Abstract
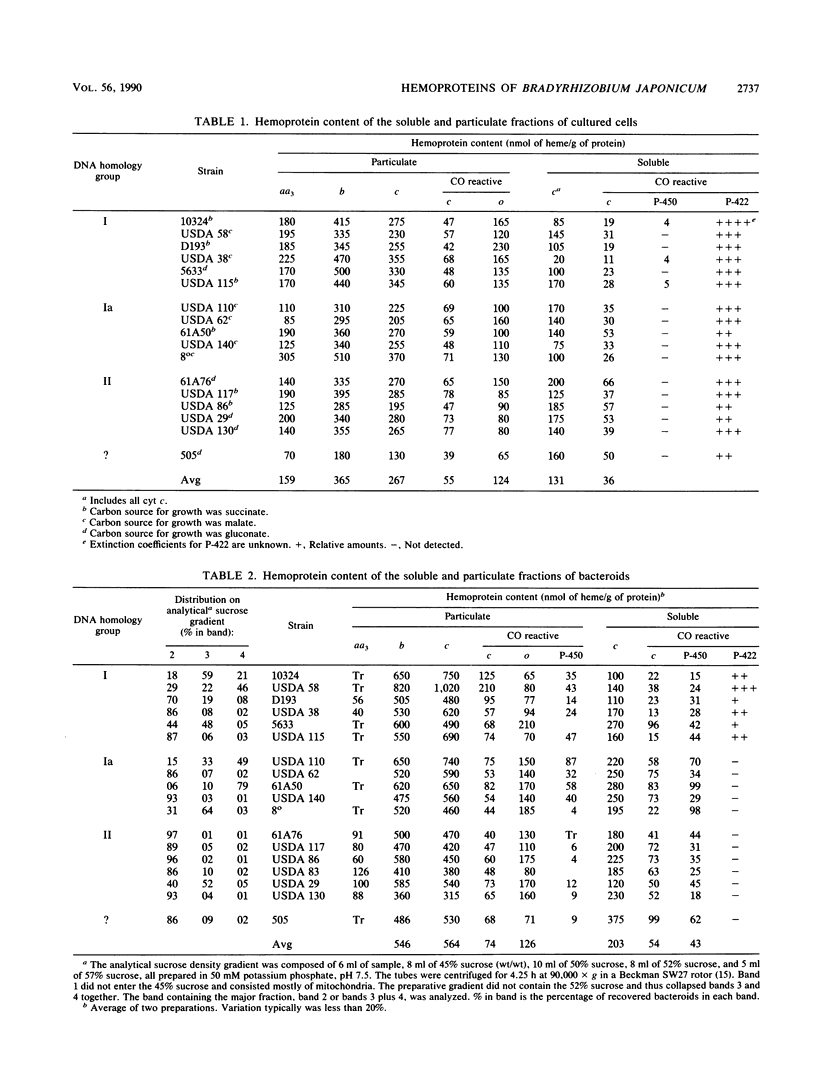
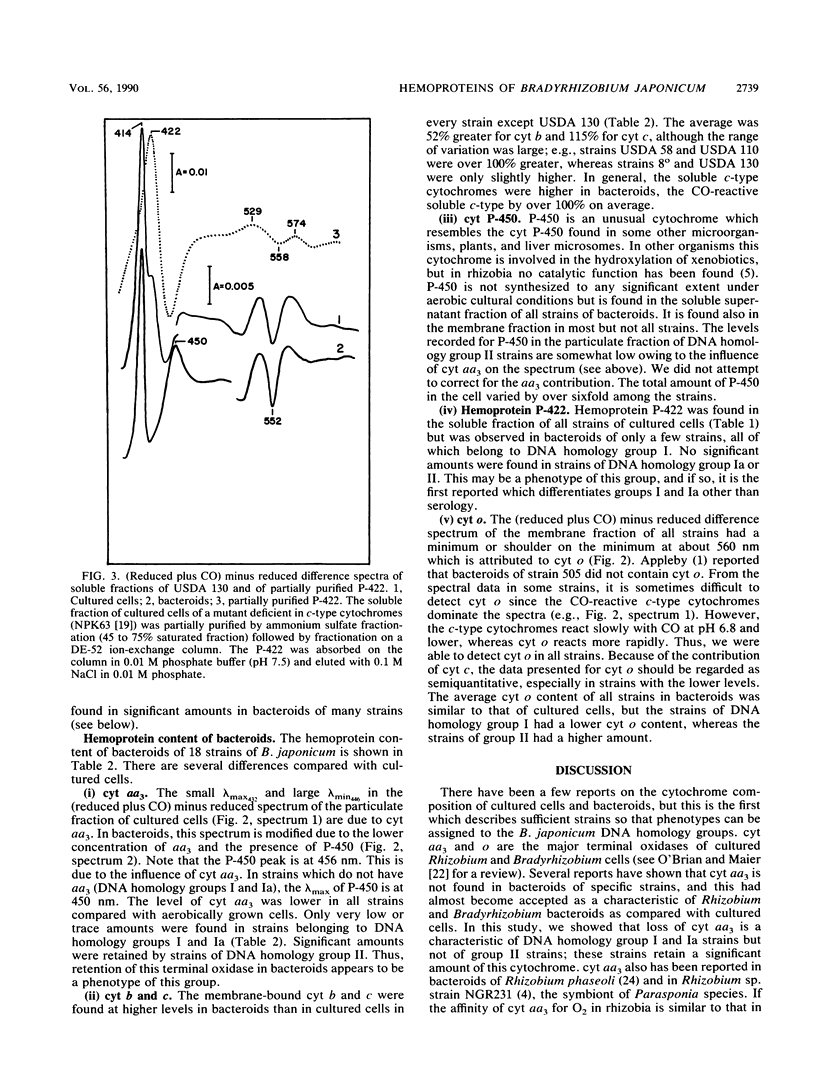
The hemoprotein content of 17 strains of Bradyrhizobium japonicum bacteroids from field-grown plants and the corresponding strains of cultured cells was determined spectrally. The major terminal oxidases, cytochromes (cyt) aa3 and o, were present in all strains of cultured cells. cyt aa3 was present in significant amounts in bacteroids only in strains of DNA homology group II. cyt o appeared to be present in bacteroids of all strains, and the average level was the same as in cultured cells. cyt b and c in the membrane fractions were higher in bacteroids of all strains compared with cultured cells. cyt P-450 was present in both the membrane and soluble fractions of bacteroids of most strains. The total P-450 content varied sixfold among strains. A CO-reactive hemoprotein, P-422, was present in the soluble fraction of all strains of cultured cells. P-422 may be a hemoglobinlike protein, and it was present in significant amounts in bacteroids only in DNA homology group I strains.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appleby C. A. Electron transport systems of Rhizobium japonicum. I. Haemoprotein P-450, other CO-reactive pigments, cytochromes and oxidases in bacteroids from N2-fixing root nodules. Biochim Biophys Acta. 1969 Jan 14;172(1):71–87. doi: 10.1016/0005-2728(69)90093-0. [DOI] [PubMed] [Google Scholar]
- Appleby C. A. Electron transport systems of Rhizobium japonicum. II. Rhizobium haemoglobin, cytochromes and oxidases in free-living (cultured) cells. Biochim Biophys Acta. 1969 Jan 14;172(1):88–105. doi: 10.1016/0005-2728(69)90094-2. [DOI] [PubMed] [Google Scholar]
