Abstract
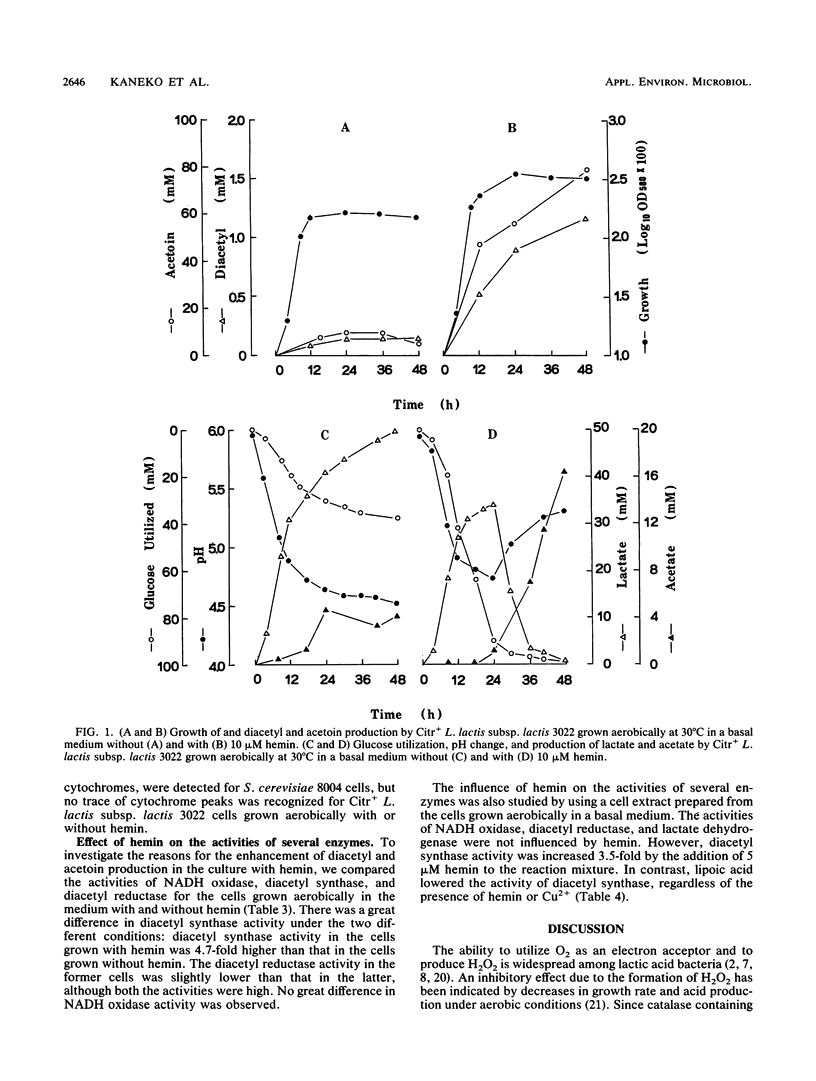
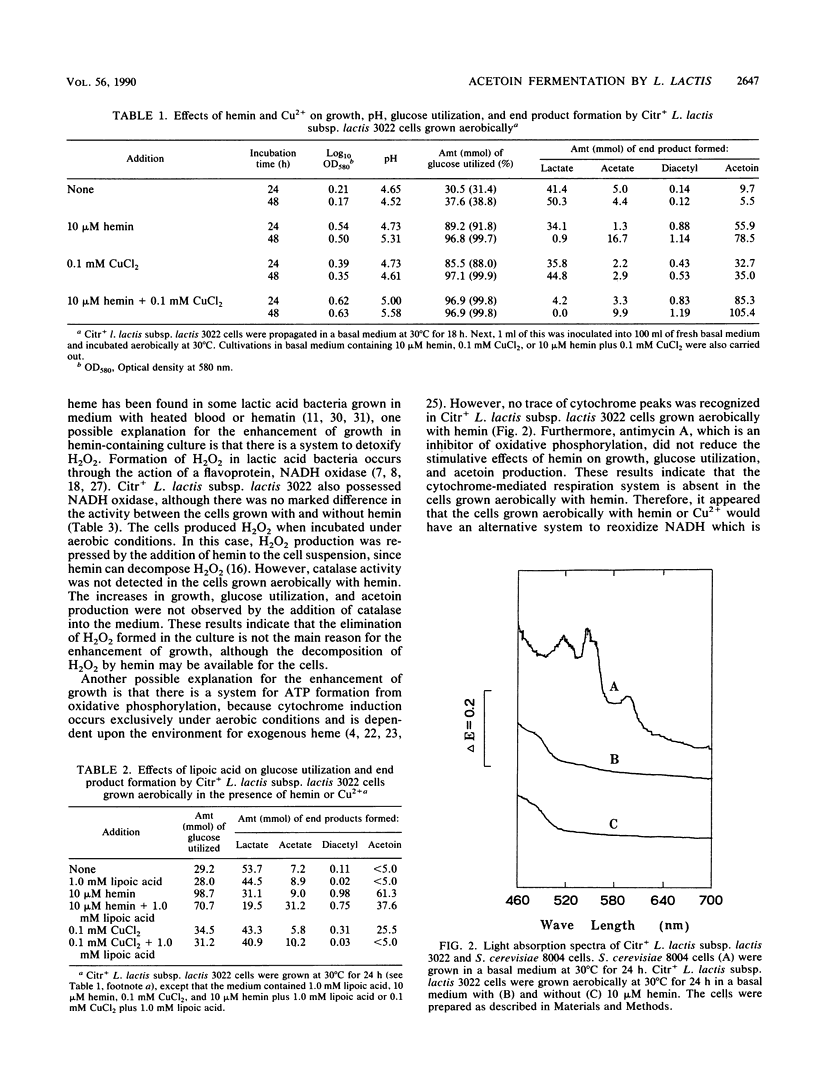
Citr+Lactococcus lactis subsp. lactis 3022 produced more biomass and converted most of the glucose substrate to diacetyl and acetoin when grown aerobically with hemin and Cu2+. The activity of diacetyl synthase was greatly stimulated by the addition of hemin or Cu2+, and the activity of NAD-dependent diacetyl reductase was very high. Hemin did not affect the activities of NADH oxidase and lactate dehydrogenase. These results indicated that the pyruvate formed via glycolysis would be rapidly converted to diacetyl and that the diacetyl would then be converted to acetoin by the NAD-dependent diacetyl reductase to reoxidize NADH when the cells were grown aerobically with hemin or Cu2+. On the other hand, the YGlu value for the hemincontaining culture was lower than for the culture without hemin, because acetate production was repressed when an excess of glucose was present. However, in the presence of lipoic acid, an essential cofactor of the dihydrolipoamide acetyltransferase part of the pyruvate dehydrogenase complex, hemin or Cu2+ enhanced acetate production and then repressed diacetyl and acetoin production. The activity of diacetyl synthase was lowered by the addition of lipoic acid. These results indicate that hemin or Cu2+ stimulates acetyl coenzyme A (acetyl-CoA) formation from pyruvate and that lipoic acid inhibits the condensation of acetyl-CoA with hydroxyethylthiamine PPi. In addition, it appears that acetyl-CoA not used for diacetyl synthesis is converted to acetate.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbe K., Takahashi S., Yamada T. Involvement of oxygen-sensitive pyruvate formate-lyase in mixed-acid fermentation by Streptococcus mutans under strictly anaerobic conditions. J Bacteriol. 1982 Oct;152(1):175–182. doi: 10.1128/jb.152.1.175-182.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruhn J. C., Collins E. B. Reduced nicotinamide adenine dinucleotide oxidase of Streptococcus diacetilactis. J Dairy Sci. 1970 Jul;53(7):857–860. doi: 10.3168/jds.S0022-0302(70)86307-X. [DOI] [PubMed] [Google Scholar]
