Abstract
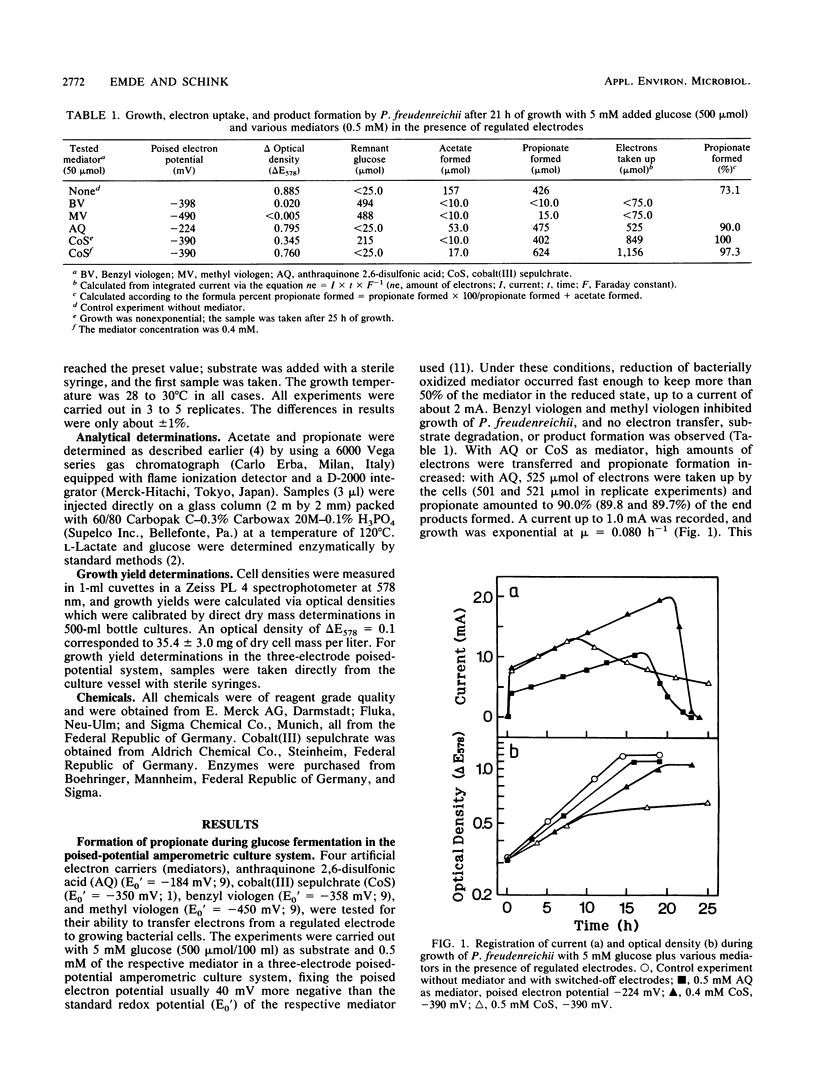
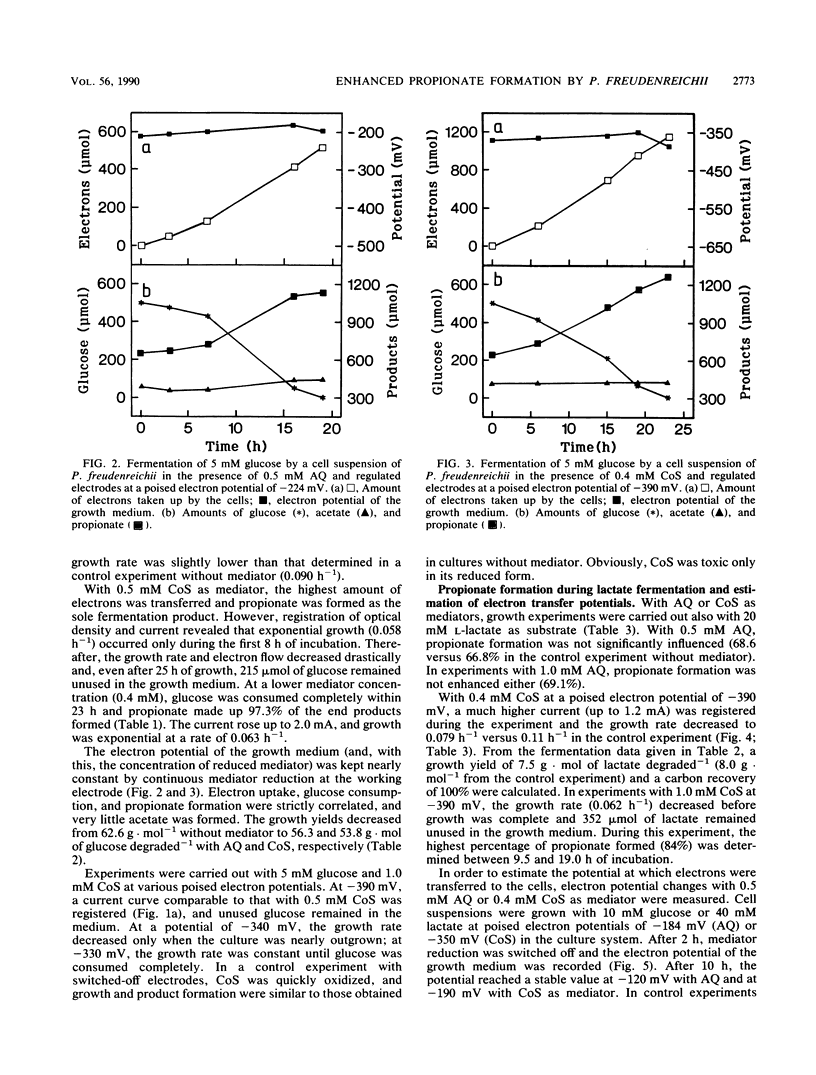
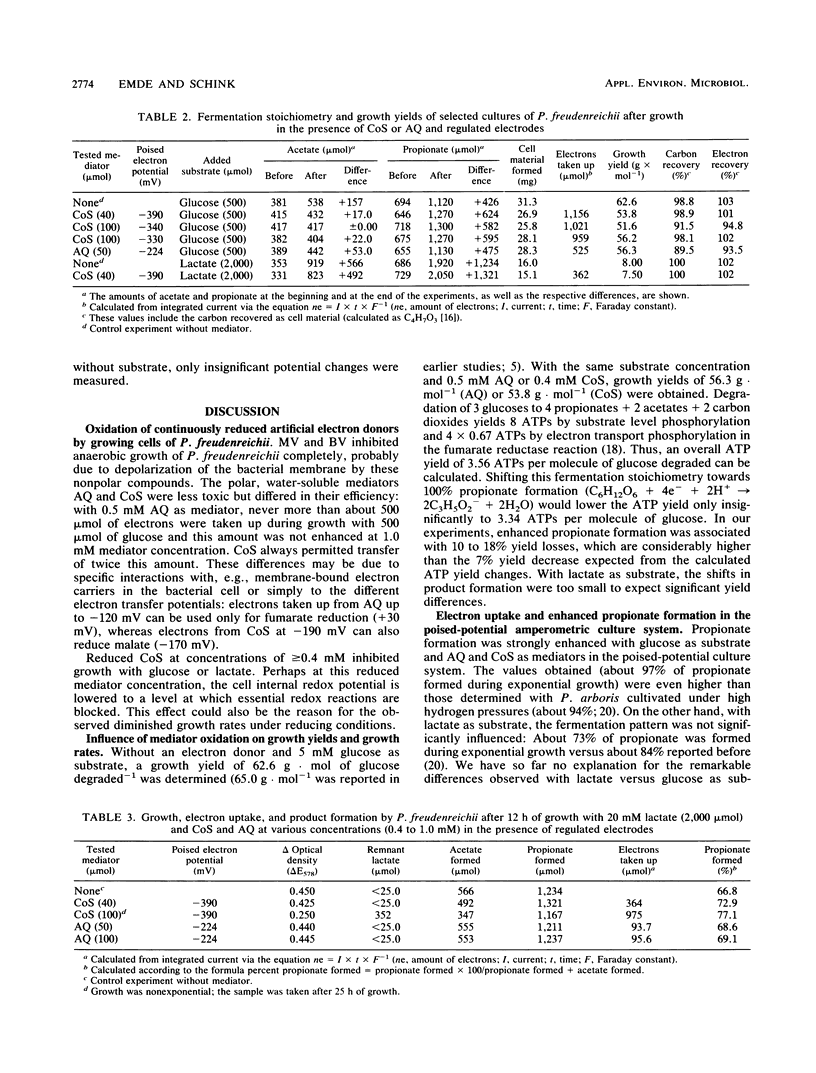
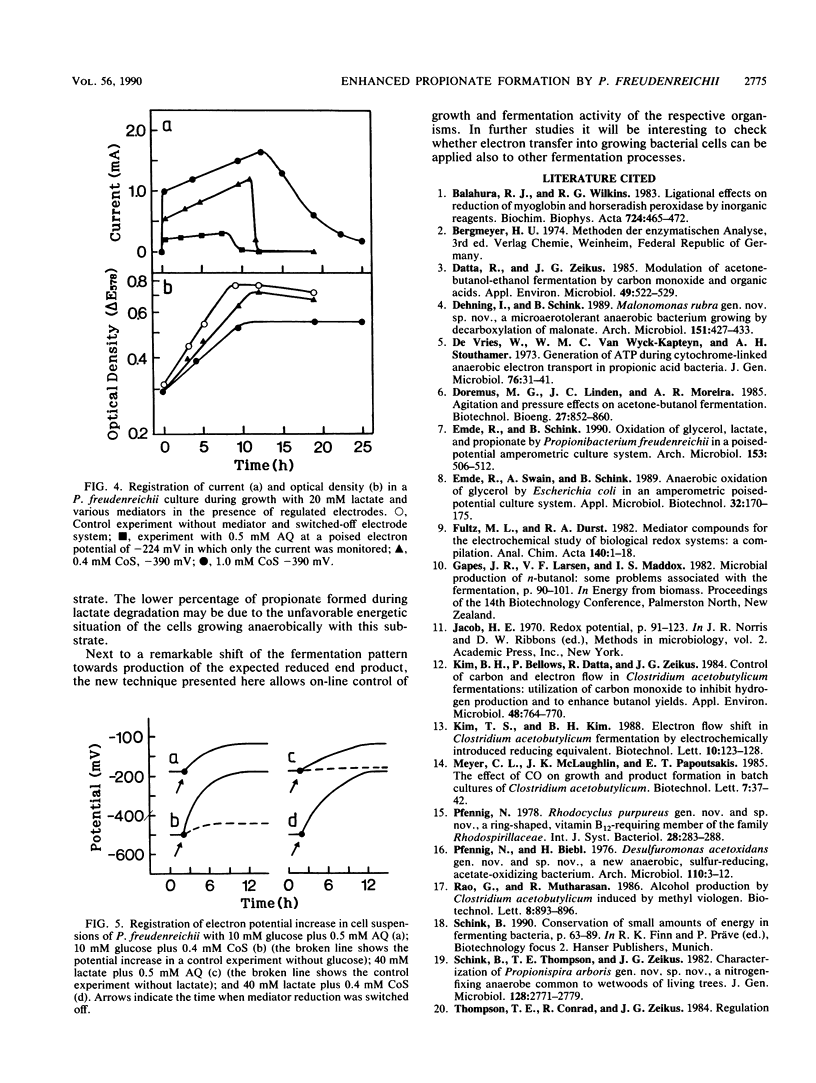
In order to influence the fermentation pattern of Propionibacterium freudenreichii towards enhanced propionate formation, growth and product formation with glucose and lactate as energy sources were studied in a three-electrode poised-potential amperometric culture system. With anthraquinone 2,6-disulfonic acid (E0′ = −184 mV; poised electron potential = −224 mV) or cobalt sepulchrate (E0′ = −350 mV; −390 mV) as mediator and an activated platinum working electrode, reduction of bacterially oxidized mediator occurred fast enough to keep more than 50% of the respective mediator (in minimum 0.4 mM) in the reduced state, up to a current of 2 mA. With glucose as substrate, 90.0 or 97.3% propionate was formed during exponential growth in the presence of 0.5 mM anthraquinone 2,6-disulfonic acid or 0.4 mM cobalt sepulchrate, respectively. Growth yields of 56.3 or 53.8 g of cell material per mol of substrate degraded were calculated, respectively, and