Abstract
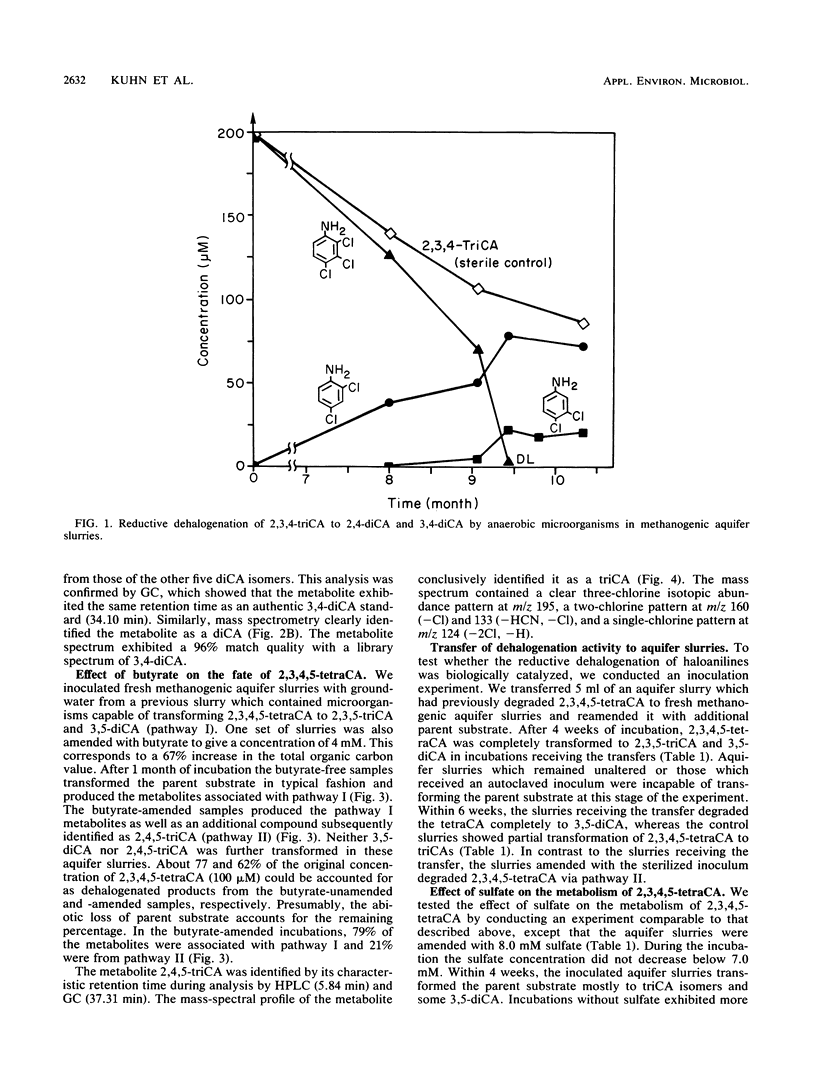
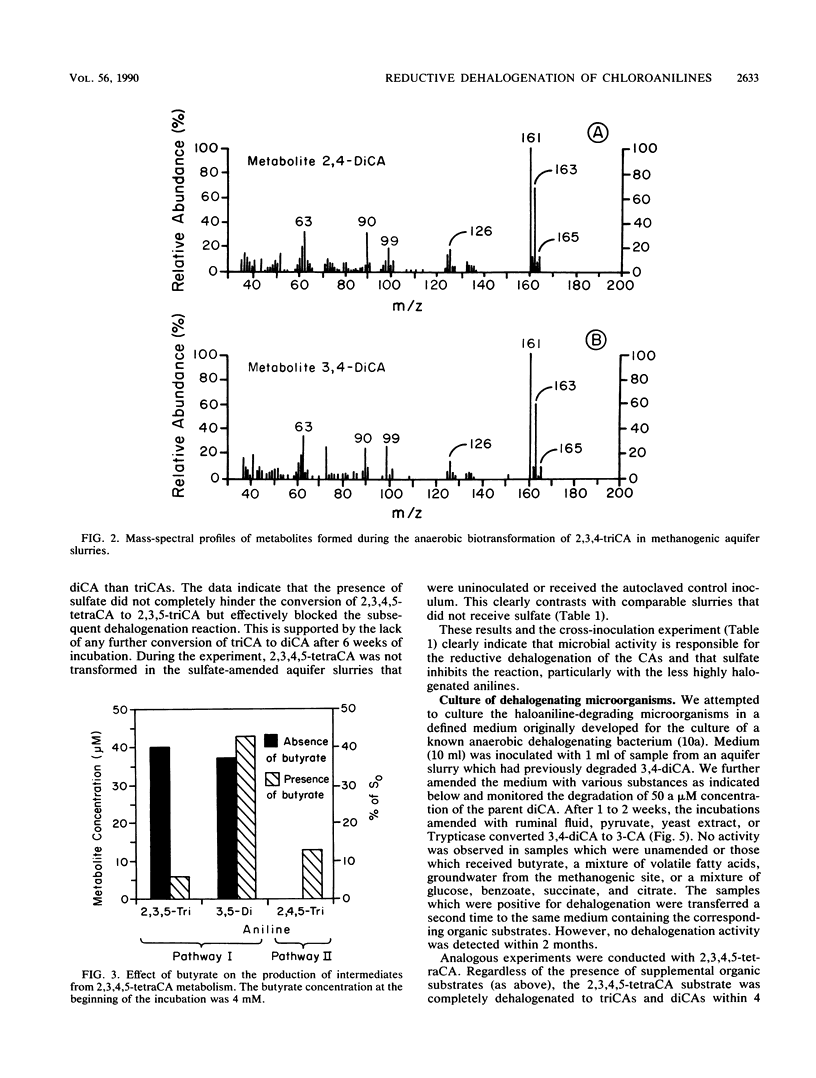
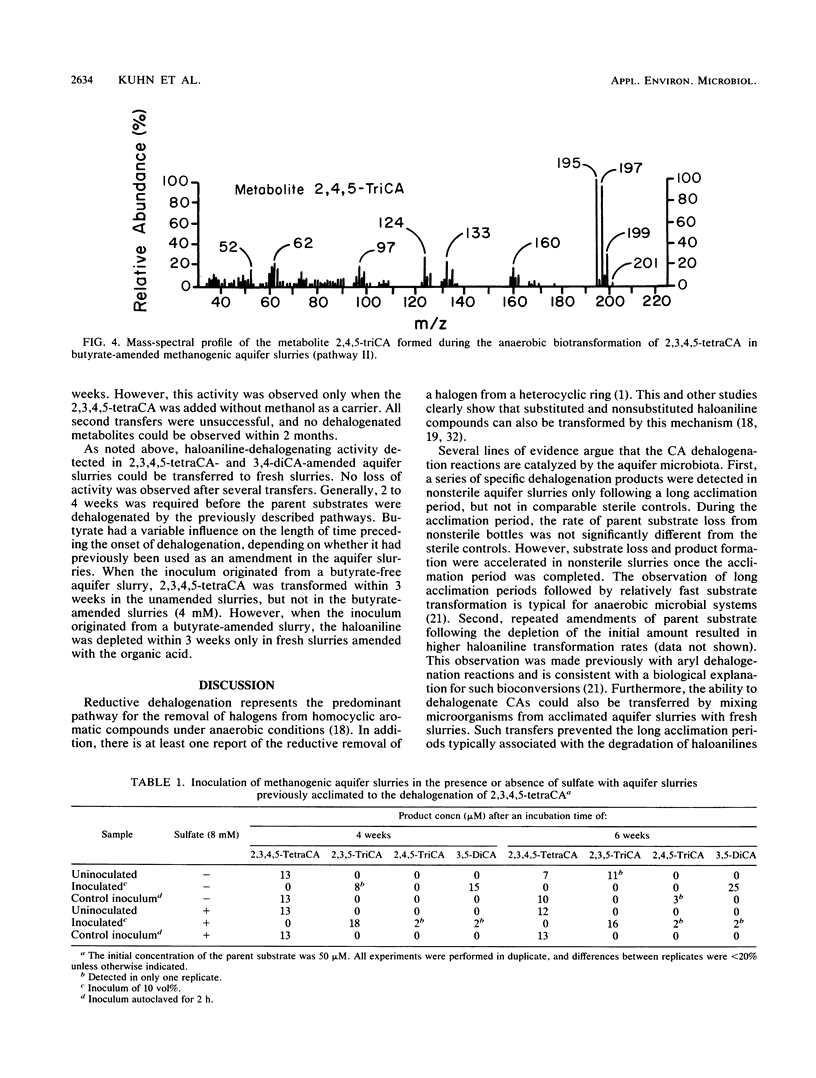
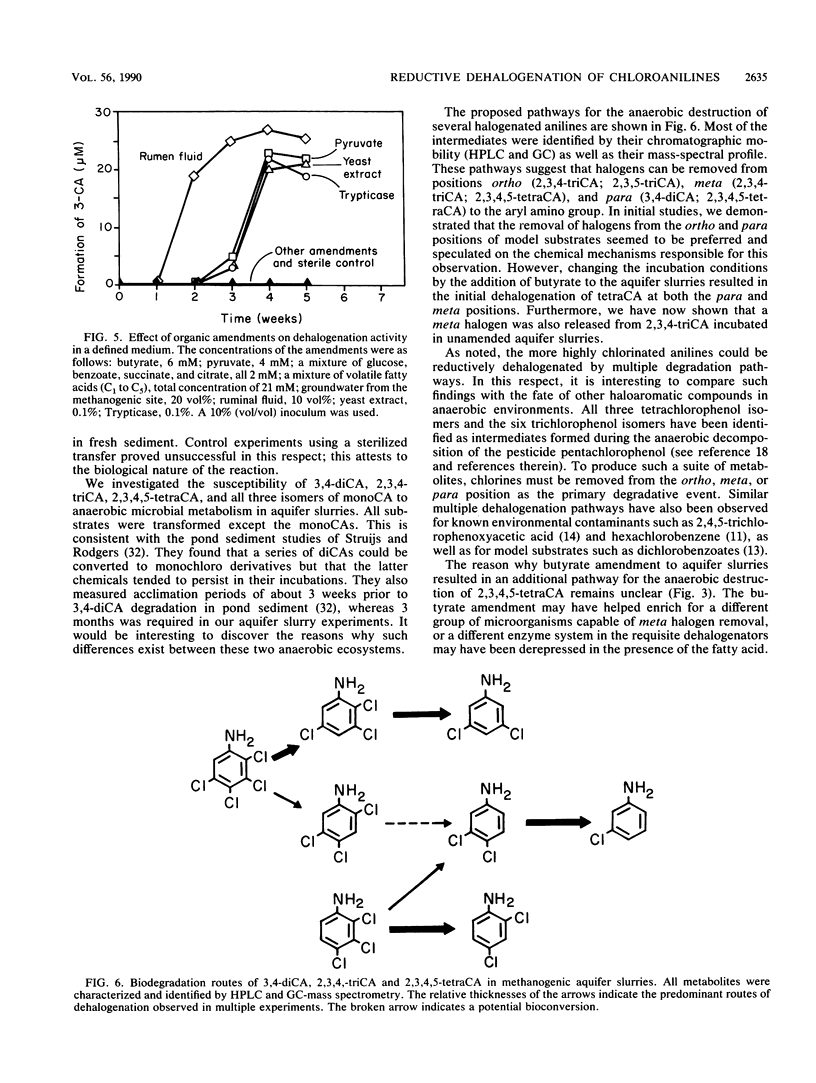
When di-, tri-, and tetrachloroaniline were incubated in methanogenic groundwater slurries, they were reductively dehalogenated by the aquifer microbiota. 2,3,4-Trichloroaniline was metabolized by two pathways. Primary dehalogenation occurred at either the meta or ortho position of this substrate to form 2,4- and 3,4-dichloroaniline, respectively. The latter chemical could be stoichiometrically converted to 3-chloroaniline. 2,3,4,5-Tetrachloroaniline was degraded by the sequential removal of halogens from the para and then the ortho position to form 3,5-dichloroaniline. An additional pathway was observed with this substrate when the aquifer slurries were amended with butyrate. That is, halogens could be removed from both the meta and ortho positions of tetrachloroaniline. The amendment of sulfate to methanogenic aquifer slurries slowed the rate of 2,3,4,5-tetrachloroaniline degradation and increased the amount of substrate channeled through the additional pathway. The reported intermediates or end products are identified by their chromatographic mobility and mass-spectral profiles.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian N. R., Suflita J. M. Reductive dehalogenation of a nitrogen heterocyclic herbicide in anoxic aquifer slurries. Appl Environ Microbiol. 1990 Jan;56(1):292–294. doi: 10.1128/aem.56.1.292-294.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balba M. T., Evans W. C. Methanogenic fermentation of the naturally occurring aromatic amino acids by a microbial consortium. Biochem Soc Trans. 1980 Oct;8(5):625–627. doi: 10.1042/bst0080625. [DOI] [PubMed] [Google Scholar]
