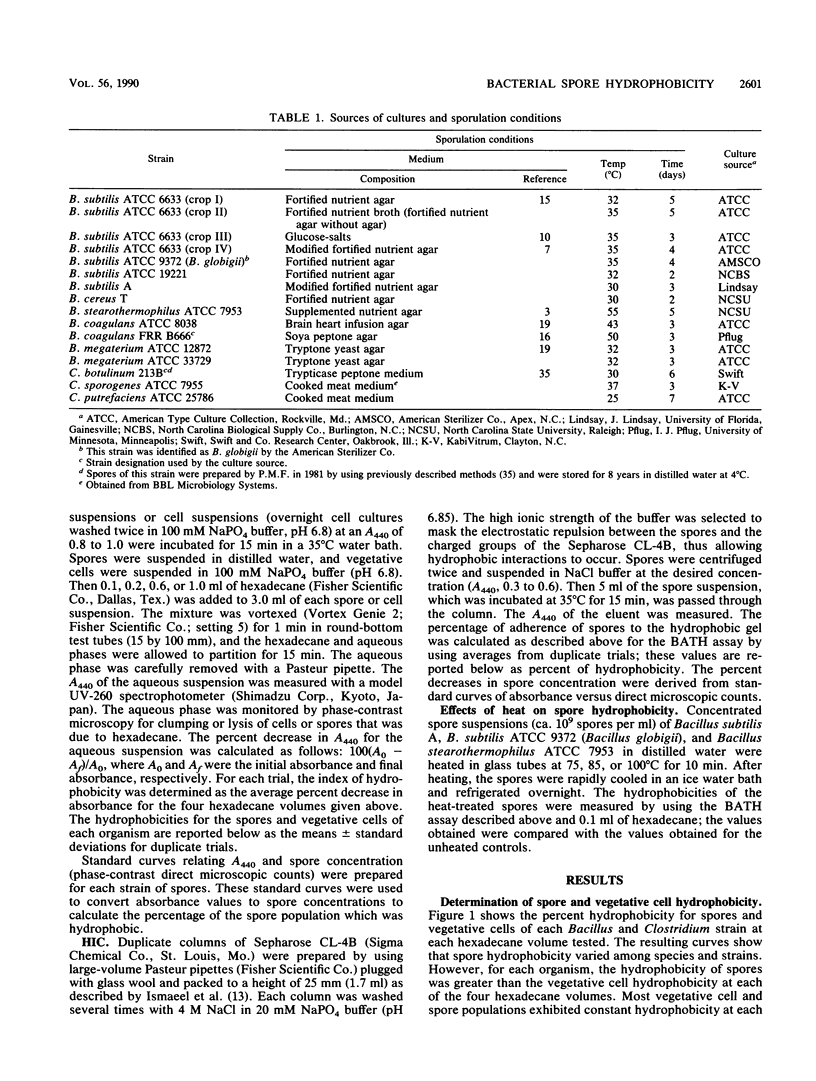
Abstract
The hydrophobicities of spores and vegetative cells of several species of the genera Bacillus and Clostridium were measured by using the bacterial adherence to hexadecane assay and hydrophobic interaction chromatography. Although spore hydrophobicity varied among species and strains, the spores of each organism were more hydrophobic than the vegetative cells. The relative hydrophobicities determined by the two methods generally agreed. Sporulation media and conditions appeared to have little effect on spore hydrophobicity. However, exposure of spore suspensions to heat treatment caused a considerable increase in spore hydrophobicity. The hydrophobic nature of Bacillus and Clostridium spores suggests that hydrophobic interactions may play a role in the adhesion of these spores to surfaces.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beck G., Puchelle E., Plotkowski C., Peslin R. Effect of growth on surface charge and hydrophobicity of Staphylococcus aureus. Ann Inst Pasteur Microbiol. 1988 Nov-Dec;139(6):655–664. doi: 10.1016/0769-2609(88)90070-1. [DOI] [PubMed] [Google Scholar]
- COOK A. M., BROWN M. R. THE RELATION BETWEEN HEAT ACTIVATION AND COLONY FORMATION FOR THE SPORES OF BACILLUS STEAROTHERMOPHILUS. J Pharm Pharmacol. 1964 Nov;16:725–732. doi: 10.1111/j.2042-7158.1964.tb07396.x. [DOI] [PubMed] [Google Scholar]
- Craven S. E., Blankenship L. C. Changes in the hydrophobic characteristics of Clostridium perfringens spores and spore coats by heat. Can J Microbiol. 1987 Sep;33(9):773–776. doi: 10.1139/m87-132. [DOI] [PubMed] [Google Scholar]
- Dickson J. S., Koohmaraie M. Cell surface charge characteristics and their relationship to bacterial attachment to meat surfaces. Appl Environ Microbiol. 1989 Apr;55(4):832–836. doi: 10.1128/aem.55.4.832-836.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards J. L., Jr, Busta F. F., Speck M. L. Thermal inactivation characteristics of Bacillus subtilis spores at ultrahigh temperatures. Appl Microbiol. 1965 Nov;13(6):851–857. doi: 10.1128/am.13.6.851-857.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges N. A., Melling J., Parker S. J. A comparison of chemically defined and complex media for the production of Bacillus subtilis spores having reproducible resistance and germination characteristics. J Pharm Pharmacol. 1980 Feb;32(2):126–130. doi: 10.1111/j.2042-7158.1980.tb12867.x. [DOI] [PubMed] [Google Scholar]
- Hogg S. D., Manning J. E. The hydrophobicity of 'viridans' streptococci isolated from the human mouth. J Appl Bacteriol. 1987 Oct;63(4):311–318. doi: 10.1111/j.1365-2672.1987.tb02708.x. [DOI] [PubMed] [Google Scholar]
- Hogt A. H., Dankert J., de Vries J. A., Feijen J. Adhesion of coagulase-negative staphylococci to biomaterials. J Gen Microbiol. 1983 Sep;129(9):2959–2968. doi: 10.1099/00221287-129-9-2959. [DOI] [PubMed] [Google Scholar]
- Jones A. T., Pflug I. J. Bacillus coagulans, FRR B666, as a potential biological indicator organism. J Parenter Sci Technol. 1981 May-Jun;35(3):82–87. [PubMed] [Google Scholar]