- Bryan-Jones D. G., Whittenbury R. Haematin-dependent oxidative phosphorylation in Streptococcus faecalis. J Gen Microbiol. 1969 Oct;58(2):247–260. doi: 10.1099/00221287-58-2-247. [DOI] [PubMed] [Google Scholar]
- Cogan T. M. Citrate utilization in milk by Leuconostoc cremoris and Streptococcus diacetilactis. J Dairy Res. 1975 Feb;42(1):139–146. doi: 10.1017/s0022029900015168. [DOI] [PubMed] [Google Scholar]
- Collins E. B., Aramaki K. Production of Hydrogen peroxide by Lactobacillus acidophilus. J Dairy Sci. 1980 Mar;63(3):353–357. doi: 10.3168/jds.S0022-0302(80)82938-9. [DOI] [PubMed] [Google Scholar]
- Drinan D. F., Robin S., Cogan T. M. Citric acid metabolism in hetero- and homofermentative lactic acid bacteria. Appl Environ Microbiol. 1976 Apr;31(4):481–486. doi: 10.1128/aem.31.4.481-486.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSTON M. A., DELWICHE E. A. DISTRIBUTION AND CHARACTERISTICS OF THE CATALASES OF LACTOBACILLACEAE. J Bacteriol. 1965 Aug;90:347–351. doi: 10.1128/jb.90.2.347-351.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko T., Suzuki H., Takahashi T. Influences of cellular components and redox potential of liquid concentrated culture of Lactobacillus bulgaricus on acid-producing activity and viability. J Dairy Sci. 1987 Jun;70(6):1128–1133. doi: 10.3168/jds.S0022-0302(87)80122-4. [DOI] [PubMed] [Google Scholar]
- Kremer M. L. The reaction of hemin with H2O2. Eur J Biochem. 1989 Nov 20;185(3):651–658. doi: 10.1111/j.1432-1033.1989.tb15162.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Murphy M. G., Condon S. Correlation of oxygen utilization and hydrogen peroxide accumulation with oxygen induced enzymes in Lactobacillus plantarum cultures. Arch Microbiol. 1984 May;138(1):44–48. doi: 10.1007/BF00425405. [DOI] [PubMed] [Google Scholar]
