Abstract
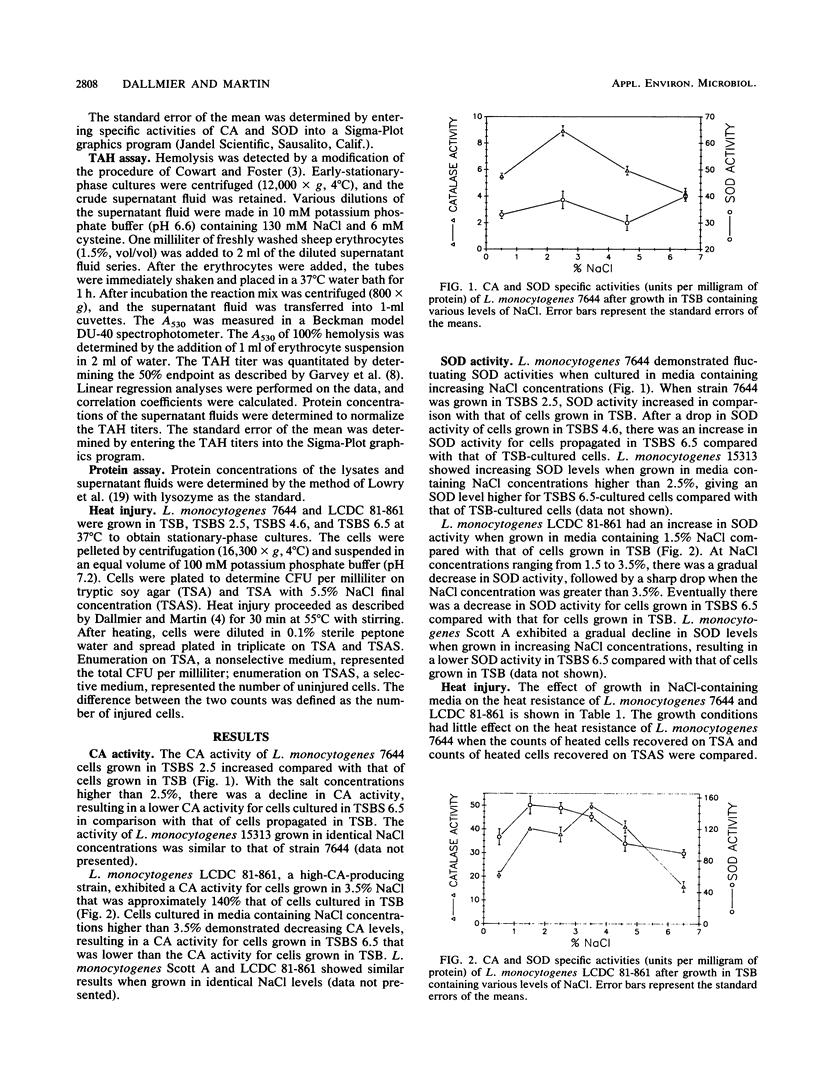
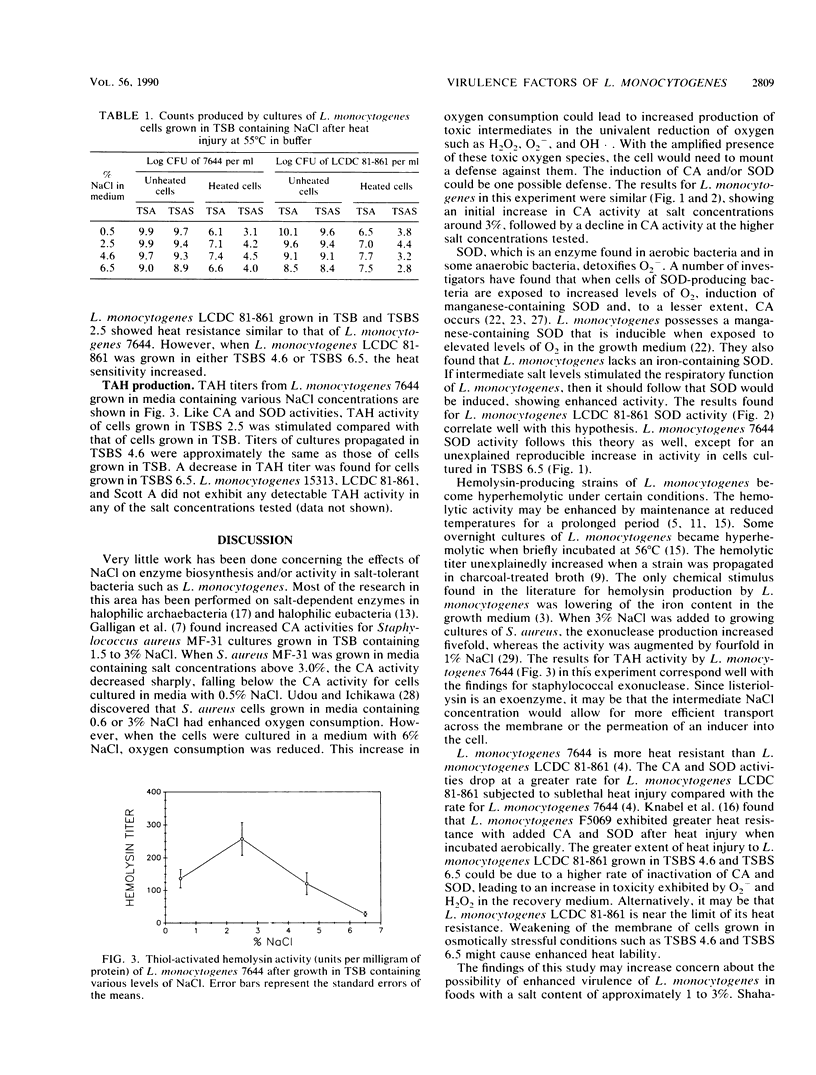
The activities of catalase, superoxide dismutase, and a thiol-activated hemolysin produced by four strains of Listeria monocytogenes propagated in media containing various concentrations of sodium chloride were examined. L. monocytogenes 7644 showed an increase in catalase, superoxide dismutase, and thiol-activated hemolysin activities when grown in a medium containing 2.5% (wt/vol) NaCl followed by a decrease in activities when propagated in media containing salt concentrations higher than 2.5%. L. monocytogenes LCDC 81-861 demonstrated enhanced catalase activity when grown in media containing NaCl ranging from 1.5 to 4.6% and increased superoxide dismutase activity when propagated in media containing 1.5 to 3.5% NaCl. L. monocytogenes LCDC 81-861 did not exhibit any detectable hemolysin activity under the conditions tested. After growth in various NaCl-containing media, both strains were subjected to sublethal heat injury for 30 min at 55 degrees C. L. monocytogenes LCDC 81-861 showed increased sensitivity to the heat treatment when grown in media containing 4.6 and 6.5% NaCl, whereas L. monocytogenes 7644 did not exhibit enhanced heat lability.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEERS R. F., Jr, SIZER I. W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952 Mar;195(1):133–140. [PubMed] [Google Scholar]
- Chakraborty T., Goebel W. Recent developments in the study of virulence in Listeria monocytogenes. Curr Top Microbiol Immunol. 1988;138:41–58. [PubMed] [Google Scholar]
- Dallmier A. W., Martin S. E. Catalase and superoxide dismutase activities after heat injury of Listeria monocytogenes. Appl Environ Microbiol. 1988 Feb;54(2):581–582. doi: 10.1128/aem.54.2.581-582.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durst J. The role of temperature factors in the epidemiology of listeriosis. Zentralbl Bakteriol Orig A. 1975 Sep;233(1):72–74. [PubMed] [Google Scholar]