- Koshikawa T., Yamazaki M., Yoshimi M., Ogawa S., Yamada A., Watabe K., Torii M. Surface hydrophobicity of spores of Bacillus spp. J Gen Microbiol. 1989 Oct;135(10):2717–2722. doi: 10.1099/00221287-135-10-2717. [DOI] [PubMed] [Google Scholar]
- Kutima P. M., Foegeding P. M. Involvement of the spore coat in germination of Bacillus cereus T spores. Appl Environ Microbiol. 1987 Jan;53(1):47–52. doi: 10.1128/aem.53.1.47-52.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marszalek D. S., Gerchakov S. M., Udey L. R. Influence of substrate composition on marine microfouling. Appl Environ Microbiol. 1979 Nov;38(5):987–995. doi: 10.1128/aem.38.5.987-995.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matz L. L., Beaman T. C., Gerhardt P. Chemical composition of exosporium from spores of Bacillus cereus. J Bacteriol. 1970 Jan;101(1):196–201. doi: 10.1128/jb.101.1.196-201.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M. J., Ahearn D. G. Adherence of Pseudomonas aeruginosa to hydrophilic contact lenses and other substrata. J Clin Microbiol. 1987 Aug;25(8):1392–1397. doi: 10.1128/jcm.25.8.1392-1397.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minagi S., Miyake Y., Fujioka Y., Tsuru H., Suginaka H. Cell-surface hydrophobicity of Candida species as determined by the contact-angle and hydrocarbon-adherence methods. J Gen Microbiol. 1986 Apr;132(4):1111–1115. doi: 10.1099/00221287-132-4-1111. [DOI] [PubMed] [Google Scholar]
- Minagi S., Miyake Y., Inagaki K., Tsuru H., Suginaka H. Hydrophobic interaction in Candida albicans and Candida tropicalis adherence to various denture base resin materials. Infect Immun. 1985 Jan;47(1):11–14. doi: 10.1128/iai.47.1.11-14.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers A. H., Pilowsky K., Zilm P. S. The effect of growth rate on the adhesion of the oral bacteria Streptococcus mutans and Streptococcus milleri. Arch Oral Biol. 1984;29(2):147–150. doi: 10.1016/0003-9969(84)90119-5. [DOI] [PubMed] [Google Scholar]
- Takubo Y., Atarashi M., Nishihara T., Kondo M. Isolation and characterization of outermost layer deficient mutant spores of Bacillus megaterium. Microbiol Immunol. 1988;32(9):973–979. doi: 10.1111/j.1348-0421.1988.tb01460.x. [DOI] [PubMed] [Google Scholar]
- Takumi K., Kinouchi T., Kawata T. Isolation and partial characterization of exosporium from spores of a highly sporogenic mutant of Clostridium botulinum type A. Microbiol Immunol. 1979;23(6):443–454. doi: 10.1111/j.1348-0421.1979.tb00484.x. [DOI] [PubMed] [Google Scholar]
- van Loosdrecht M. C., Lyklema J., Norde W., Schraa G., Zehnder A. J. The role of bacterial cell wall hydrophobicity in adhesion. Appl Environ Microbiol. 1987 Aug;53(8):1893–1897. doi: 10.1128/aem.53.8.1893-1897.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Pelt A. W., Weerkamp A. H., Uyen M. H., Busscher H. J., de Jong H. P., Arends J. Adhesion of Streptococcus sanguis CH3 to polymers with different surface free energies. Appl Environ Microbiol. 1985 May;49(5):1270–1275. doi: 10.1128/aem.49.5.1270-1275.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]