- Pritchard G. G., Wimpenny J. W. Cytochrome formation, oxygen-induced proton extrusion and respiratory activity in Streptococcus faecalis var. zymogenes grown in the presence of haematin. J Gen Microbiol. 1978 Jan;104(1):15–22. doi: 10.1099/00221287-104-1-15. [DOI] [PubMed] [Google Scholar]
- Ritchey T. W., Seeley H. W. Cytochromes in Streptococcus faecalis var. zymogenes grown in a haematin-containing medium. J Gen Microbiol. 1974 Dec;85(2):220–228. doi: 10.1099/00221287-85-2-220. [DOI] [PubMed] [Google Scholar]
- SHIBATA K., BENSON A. A., CALVIN M. The absorption spectra of suspensions of living micro-organisms. Biochim Biophys Acta. 1954 Dec;15(4):461–470. doi: 10.1016/0006-3002(54)90002-5. [DOI] [PubMed] [Google Scholar]
- Sijpesteijn A. K. Induction of cytochrome formation and stimulation of oxidative dissimilation by hemin in Streptococcus lactis and Leuconostoc mesenteroides. Antonie Van Leeuwenhoek. 1970;36(3):335–348. doi: 10.1007/BF02069035. [DOI] [PubMed] [Google Scholar]
- Smart J. B., Thomas T. D. Effect of oxygen on lactose metabolism in lactic streptococci. Appl Environ Microbiol. 1987 Mar;53(3):533–541. doi: 10.1128/aem.53.3.533-541.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speckman R. A., Collins E. B. Diacetyl biosynthesis in Streptococcus diacetilactis and Leuconostoc citrovorum. J Bacteriol. 1968 Jan;95(1):174–180. doi: 10.1128/jb.95.1.174-180.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas T. D., Turner K. W. Carbohydrate Fermentation by Streptococcus cremoris and Streptococcus lactis Growing in Agar Gels. Appl Environ Microbiol. 1981 Jun;41(6):1289–1294. doi: 10.1128/aem.41.6.1289-1294.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITTENBURY R. HYDROGEN PEROXIDE FORMATION AND CATALASE ACTIVITY IN THE LACTIC ACID BACTERIA. J Gen Microbiol. 1964 Apr;35:13–26. doi: 10.1099/00221287-35-1-13. [DOI] [PubMed] [Google Scholar]


