Abstract
In this report we document the creation of transgenic mice in which the native ratio of A and B forms of progesterone receptor (PR) has been altered by the introduction of additional A form as transgene. We also show that in these mice there is an aberration in mammary development. In ovariectomized prepubertal PR-A transgenic mice, end buds with unusual morphology persist after ovariectomy, and in young adult nonovariectomized mice, mammary glands have extensive lateral branching. The glands of adult mice also exhibit ductal hyperplasia with a disorganized basement membrane and decreased cell–cell adhesion, features commonly associated with neoplasia. Because progesterone is a mitogenic hormone in mammary glands and PR is required for mammary development, these data provide direct evidence that in vivo a regulated expression of the two isoforms of PR is critical for appropriate cellular response to progesterone and that for mammary glands this may have major implications to carcinogenesis.
Progesterone receptor (PR) belongs to the superfamily of steroid receptors and mediates the action of progesterone in its target tissues (1, 2). In normal mammary glands of both rodents and humans, progesterone promotes the proliferation of epithelial cells (3–6). High levels of PR gene expression are associated with the end bud cells of the growing duct (7), the putative progenitors of the ductal cells (8), and in the mature female, the epithelial cells of the duct that give rise to lobulo-alveolar outgrowths also express high levels of PR (7). Direct evidence for the importance of PR in mammary development is revealed in PR-null mutant mice that exhibit a marked impairment in lobulo-alveolar development (9).
PR exists in two molecular forms, the A and B forms whose expression is regulated by two promoters (10, 11). The ratio of the two forms varies among target tissues (2), suggesting that their differential expression may be critical for appropriate cellular responsiveness to progesterone (12). In the same cell, the A and B forms can have different functions and the activity of the individual form of the receptor varies among different types of cells (13, 14). Also, depending on the cell and promoter context, the A form can either inhibit or enhance the activity of the B form (14). The A and B forms of PR also modulate estrogen receptor (ER) and estrogen-dependent gene expression (15–17). All these observations strongly suggest that an imbalance in the expression and/or activities of the two forms of PR can have important consequences to normal mammary development, which requires a coordinated action of estrogen and progesterone among its various cell types (18). Also, to the extent that an aberration in normal developmental processes can serve as a trigger for carcinogenesis, an imbalance in the expression and/or activities of the two forms of PR can also have implications to mammary carcinogenesis.
Almost all studies to date have employed in vitro models to investigate the relative actions of the A and B forms of PR, by using either immortalized or tumorigenic cell lines; such studies, although informative, however, cannot be extrapolated to normal developmental processes. Therefore, we have created transgenic mice in which the native ratio of A/B forms of PR has been altered by introduction of additional A form as transgene. In these mice, there is an aberration in mammary development characterized by extensive lateral branching. These glands also exhibit ductal hyperplasia and a disorganized basement membrane, features commonly associated with neoplasia.
MATERIALS AND METHODS
Construction of Transgenic Mice.
There was a possibility that in transgenic mice, carrying an imbalance in the normal ratio of the two forms of PR, pregnancy might be jeopardized. Therefore, we used a binary transgenic system in which the GAL-4 gene, driven by the murine cytomegalovirus (CMV) promoter (CMV-GAL-4 mice), served as the transactivator of the PR-A gene, carrying four GAL-4 binding sites (UAS; UAS-PR-A mice). Crossing the CMV-GAL-4 mice with UAS-PR-A mice resulted in bigenic mice carrying additional PR-A gene.
For construction of CMV-GAL-4 plasmids, the GAL-4 gene was excised from the plasmid of pGATB (19) as a HindIII fragment and the HindIII site was modified to EcoRI prior to insertion into the unique EcoRI site of plasmid pSV2NeoCMV; this vector, derived from pSV2 Neo (20), carries the murine CMV promoter (21) upstream from the inserted sequence. For construction of UAS-TATA-PR-A plasmid, the first intron of mouse PR (cloned by this laboratory) was inserted into its proper position in the PR cDNA (22), previously digested with BamHI to remove the first ATG; this fragment was then fused to the UAS-TATA fragment excised from pUAST (19) and was inserted in place of the CMV-PR cDNA in pCNmPR3, previously constructed by this laboratory (22). A schematic representation of the two constructs is shown in Fig. 1. Both DNA constructs were tested in cultured cells to confirm the GAL-4/UAS transactivation (data not shown). The parent plasmids containing the respective transgenes were digested with appropriate restriction enzymes to release the transgene(s) and purified prior to microinjection into the pronuclei of mouse zygotes. Transgenic mice were identified initially by Southern blot analysis, and once the founder lines had been established, they were routinely screened by PCR using tail DNA.