the electrons were transferred quantitatively from the working electrode to the bacterial cells. With l-lactate, only 68.6 or 72.9% propionate was formed with the same mediators. The results are discussed with respect to energetics, electron transfer potentials, and potential application of the new technique in technical propionate production.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balahura R. J., Wilkins R. G. Ligational effects on reduction of myoglobin and horseradish peroxidase by inorganic reagents. Biochim Biophys Acta. 1983 Sep 30;724(3):465–472. doi: 10.1016/0005-2728(83)90107-x. [DOI] [PubMed] [Google Scholar]
- Datta R., Zeikus J. G. Modulation of acetone-butanol-ethanol fermentation by carbon monoxide and organic acids. Appl Environ Microbiol. 1985 Mar;49(3):522–529. doi: 10.1128/aem.49.3.522-529.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B. H., Bellows P., Datta R., Zeikus J. G. Control of Carbon and Electron Flow in Clostridium acetobutylicum Fermentations: Utilization of Carbon Monoxide to Inhibit Hydrogen Production and to Enhance Butanol Yields. Appl Environ Microbiol. 1984 Oct;48(4):764–770. doi: 10.1128/aem.48.4.764-770.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfennig N., Biebl H. Desulfuromonas acetoxidans gen. nov. and sp. nov., a new anaerobic, sulfur-reducing, acetate-oxidizing bacterium. Arch Microbiol. 1976 Oct 11;110(1):3–12. doi: 10.1007/BF00416962. [DOI] [PubMed] [Google Scholar]
- Widdel F., Pfennig N. Studies on dissimilatory sulfate-reducing bacteria that decompose fatty acids. I. Isolation of new sulfate-reducing bacteria enriched with acetate from saline environments. Description of Desulfobacter postgatei gen. nov., sp. nov. Arch Microbiol. 1981 Jul;129(5):395–400. doi: 10.1007/BF00406470. [DOI] [PubMed] [Google Scholar]
- Zeikus J. G. Chemical and fuel production by anaerobic bacteria. Annu Rev Microbiol. 1980;34:423–464. doi: 10.1146/annurev.mi.34.100180.002231. [DOI] [PubMed] [Google Scholar]
- de Vries W., van Wyck-Kapteyn W. M., Stouthamer A. H. Generation of ATP during cytochrome-linked anaerobic electron transport in propionic acid bacteria. J Gen Microbiol. 1973 May;76(1):31–41. doi: 10.1099/00221287-76-1-31. [DOI] [PubMed] [Google Scholar]