- GIRARD K. F., SBARRA A. J., BARDAWIL W. A. Serology of Listeria monocytogenes. I. Characteristics of the soluble hemolysin. J Bacteriol. 1963 Feb;85:349–355. doi: 10.1128/jb.85.2.349-355.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard J. L., Berche P., Sansonetti P. Transposon mutagenesis as a tool to study the role of hemolysin in the virulence of Listeria monocytogenes. Infect Immun. 1986 Apr;52(1):50–55. doi: 10.1128/iai.52.1.50-55.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geoffroy C., Gaillard J. L., Alouf J. E., Berche P. Production of thiol-dependent haemolysins by Listeria monocytogenes and related species. J Gen Microbiol. 1989 Mar;135(3):481–487. doi: 10.1099/00221287-135-3-481. [DOI] [PubMed] [Google Scholar]
- Gill P. Is listeriosis often a foodborne illness? J Infect. 1988 Jul;17(1):1–5. doi: 10.1016/s0163-4453(88)92212-8. [DOI] [PubMed] [Google Scholar]
- Junttila J., Brander M. Listeria monocytogenes septicemia associated with consumption of salted mushrooms. Scand J Infect Dis. 1989;21(3):339–342. doi: 10.3109/00365548909035707. [DOI] [PubMed] [Google Scholar]
- Kathariou S., Metz P., Hof H., Goebel W. Tn916-induced mutations in the hemolysin determinant affecting virulence of Listeria monocytogenes. J Bacteriol. 1987 Mar;169(3):1291–1297. doi: 10.1128/jb.169.3.1291-1297.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kathariou S., Rocourt J., Hof H., Goebel W. Levels of Listeria monocytogenes hemolysin are not directly proportional to virulence in experimental infections of mice. Infect Immun. 1988 Feb;56(2):534–536. doi: 10.1128/iai.56.2.534-536.