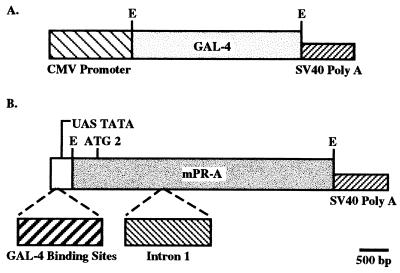
Figure 1.
Schematic representation of plasmid construction for the binary system. (A) Insertion of the GAL-4 gene into the CMV promoter expression plasmid containing simian virus 40 splice and polyadenylylation sequences. (B) mPR cDNA (A form with only ATG 2) containing intron 1 and simian virus 40 splice and polyadenylylation sequences fused to UAS-TATA fragment containing four GAL-4 binding sites. E, EcoRI.
Analysis for Transgene and Endogenous Genes’ Expression.
Transgene and endogenous PR expression was examined by reverse transcription-coupled PCR. The oligonucleotide primers for detecting various transgene expression were as follows: PR-A transgene (forward from PR cDNA, PR-2527, 5′-CGAATTGATCAAGGCAATTGGT-3′; reverse from the simian virus 40 termination sequence, 5′-AGACACTCTATGCCTGTGTGGAG-3′), GAL-4 transgene (forward from GAL-4 untranslated leader (UTL), 5′-GAAGCAAGCCTCCTGAAAGA-3′; reverse GAL-4 784, 5′-CACTGAAGCCAATCTATCTG-3′), and endogenous PR gene (forward from mPR UTL, 5′-AAAAGGGGAGCTTGGGTCGT-3′; reverse, PR-440, 5′-CAAAGAGACACCAGGAAGTG-3′).
Indirect Immunofluorescence Assay.
For examining the immunolocalization of PR, E-cadherin, and laminin in individual mammary glands, an indirect immunofluorescence assay was performed with a secondary antibody conjugated to fluorescein isothiocyanate, as described (22). The antibody used for analysis of PR was prepared against synthetic peptide corresponding to amino acid residues 376–394, selected from the amino-terminal half of the mouse PR sequence (23); this antibody reacts with both the A and B forms of murine PR (22). Antibody to E-cadherin, originally obtained from M. Takeichi (Kyoto University, Japan) was provided by C. Daniel (University of California, Santa Cruz). Antibody to laminin was purchased from Telios Pharmaceuticals (San Diego).
Whole-Mount Preparation and Histological Analysis.
The entire number 4 inguinal mammary gland was removed and fixed in Carnoy’s solution (acidic ethanol) at room temperature. The tissues were washed in 70% ethanol, rinsed in distilled water, and stained overnight in carmine solution [0.2% carmine/0.5% aluminum potassium sulfate (both from Sigma)] at room temperature. The stained tissue was dehydrated through graded series of ethanol, cleared in toluene, and stored in methyl salicylate. For histological examination, structures of interest in whole mounts were excised and embedded in paraffin, sectioned at 4 mm, and stained with hematoxylin/eosin by standard procedures.
RESULTS
Analysis for PR Transgene Expression.
At present, we have examined two sets of bigenic mice (TG 32/91 and TG 32/42) obtained by crossing the same GAL-4 founder with two different lines of UAS/PR-A trangenic mice that, so far, have not shown any appreciable differences with respect to their mammary morphology and histology. TG 32/91, the best characterized bigenic mice, will be described herein. In all experiments, littermates negative for PR-A transgene were used as controls and on occasion, when these were not available, transgene-negative mice of same age were used.