- Appleby C. A., Turner G. L., Macnicol P. K. Involvement of oxyleghaemoglobin and cytochrome P-450 in an efficient oxidative phosphorylation pathway which supports nitrogen fixation in Rhizobium. Biochim Biophys Acta. 1975 Jun 17;387(3):461–474. doi: 10.1016/0005-2728(75)90086-9. [DOI] [PubMed] [Google Scholar]
- Bergersen F. J., Turner G. L. Leghaemoglobin and the supply of O2 to nitrogen-fixing root nodule bacteroids: presence of two oxidase systems and ATP production at low free O2 concentration. J Gen Microbiol. 1975 Dec;91(2):345–354. doi: 10.1099/00221287-91-2-345. [DOI] [PubMed] [Google Scholar]
- Ching T. M., Hedtke S. Isolation of bacteria, transforming bacteria, and bacteroids from soybean nodules. Plant Physiol. 1977 Nov;60(5):771–774. doi: 10.1104/pp.60.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel R. M., Appleby C. A. Anaerobic-nitrate, symbiotic and aerobic growth of Rhizobium japonicum: effects on cytochrome P 450 , other haemoproteins, nitrate and nitrite reductases. Biochim Biophys Acta. 1972 Sep 20;275(3):347–354. doi: 10.1016/0005-2728(72)90215-0. [DOI] [PubMed] [Google Scholar]
- Daniel R. M. The electron transport system of Acetobacter suboxydans with particular reference to cytochrome. Biochim Biophys Acta. 1970 Sep 1;216(2):328–341. doi: 10.1016/0005-2728(70)90224-0. [DOI] [PubMed] [Google Scholar]
- Fuchs R. L., Keister D. L. Comparative properties of glutamine synthetases I and II in Rhizobium and Agrobacterium spp. J Bacteriol. 1980 Nov;144(2):641–648. doi: 10.1128/jb.144.2.641-648.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keister D. L., Marsh S. S., El Mokadem M. T. Cytochromes of Rhizobium japonicum 61A76 Bacteroids from Soybean Nodules. Plant Physiol. 1983 Jan;71(1):194–196. doi: 10.1104/pp.71.1.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretovich W. L., Melik-Sarkissian S. S., Raikchinstein M. V., Archakov A. I. The binding of microsomal hydroxylation substrates to cytochrome P-450Rh and its effect on the nitrogen fixation by lupin bacteroids. FEBS Lett. 1974 Aug 30;44(3):305–308. [PubMed] [Google Scholar]