- Bartha R. Fate of herbicide-derived chloroanilines in soil. J Agric Food Chem. 1971 Mar-Apr;19(2):385–387. doi: 10.1021/jf60174a024. [DOI] [PubMed] [Google Scholar]
- Beeman R. E., Suflita J. M. Environmental factors influencing methanogenesis in a shallow anoxic aquifer: a field and laboratory study. J Ind Microbiol. 1990 Jan;5(1):45–57. doi: 10.1007/BF01569605. [DOI] [PubMed] [Google Scholar]
- Berry D. F., Francis A. J., Bollag J. M. Microbial metabolism of homocyclic and heterocyclic aromatic compounds under anaerobic conditions. Microbiol Rev. 1987 Mar;51(1):43–59. doi: 10.1128/mr.51.1.43-59.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fathepure B. Z., Tiedje J. M., Boyd S. A. Reductive dechlorination of hexachlorobenzene to tri- and dichlorobenzenes in anaerobic sewage sludge. Appl Environ Microbiol. 1988 Feb;54(2):327–330. doi: 10.1128/aem.54.2.327-330.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genthner B. R., Price W. A., Pritchard P. H. Anaerobic Degradation of Chloroaromatic Compounds in Aquatic Sediments under a Variety of Enrichment Conditions. Appl Environ Microbiol. 1989 Jun;55(6):1466–1471. doi: 10.1128/aem.55.6.1466-1471.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson S. A., Suflita J. M. Anaerobic biodegradation of 2,4,5-trichlorophenoxyacetic Acid in samples from a methanogenic aquifer: stimulation by short-chain organic acids and alcohols. Appl Environ Microbiol. 1990 Jun;56(6):1825–1832. doi: 10.1128/aem.56.6.1825-1832.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson S. A., Suflita J. M. Extrapolation of biodegradation results to groundwater aquifers: reductive dehalogenation of aromatic compounds. Appl Environ Microbiol. 