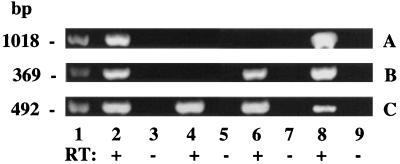
PR-A transgene expression was found in mammary glands of bigenic TG 32/91 mice (Fig. 2A, lanes 2 and 8) and not in glands of monogenic TG 32 mice carrying only the GAL-4 gene (Fig. 2A, lane 6) or in glands of mice negative for both GAL-4 and PR-A transgenes (Fig. 2A, lane 4). GAL-4 gene expression was found in the mammary glands of both TG 32/91 and TG 32 mice (Fig. 2B, lanes 2, 6, and 8) and not in the glands of transgene-negative mice (Fig. 2B, lane 4). In contrast, as expected, endogenous PR expression was found in the mammary glands of all mice (Fig. 2C). The identity of all PCR products were confirmed by Southern blot analysis (data not shown). The transgene expression was unaffected by ovariectomy (Fig. 2A, compare lane 2 with lane 8), although, as expected, endogenous PR expression was lower in the glands of ovariectomized mice (Fig. 2C, compare lane 2 with lane 8).
Figure 2.
Reverse transcription-coupled PCR analysis of gene expression. RNA from mammary glands of nonovariectomized bigenic (lanes 2 and 3), bigenic ovariectomized for 2 weeks (lanes 8 and 9), nonovariectomized monogenic GAL-4 (lanes 6 and 7), and transgene-negative (lanes 4 and 5) mice was subjected to PCR analysis either without (−RT) or (+RT) after reverse transcription. (A) PR-A transgene expression corresponding to the expected fragment of 1031 bp. (B) GAL-4 gene expression corresponding to the expected fragment of 360 bp. (C) Endogenous PR expression corresponding to the expected fragment of 460 bp. Lanes 1 represent standard DNA with molecular weights indicated on the left.
Analysis for PR by Indirect Immunofluorescence.
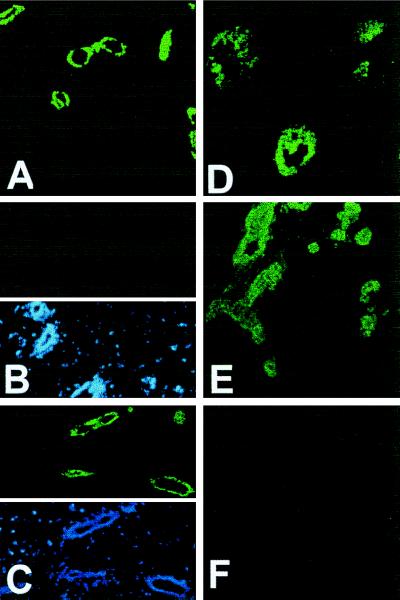
Mammary glands of nonpregnant females are composed primarily of nonepithelial cells and PR is present only in a subpopulation (23) of the epithelial cells; this results in a low overall concentration of PR so that analyses of PR in individual mammary glands by biochemical assays is not feasible. Therefore, to verify that the introduction of additional A form of PR as transgene had indeed increased the steady-state levels of PR, an indirect immunofluorescence assay was performed in individual mammary glands of ovariectomized prepubertal and adult mice. Ovariectomy was performed to reduce the contribution of the endogenous PR gene expression and at the same time allow the detection of PR arising from the transgene. As expected, the levels of PR were greatly diminished and virtually undetectable in the glands of transgene negative mice ovariectomized at 5 weeks of age (Fig. 3B) as compared with the glands of control intact transgene negative mice (Fig. 3A). Analysis of mammary glands of TG 32/91 (hereafter referred to as PR-A transgenic) mice, also ovariectomized at 5 weeks of age, readily revealed that they contained much higher levels of PR as compared with their transgene-negative counterparts (Fig. 3, compare B and C). Similarly, the mammary glands of adult ovariectomized PR-A transgenic mice also contained more immunoreactive PR than glands of ovariectomized adult transgene negative mice (data not shown). Thus, overall, the immunolocalization of PR confirmed a higher level of PR expression in PR-A transgenic mice.
Figure 3.
Immunolocalization of PR. Mammary glands from control PR-A transgene-negative (A and B) and PR-A transgenic (C–F) were analyzed for PR (green color) by indirect immunofluorescence. (B–D) Glands from mice ovariectomized at 5 weeks of age and analyzed 2 weeks later. (A) Glands from intact 7 week old. (E and F) Glands from intact 14 week old mice. (B Lower and C Lower) Nuclei (in the same sections as Upper) stained with 4,6-diamidino-2-phenylindole (blue color) to illustrate that the lack of immunoreactivity in B was not due to the lack of ductal epithelium. In all cases, without the primary antibody, there was no immunoreactivity (F). (Original magnification: ×100.)