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knabel S. J., Walker H. W., Hartman P. A., Mendonca A. F. Effects of growth temperature and strictly anaerobic recovery on the survival of Listeria monocytogenes during pasteurization. Appl Environ Microbiol. 1990 Feb;56(2):370–376. doi: 10.1128/aem.56.2.370-376.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lamont R. J., Postlethwaite R., MacGowan A. P. Listeria monocytogenes and its role in human infection. J Infect. 1988 Jul;17(1):7–28. doi: 10.1016/s0163-4453(88)92236-0. [DOI] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Schiavone J. R., Hassan H. M. The role of redox in the regulation of manganese-containing superoxide dismutase biosynthesis in Escherichia coli. J Biol Chem. 1988 Mar 25;263(9):4269–4273. [PubMed] [Google Scholar]
- Shahamat M., Seaman A., Woodbine M. Survival of Listeria monocytogenes in high salt concentrations. Zentralbl Bakteriol A. 1980;246(4):506–511. [PubMed] [Google Scholar]
- Takahashi M., Nagano T., Hirobe M. Dioxathiadiaza-heteropentalenes mediate superoxide and hydrogen peroxide production in Escherichia coli. Arch Biochem Biophys. 1989 Jan;268(1):137–143. doi: 10.1016/0003-9861(89)90574-2. [DOI] [PubMed] [Google Scholar]
- Udou T., Ichik'awa Y. Effect of sodium chloride on the activity and production of staphylococcal exonuclease. J Gen Microbiol. 1980 Jan;116(1):69–74. doi: 10.1099/00221287-116-1-69. [DOI] [PubMed] [Google Scholar]
- Udou T., Ichikawa Y. Effect of sodium chloride on the respiratory function of Staphylococcus aureus. Experientia. 1979 Sep 15;35(9):1144–1145. doi: 10.1007/BF01963249. [DOI] [PubMed] [Google Scholar]
- Wood L. V., Woodbine M. Low temperature virulence of Listeria monocytogenes in the avian embryo. Zentralbl Bakteriol Orig A. 1979 Mar;243(1):74–81. [PubMed] [Google Scholar]



