006-2952(96)00181-5. [DOI] [PubMed] [Google Scholar]
- 32.Chiu C-H, McEntee M F, Whelan J. Cancer Res. 1997;57:4267–4273. [PubMed] [Google Scholar]
- 33.Oshima M, Dinchuk J E, Kargman S L, Oshima H, Hancock B, Kwong E, Trzaskos J M, Evans J F, Taketo M M. Cell. 1996;87:803–809. doi: 10.1016/s0092-8674(00)81988-1. [DOI] [PubMed] [Google Scholar]
- 34.Dietrich W F, Lander E S, Smith J S, Moser A R, Gould K A, Luongo C, Borenstein N, Dove W. Cell. 1993;75:631–639. doi: 10.1016/0092-8674(93)90484-8. [DOI] [PubMed] [Google Scholar]
- 35.MacPhee M, Chepenik K, Liddell R, Nelson K, Siracusa L, Buchberg A. Cell. 1995;81:957–966. doi: 10.1016/0092-8674(95)90015-2. [DOI] [PubMed] [Google Scholar]
- 36.Cormier R T, Hong K H, Halberg R B, Hawkins T L, Richardson P, Mulherkar R, Dove W F, Lander E S. Nat Genet. 1997;17:88–91. doi: 10.1038/ng0997-88. [DOI] [PubMed] [Google Scholar]
- 37.Jayadev S, Hayter H L, Andrieu N, Gamard C J, Liu B, Balu R, Hayakawa M, Ito F, Hanun Y A. J Biol Chem. 1997;272:17196–17203. doi: 10.1074/jbc.272.27.17196. [DOI] [PubMed] [Google Scholar]
- 38.DuBois R N, Giardiello F M, Smalley W E. Gastroenterol Clin North Am. 1996;25:773–791. doi: 10.1016/s0889-8553(05)70274-0. [DOI] [PubMed] [Google Scholar]
- 39.Hannun Y A. Science. 1996;274:1855–1859. doi: 10.1126/science.274.5294.1855. [DOI] [PubMed] [Google Scholar]
- 40.Duggan D E, Hooke K F, Hwang S S. Drug Metab Dispos. 1980;8:241–246. [PubMed] [Google Scholar]
- 41.Willett W C, Stampfer M J, Colditz G A, Rosner B A, Speizer F E. N Engl J Med. 1990;323:1664–1672. doi: 10.1056/NEJM199012133232404. [DOI] [PubMed] [Google Scholar]
- 42.Giovannucci E, Willett W C. Ann Med. 1994;26:443–452. doi: 10.3109/07853899409148367. [DOI] [PubMed] [Google Scholar]
- 43.Reddy B S. Cancer Metastasis Rev. 1994;13:285–302. doi: 10.1007/BF00666099. [DOI] [PubMed] [Google Scholar]