In the glands of ovariectomized prepubertal PR-A transgenic mice, immunostaining was also observed in structures resembling end buds (Fig. 3D). This was surprising because end buds usually regress when there is a cessation in growth after ovariectomy or after antiestrogen treatment (24). Also, in the glands of nonovariectomized adult PR-A transgenic mice, immunoreactive PR was present in several ducts composed of more than one layer of cells (Fig. 3E). This was unusual because normal mammary ducts in young nulliparous mice are composed of a single layer of epithelial cells (see Fig. 3A), suggesting that in PR-A transgenic mice there may be an aberration in mammary development.
Morphological and Histological Characterization.
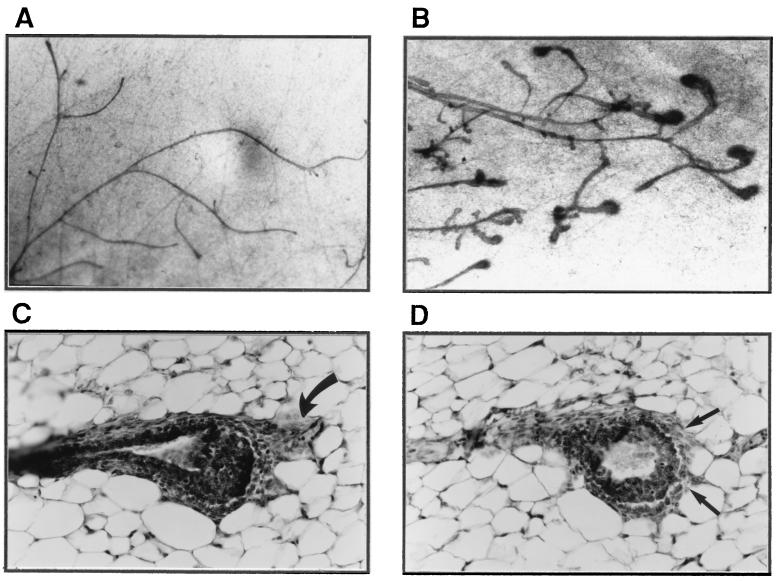
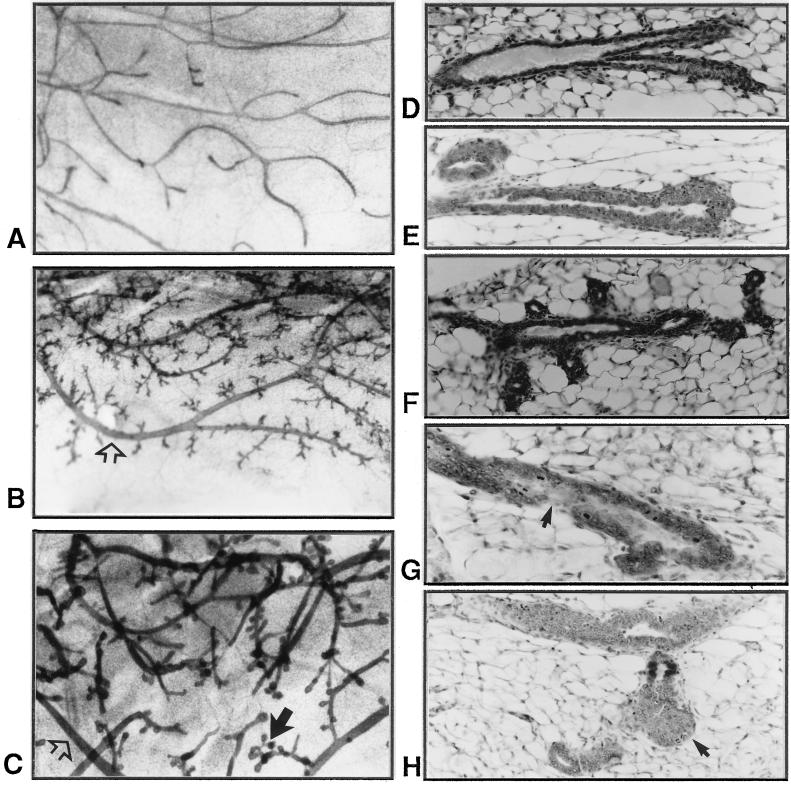
An overall comparison of mammary glands of 5- to 6-week-old prepubertal PR-A transgenic females with their transgene negative counterparts did not reveal any major differences; in both cases, 50–60% of the fat pads had been filled with ducts and several end buds, characteristic of a growing tissue were present (data not shown). However, after ovariectomy, the end buds in the mammary glands of control mice regressed (indicating a cessation in growth), whereas the end buds in transgenic mice persisted (Fig. 4, compare A with B). The degree of end-bud persistence was variable among individual mice but after prolonged ovariectomy, end buds disappeared in all mice. Histological analyses revealed that some of these end buds had unusual fibrous caps (Fig. 4C) and disruption in the continuity of cap cells (Fig. 4D).