1986 Oct;52(4):681–688. doi: 10.1128/aem.52.4.681-688.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häggblom M. M., Janke D., Salkinoja-Salonen M. S. Hydroxylation and Dechlorination of Tetrachlorohydroquinone by Rhodococcus sp. Strain CP-2 Cell Extracts. Appl Environ Microbiol. 1989 Feb;55(2):516–519. doi: 10.1128/aem.55.2.516-519.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King G. M. Dehalogenation in marine sediments containing natural sources of halophenols. Appl Environ Microbiol. 1988 Dec;54(12):3079–3085. doi: 10.1128/aem.54.12.3079-3085.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohring G. W., Zhang X. M., Wiegel J. Anaerobic dechlorination of 2,4-dichlorophenol in freshwater sediments in the presence of sulfate. Appl Environ Microbiol. 1989 Oct;55(10):2735–2737. doi: 10.1128/aem.55.10.2735-2737.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M. J., Zinder S. H. Hydrogen partial pressures in a thermophilic acetate-oxidizing methanogenic coculture. Appl Environ Microbiol. 1988 Jun;54(6):1457–1461. doi: 10.1128/aem.54.6.1457-1461.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linkfield T. G., Suflita J. M., Tiedje J. M. Characterization of the acclimation period before anaerobic dehalogenation of halobenzoates. Appl Environ Microbiol. 1989 Nov;55(11):2773–2778. doi: 10.1128/aem.55.11.2773-2778.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linkfield T. G., Tiedje J. M. Characterization of the requirements and substrates for reductive dehalogenation by strain DCB-1. J Ind Microbiol. 1990 Jan;5(1):9–15. doi: 10.1007/BF01569601. [DOI] [PubMed] [Google Scholar]