- Nautiyal C. S., van Berkum P., Sadowsky M. J., Keister D. L. Cytochrome mutants of bradyrhizobium induced by transposon tn5. Plant Physiol. 1989 Jun;90(2):553–559. doi: 10.1104/pp.90.2.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brian M. R., Kirshbom P. M., Maier R. J. Tn5-induced cytochrome mutants of Bradyrhizobium japonicum: effects of the mutations on cells grown symbiotically and in culture. J Bacteriol. 1987 Mar;169(3):1089–1094. doi: 10.1128/jb.169.3.1089-1094.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brian M. R., Maier R. J. Involvement of cytochromes and a flavoprotein in hydrogen oxidation in Rhizobium japonicum bacteroids. J Bacteriol. 1983 Aug;155(2):481–487. doi: 10.1128/jb.155.2.481-487.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brian M. R., Maier R. J. Molecular aspects of the energetics of nitrogen fixation in Rhizobium-legume symbioses. Biochim Biophys Acta. 1989 May 30;974(3):229–246. doi: 10.1016/s0005-2728(89)80239-7. [DOI] [PubMed] [Google Scholar]
- Soberón M., Williams H. D., Poole R. K., Escamilla E. Isolation of a Rhizobium phaseoli cytochrome mutant with enhanced respiration and symbiotic nitrogen fixation. J Bacteriol. 1989 Jan;171(1):465–472. doi: 10.1128/jb.171.1.465-472.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Hollander J. A., Bettenhaussen C. W., Stouthamer A. H. Growth yields, polysaccharide production and energy conservation in chemostat cultures of Rhizobium trifolii. Antonie Van Leeuwenhoek. 1979;45(3):401–415. doi: 10.1007/BF00443279. [DOI] [PubMed] [Google Scholar]