Figure 4.
Morphological and histological characteristics of mammary glands in prepubertal PR-A transgenic mice. Micrographs (A and B) of and histology (C and D) of mammary glands from mice ovariectomized at 5 weeks of age and examined 2 weeks later are shown. In controls glands from PR-A transgene-negative mice (A), there are no end buds in contrast to glands of PR-A transgenic mice (B). (C) An end bud with unusual fibrous cap (curved arrow). Arrows in D show disruption in the continuity of cap cells.
From whole-mount analyses of mammary glands of young adult mice (10–14 weeks old), the degree of ductal branching appeared to be similar for both PR-A transgenic and control mice; i.e., the ducts at each level of branching appeared to be present in roughly the same numbers. However, the glands of adult PR-A transgenic mice had extensive lateral branching and also contained some very thick ducts (Fig. 5, compare B and C with A). The extensive lateral branching from mature secondary ducts sometimes resulted in a gland resembling that of an early pregnant female (Fig. 5B); however, often the lateral branches terminated in bulbous structures, and in contrast to normal ducts, the ducts in transgenic mice exhibited extraordinary numbers of buds growing from what are normally growth-quiescent zones (Fig. 5C). Peculiar morphology was also apparent at the tips of the ducts of transgenic mice that exhibited clustered buds compared with the smooth structure characteristic of normal terminal ducts (as shown in Fig. 5A).
Figure 5.
Morphological and histological characteristics of mammary glands in adult PR-A transgenic mice. Whole-mounts (A–C) and histology (D–H) of mammary glands from young (10–14 weeks old) control PR-A transgene-negative (A and D) and PR-A transgenic mice (B, C, and E–H) are shown. In B and C, open arrows show thick ducts and solid arrow shows clustered buds at the tip of ducts. In G, arrow shows an indistinct epithelial–stromal boundary, and in H, arrow shows disorganized masses of cells at the tip of a duct.
Histological analyses revealed that the thickening of the duct wall in the glands of transgenic mice was the result of ducts composed of multilayered cells, in contrast to the monolayer associated with the normal duct (Fig. 5, compare E with D). Increased budding in the mammary glands of transgenic mice was also evident when ducts of similar lengths from control and transgenic mice were compared (Fig. 5, compare F with D). Some of the ducts in transgenic mice also exhibited regions with indistinct epithelial–stromal boundary (Fig. 5G) and multiple branched outgrowths consisting of disorganized masses of epithelial cells were seen at the tip of some ducts (Fig. 5H).
Disruption of Basement Membrane Integrity and Cell–Cell Interaction in Mammary Epithelium of PR-A Transgenic Mice.