- Mogilevich N. F., Tashirev A. B., Romanova E. A. Transformatsiia n-khloranilina, vyzyvaemaia Escherichia coli v anaérobnykh usloviiakh. Mikrobiologiia. 1987 Mar-Apr;56(2):205–208. [PubMed] [Google Scholar]
- Müller R., Thiele J., Klages U., Lingens F. Incorporation of [18O]water into 4-hydroxybenzoic acid in the reaction of 4-chlorobenzoate dehalogenase from pseudomonas spec. CBS 3. Biochem Biophys Res Commun. 1984 Oct 15;124(1):178–182. doi: 10.1016/0006-291x(84)90933-1. [DOI] [PubMed] [Google Scholar]
- Neilson A. H., Allard A. S., Lindgren C., Remberger M. Transformations of chloroguaiacols, chloroveratroles, and chlorocatechols by stable consortia of anaerobic bacteria. Appl Environ Microbiol. 1987 Oct;53(10):2511–2519. doi: 10.1128/aem.53.10.2511-2519.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J. A., Strayer R. F., Tiedje J. M. Method for measuring dissolved hydrogen in anaerobic ecosystems: application to the rumen. Appl Environ Microbiol. 1981 Feb;41(2):545–548. doi: 10.1128/aem.41.2.545-548.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struijs J., Rogers J. E. Reductive dehalogenation of dichloroanilines by anaerobic microorganisms in fresh and dichlorophenol-acclimated pond sediment. Appl Environ Microbiol. 1989 Oct;55(10):2527–2531. doi: 10.1128/aem.55.10.2527-2531.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Tweel W. J., Kok J. B., de Bont J. A. Reductive dechlorination of 2,4-dichlorobenzoate to 4-chlorobenzoate and hydrolytic dehalogenation of 4-chloro-, 4-bromo-, and 4-iodobenzoate by Alcaligenes denitrificans NTB-1. Appl Environ Microbiol. 1987 Apr;53(4):810–815. doi: 10.1128/aem.53.4.810-815.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]



