Abstract
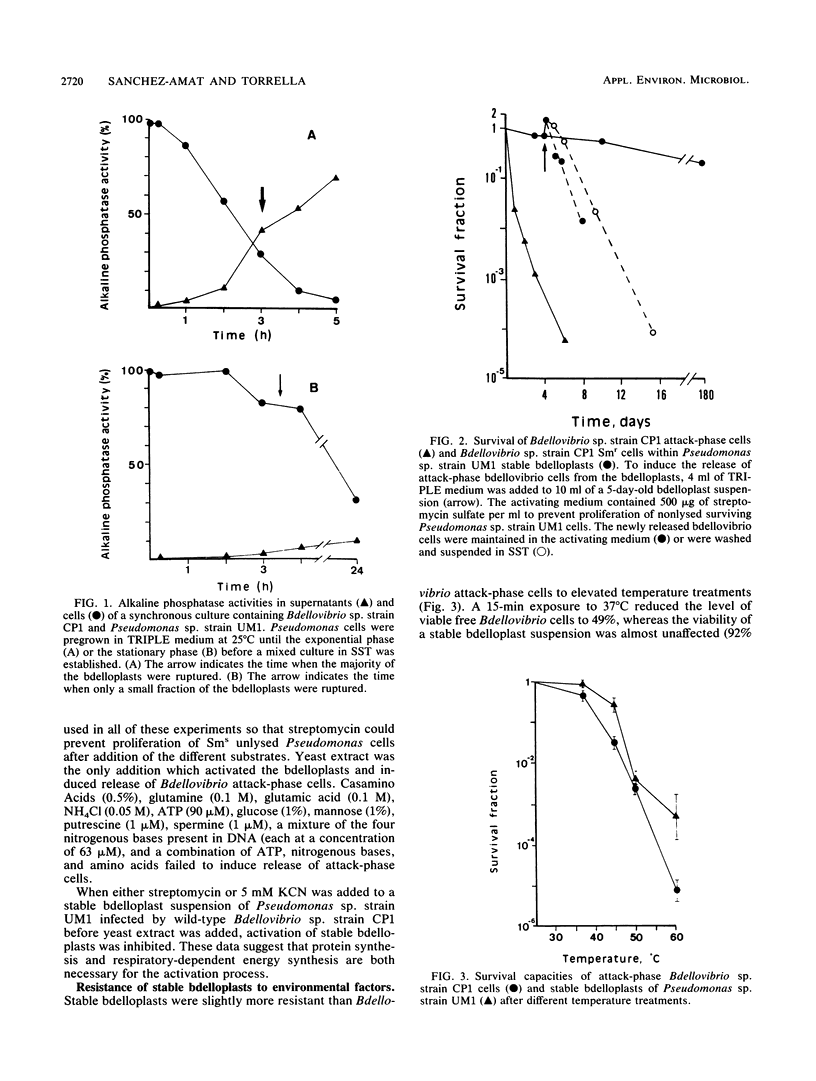
Several wild-type isolates of marine bdellovibrios formed stable bdelloplasts when they infected gram-negative bacterial prey under certain culture conditions. Synchronous predator-prey cultures and low nutrient concentrations increased the yield of stable bdelloplasts. The bdellovibrio cells retained in the stable bdelloplasts showed a high survival capacity in nutrient-depleted saline solution (10% viable Bdellovibrio cells after 3 months at 25°C), whereas Bdellovibrio attack-phase cells kept under the same starvation conditions lost viability more quickly (1% viable cells after 48 h). The addition of yeast extract to a stable bdelloplast suspension induced lysis of the bdelloplasts and release of motile infecting attack-phase Bdellovibrio cells. Other substances, such as free amino acids, protein hydrolysates, NH4+, carbohydrates, and organic amines, did not induce such a release. Stable bdelloplasts were highly hydrophobic and had a lower endogenous respiration rate than attack-phase cells. In general, stable bdelloplasts were almost as sensitive to temperature changes, desiccation, sonication, tannic acid, and Triton X-100 treatment as attack-phase cells. Electron microscopy of stable bdelloplasts did not reveal any extra cell wall layer, either in the bdelloplast envelope or in the retained Bdellovibrio cells, unlike the bdellocysts of the soil bacterium Bdellovibrio sp. strain W. We propose that formation of stable bdelloplasts is a survival strategy of marine bdellovibrios which occurs in response to nutrient- and prey-poor seawater habitats.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fry J. C., Staples D. G. Distribution of Bdellovibrio bacteriovorus in sewage works, river water, and sediments. Appl Environ Microbiol. 1976 Apr;31(4):469–474. doi: 10.1128/aem.31.4.469-474.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hespell R. B., Thomashow M. F., Rittenberg S. C. Changes in cell composition and viability of Bdellovibrio bacteriovorus during starvation. Arch Microbiol. 1974 May 20;97(4):313–327. doi: 10.1007/BF00403070. [DOI] [PubMed] [Google Scholar]
- KELLENBERGER E., RYTER A., SECHAUD J. Electron microscope study of DNA-containing plasms. II. Vegetative and mature phage DNA as compared with normal bacterial nucleoids in different physiological states. J Biophys Biochem Cytol. 1958 Nov 25;4(6):671–678. doi: 10.1083/jcb.4.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjelleberg S., Hermansson M., Mårdén P., Jones G. W. The transient phase between growth and nongrowth of heterotrophic bacteria, with emphasis on the marine environment. Annu Rev Microbiol. 1987;41:25–49. doi: 10.1146/annurev.mi.41.100187.000325. [DOI] [PubMed] [Google Scholar]
- Odelson D. A., Patterson M. A., Hespell R. B. Periplasmic enzymes in Bdellovibrio bacteriovorus and Bdellovibrio stolpii. J Bacteriol. 1982 Aug;151(2):756–763. doi: 10.1128/jb.151.2.756-763.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roszak D. B., Colwell R. R. Survival strategies of bacteria in the natural environment. Microbiol Rev. 1987 Sep;51(3):365–379. doi: 10.1128/mr.51.3.365-379.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby E. G., McCabe J. B. Metabolism of periplasmic membrane-derived oligosaccharides by the predatory bacterium Bdellovibrio bacteriovorus 109J. J Bacteriol. 1988 Feb;170(2):646–652. doi: 10.1128/jb.170.2.646-652.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidler R. J., Starr M. P. Isolation and characterization of host-independent Bdellovibrios. J Bacteriol. 1969 Nov;100(2):769–785. doi: 10.1128/jb.100.2.769-785.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tudor J. J., Conti S. F. Characterization of bdellocysts of Bdellovibrio sp. J Bacteriol. 1977 Jul;131(1):314–322. doi: 10.1128/jb.131.1.314-322.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tudor J. J., Conti S. F. Characterization of germination and activation of Bdellovibrio bdellocysts. J Bacteriol. 1978 Jan;133(1):130–138. doi: 10.1128/jb.133.1.130-138.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tudor J. J., Conti S. F. Ultrastructural changes during encystment and germination of Bdellovibrio sp. J Bacteriol. 1977 Jul;131(1):323–330. doi: 10.1128/jb.131.1.323-330.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varon M., Zeigler B. P. Bacterial predator-prey interaction at low prey density. Appl Environ Microbiol. 1978 Jul;36(1):11–17. doi: 10.1128/aem.36.1.11-17.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams H. N., Falkler W. A., Jr, Shay D. E. Incidence of marine bdellovibrios lytic against Vibrio parahaemolyticus in Chesapeake Bay. Appl Environ Microbiol. 1980 Nov;40(5):970–972. doi: 10.1128/aem.40.5.970-972.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]