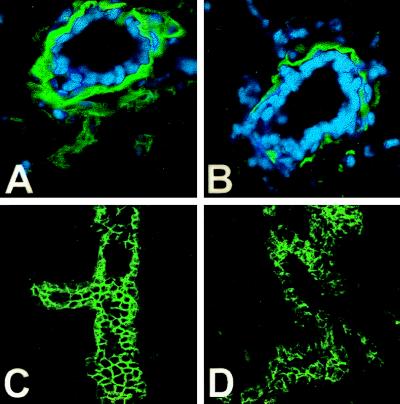
In normal mouse mammary glands during puberty, the end bud body cells (which give rise to ductal cells) express E-cadherin and exposure to anti-E-cadherin antibodies causes a disorganization of ductal epithelial cells and their detachment (25). A decreased expression of E-cadherin has also been shown to be associated with a reduced ability by human mammary epithelial cells to undergo morphogenesis in vitro (26). Cadherins are cell adhesion molecules (27) and play an important role in cell–cell interaction and also in cell–matrix interactions; this is because cells interact with basement membrane by means of adhesion receptors that allow the cells to migrate on extracellular matrix components, such as laminin. Therefore, to determine whether the disruption in the architecture of mammary glands in PR-A transgenic mice was the result of a derangement in the mechanisms regulating normal cell–cell adhesion and cell–matrix interactions, we examined the pattern of immunostaining for E-cadherin and laminin. As shown in Fig. 6, in the mammary glands of PR-A transgenic mice, laminin staining was discontinuous at the base of epithelial cells, indicative of a disruption in the basement membrane (Fig. 6, compare A with B). Similarly, in the mammary glands of PR-A transgenic mice, E-cadherin staining exhibited a disorganized pattern (Fig. 6D), whereas in the glands of control mice, E-cadherin was present in an organized manner outlining the epithelial cells (Fig. 6C).
Figure 6.
Immunolocalization of E-cadherin and laminin. Laminin immunoreactivity (A and B), green color (nuclei are blue), in mammary-duct cross-sections circumscribes the mammary epithelium of PR-A transgene-negative mice (A) but is discontinuous and decreased in the gland of PR-A transgenic mice (B). Cadherin immunoreactivity (green color) delineates the epithelial cells in this branching duct of transgene negative mice (C), which is greatly diminished and disorganized in the epithelium of PR-A transgenic mice (D). In all cases, without primary antibody, there was no immunoreactivity (data not shown).
DISCUSSION
In this report, we document the creation of transgenic mice in which the native ratio of A to B forms of PR has been altered by introduction of additional A form of PR as transgene. We also show that the mammary glands of these PR-A transgenic mice have an aberrant morphology. Normal mammary development requires a coordinated action of estrogen, progesterone, and glucocorticoids. Also, the action of each of these steroids has a relative dominance depending on the developmental state (28). Studies with human PR have established that, in vitro, PR-A can modulate the action of PR-B, ER, and also other steroid receptors (12). Furthermore, it also appears that in the mammary epithelium, there may be distinct lineage limited progenitor cells capable of giving rise to either ductal or lobulo-alveolar growth (29) and PR is present only in a subpopulation of mammary epithelial cells (23). Therefore, when the complexity of steroid hormonal regulation of normal mammary development and the complexity of PR-A action is considered, at present, we can only speculate on the mechanisms whereby an overexpression of PR-A can result in an abnormal mammary phenotype.
It is well established that for ductal growth accompanying puberty, ER and estrogen are essential (24, 30). End buds are the indicators of ductal growth representing the site of both intense mitotic activity (24) and apoptosis (31). Indeed, an inhibition of apoptosis in the end buds can have an effect on the structural organization of end buds (31). As such, the persistence of end buds upon ovariectomy in prepubertal PR-A transgenic mice and their atypical organization suggest that during this developmental state, overexpression of PR-A may have interfered with ER action.
The mammary glands in adult PR-A transgenic mice have extensive adventitious lateral branching and also contain ducts composed of multilayered cells. Progesterone augments DNA synthesis in the epithelial cells of mouse mammary ducts (4) and extensive lateral branching usually accompanies pregnancy (3, 8) and requires PR (9). Therefore, it is likely that in PR-A transgenic mice, due to the increase in steady-state levels of PR, there is an increased responsiveness to progesterone. Indeed upon ovariectomy, there is a loss in the thickening of ductal walls, which, however, reappear upon administration of progesterone (data not shown). Regardless, it is clear that in PR-A transgenic mice, there is a derangement in the epithelial-cell replicative homeostasis. Therefore, although PR-A (directly or indirectly) can trigger the epithelial growth and lateral branching, a regulated growth may require the coordinated action of PR-A, PR-B, and ER that is disrupted with the overexpression of PR-A.
An important feature of the mammary glands of PR-A transgenic mice is the disruption in the organization of the basement membrane and a decrease in cell–cell adhesion. This is clearly abnormal because during pregnancy, when there is an extensive epithelial cell proliferation and lateral branching, the basement membrane remains intact (32). In human breast, basement membrane proteins undergo differential distribution during the menstrual cycle (33), indicating their potential regulation by ovarian hormones. In rodents, normal mammary development can be disrupted when there is an inhibition in the deposition of extracellular matrix (34, 35). Therefore, if the integrity of the basement membrane indeed requires a coordinated action of ovarian steroids, it is conceivable that this can be disrupted in PR-A transgenic mice, Mammary hyperplasia with a disorganized basement membrane and decreased cell–cell adhesion are characteristics generally associated with mammary epithelial cells that have acquired invasive properties. As such, the characteristics of mammary glands of PR-A transgenic mice strongly suggest that they may have a high predisposition to become tumors.
In summary, our present studies provide in vivo evidence that a regulated expression of the two PR isoforms is critical for appropriate responsiveness to progesterone. Furthermore, they also provide direct evidence that an aberration in the mechanisms regulating the differential expression of the two isoforms of PR can have major implications to mammary carcinogenesis.
Acknowledgments
We thank Dr. M. Strathman for providing the plasmids of pGATB and pUAST and Dr. Mary Stevens for assistance with pronuclear injections. Mr. Jaime Diaz and Ms. Sharianne G. Louie provided expert technical assistance. These studies were supported by National Institutes of Health Grant CA 66541 (to G.S.).
ABBREVIATIONS
- PR
progesterone receptor
- ER
estrogen receptor
- CMV
cytomegalovirus
References
- 1.Mangelsdorf D J, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans R M. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsai M J, O’Malley B W. Annu Rev Biochem. 1994;63:451–486. doi: 10.1146/annurev.bi.63.070194.002315. [DOI] [PubMed] [Google Scholar]
- 3.Nandi S. J Natl Cancer Inst. 1958;21:1039–1063. [PubMed] [Google Scholar]
- 4.Bresciani F. Cell Tissue Kinet. 1968;1:51–63. [Google Scholar]
- 5.Ferguson D J P, Anderson J J. Br J Cancer. 1981;44:177–181. doi: 10.1038/bjc.1981.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Going J J, Anderson J J, Battersby S, Macintyre C C A. Am J Pathol. 1988;130:193–204. [PMC free article] [PubMed] [Google Scholar]
- 7.Silberstein G B, VanHorn K, Shyamala G, Daniel C W. Cell Growth Differ. 1996;7:945–952. [PubMed] [Google Scholar]
- 8.Williams J M, Daniel C W. Dev Biol. 1983;97:274–290. doi: 10.1016/0012-1606(83)90086-6. [DOI] [PubMed] [Google Scholar]
- 9.Lydon J P, DeMayo F J, Funk C R, Mani S K, Hughes A R, Montgomery C A, Jr, Shyamala G, Conneely O M, O’Malley B W. Genes Dev. 1995;9:2266–2278. doi: 10.1101/gad.9.18.2266. [DOI] [PubMed] [Google Scholar]
- 10.Kastner P, Krust A, Turcotte B, Stropp U, Tora L, Gronemeyer H, Chambon P. EMBO J. 1990;9:1603–1614. doi: 10.1002/j.1460-2075.1990.tb08280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kraus W L, Montano M M, Katzenellenbogen G S. Mol Endocrinol. 1993;7:1603–1616. doi: 10.1210/mend.7.12.8145766. [DOI] [PubMed] [Google Scholar]
- 12.McDonnell D P. Trends Endocrinol Metab. 1995;6:133–138. doi: 10.1016/1043-2760(95)00065-p. [DOI] [PubMed] [Google Scholar]
- 13.Tora L, Gronemeyer H, Turcotte B, Gaub M P, Chambon P. Nature (London) 1988;333:185–188. doi: 10.1038/333185a0. [DOI] [PubMed] [Google Scholar]
- 14.Vegeto E, Shahbaz M M, Wen D X, Goldman M E, O’Malley B W, McDonnell D P. Mol Endocrinol. 1993;7:1244–1255. doi: 10.1210/mend.7.10.8264658. [DOI] [PubMed] [Google Scholar]
- 15.McDonnell D P, Goldman M E. J Biol Chem. 1994;269:11945–11949. [PubMed] [Google Scholar]
- 16.Chalbos D, Galtier F. J Biol Chem. 1994;269:23007–23012. [PubMed] [Google Scholar]
- 17.Kraus W L, Weis K E, Katzenellenbogen B S. Mol Cell Biol. 1995;15:1847–1857. doi: 10.1128/mcb.15.4.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shyamala G. Trends Endocrinol Metab. 1997;8:34–39. doi: 10.1016/s1043-2760(96)00207-x. [DOI] [PubMed] [Google Scholar]
- 19.Brand A H, Manoukian A S, Perrimon N. Methods Cell Biol. 1994;44:635–654. doi: 10.1016/s0091-679x(08)60936-x. [DOI] [PubMed] [Google Scholar]
- 20.Southern P J, Berg P J. Mol Appl Genet. 1982;1:327–341. [PubMed] [Google Scholar]
- 21.Dorsch-Hasler K, Keil G M, Weber F, Jasin M, Schaffner W, Koszinowski U H. Proc Natl Acad Sci USA. 1985;52:8325–8329. doi: 10.1073/pnas.82.24.8325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schott D R, Shyamala G, Schneider W, Parry G. Biochemistry. 1991;30:7014–7020. doi: 10.1021/bi00242a029. [DOI] [PubMed] [Google Scholar]
- 23.Shyamala, G., Barcellos-Hoff, M. H., Toft, D. & Yang, X. (1997) J. Steroid Biochem. Mol. Biol., in press. [DOI] [PubMed]
- 24.Silberstein G B, Van Horn K, Shyamala G, Daniel C W. Endocrinology. 1994;134:84–90. doi: 10.1210/endo.134.1.8275973. [DOI] [PubMed] [Google Scholar]
- 25.Daniel C W, Strickland P, Friedmann Y. Dev Biol. 1995;169:511–519. doi: 10.1006/dbio.1995.1165. [DOI] [PubMed] [Google Scholar]
- 26.D’Souza B, Taylor-Papadimitriou J. Proc Natl Acad Sci USA. 1994;91:7202–7206. doi: 10.1073/pnas.91.15.7202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takeichi M. Development (Cambridge, UK) 1988;102:639–655. doi: 10.1242/dev.102.4.639. [DOI] [PubMed] [Google Scholar]
- 28.Topper Y J, Freeman C S. Physiol Rev. 1980;60:1049–1106. doi: 10.1152/physrev.1980.60.4.1049. [DOI] [PubMed] [Google Scholar]
- 29.Smith G H. Breast Cancer Res Treat. 1996;39:21–31. doi: 10.1007/BF01806075. [DOI] [PubMed] [Google Scholar]
- 30.Korach K S, Crouse J F, Curtis S W, Washburn T F, Lindzey J, Kimbro K S, Eddy E M, Migliaccio S, Snedeker S M, Lubahan D B, Schomberg D W, Smith E P. Rec Prog Horm Res. 1996;51:159–188. [PubMed] [Google Scholar]
- 31.Humphreys R C, Krajewska M, Kranick S, Jaeger R, Weiter H, Krajewski S, Reed J C, Rosen J M. Development (Cambridge, UK) 1996;122:4013–4022. doi: 10.1242/dev.122.12.4013. [DOI] [PubMed] [Google Scholar]
- 32.Warburton M J, Mitchell D, Ormerod E J, Rudland P. J Histochem Cytochem. 1982;30:667–676. doi: 10.1177/30.7.6179984. [DOI] [PubMed] [Google Scholar]
- 33.Ferguson J E, Schor A M, Howell A, Ferguson M W J. Cell Tissue Res. 1992;268:167–177. doi: 10.1007/BF00338066. [DOI] [PubMed] [Google Scholar]
- 34.Daniel, C. W., Silberstein, G. B. & Strickland, P. (1987) Cancer Res. 6052–6057. [PubMed]
- 35.Wicha M S, Liotta L A, Vonderhaar B K, Kidwell W R. Dev Biol. 1980;80:253–266. doi: 10.1016/0012-1606(80)90402-9. [DOI] [PubMed] [Google Scholar]