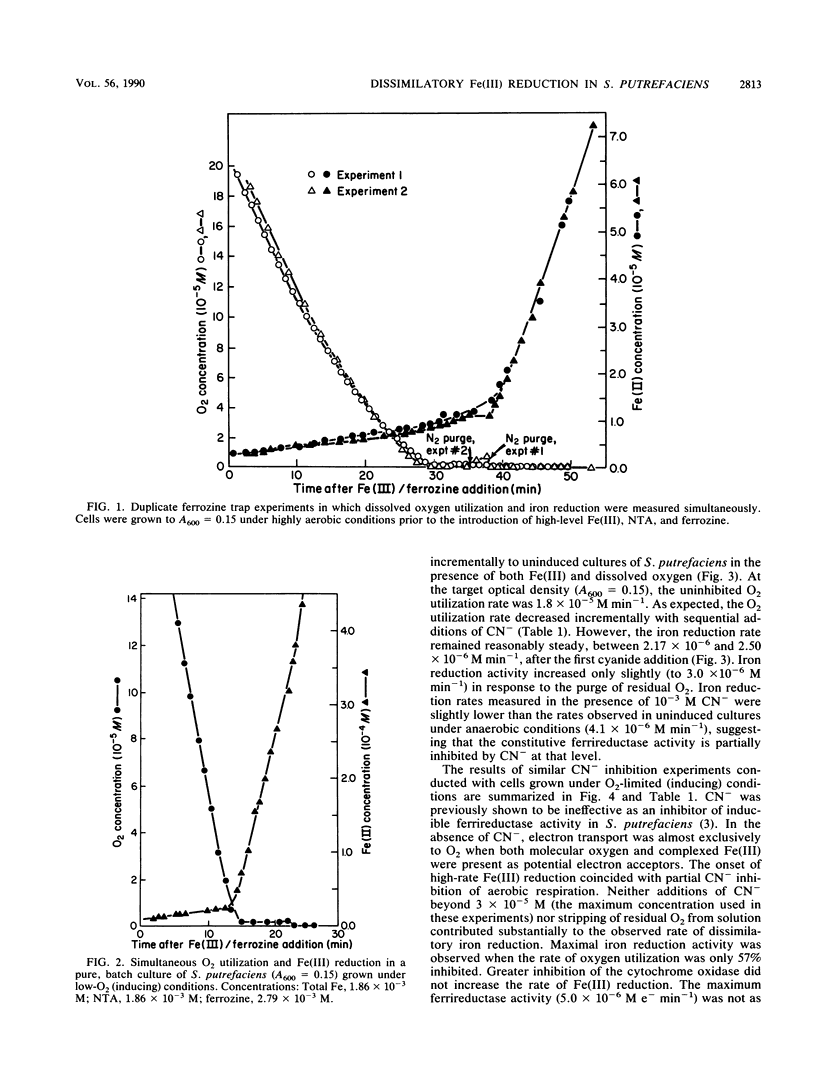
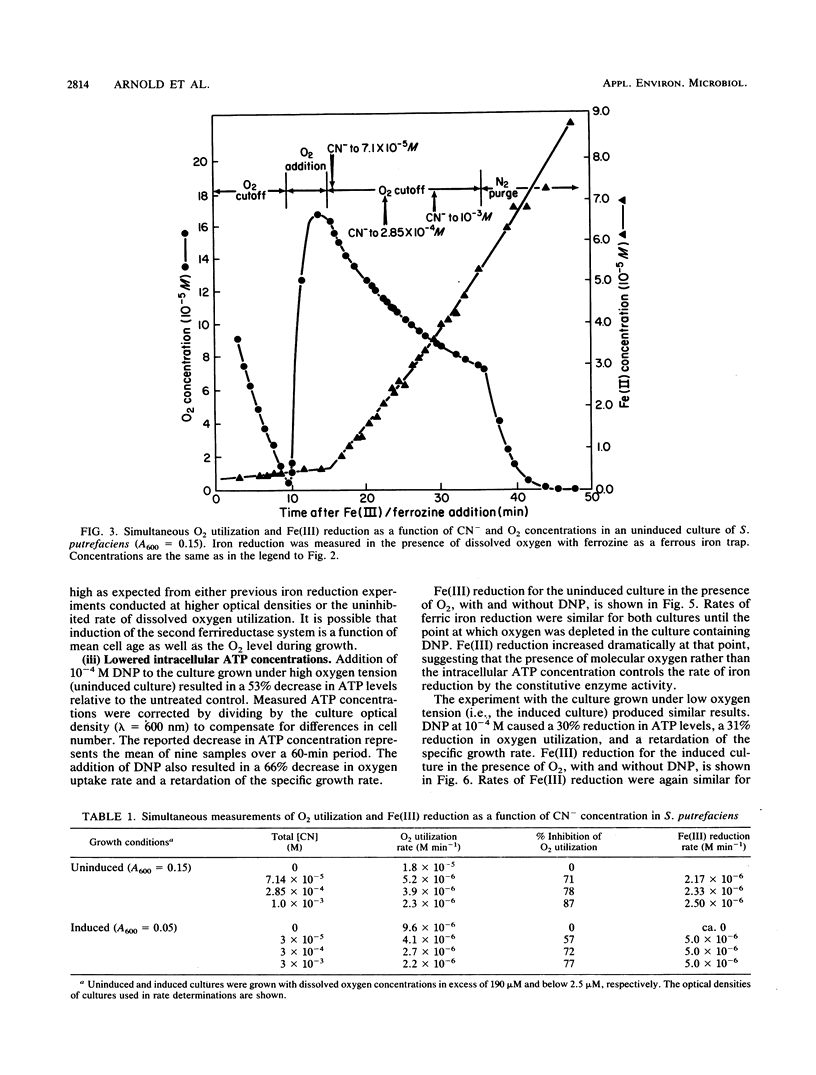
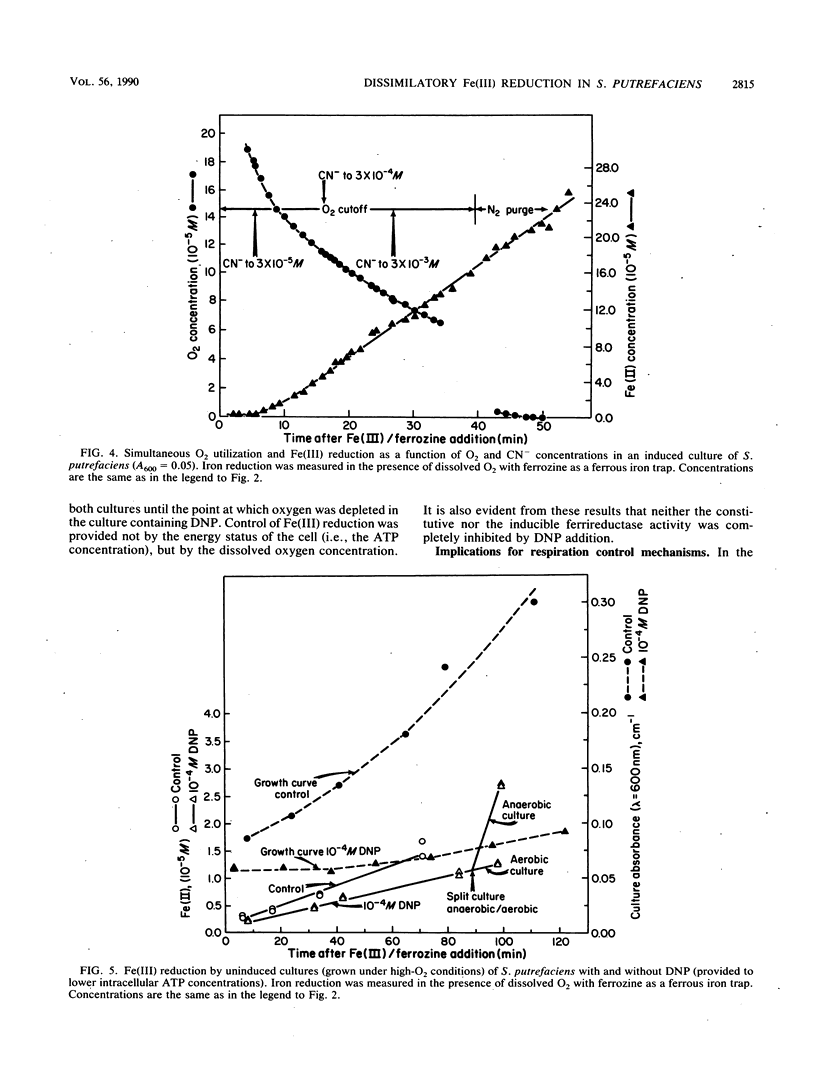
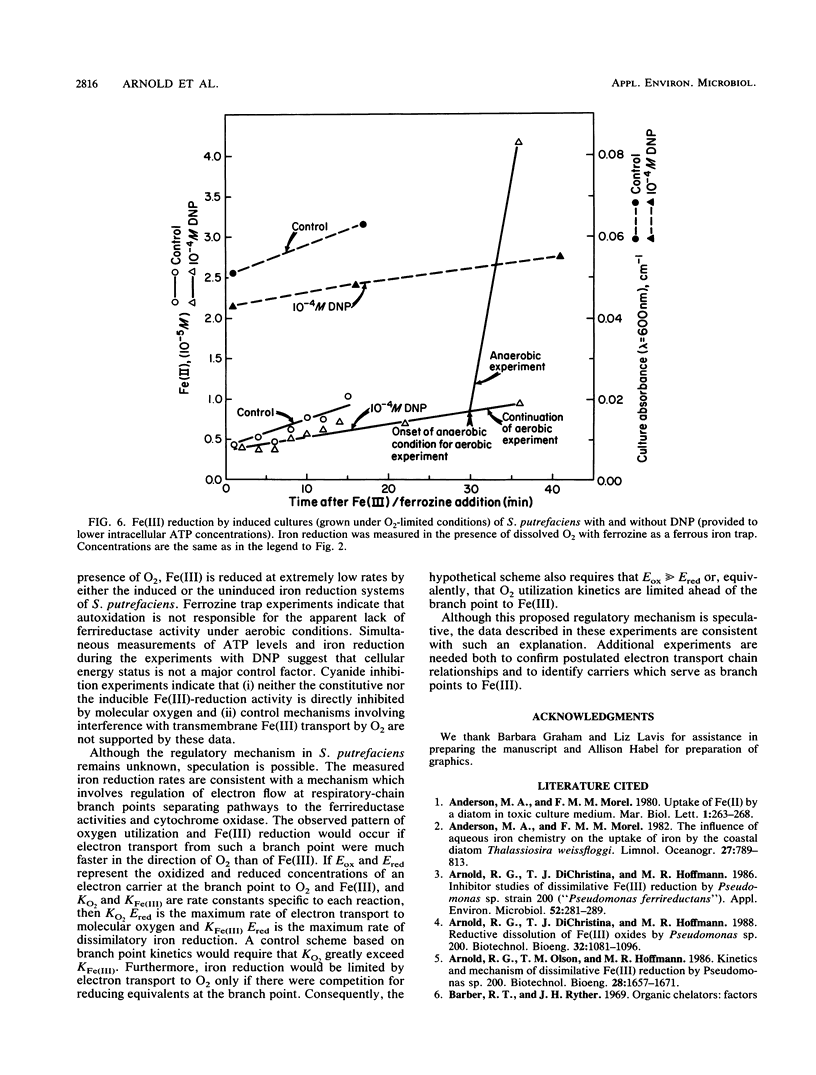
Abstract
Under anaerobic conditions, Shewanella putrefaciens is capable of respiratory-chain-linked, high-rate dissimilatory iron reduction via both a constitutive and inducible Fe(III)-reducing system. In the presence of low levels of dissolved oxygen, however, iron reduction by this microorganism is extremely slow. Fe(II)-trapping experiments in which Fe(III) and O2 were presented simultaneously to batch cultures of S. putrefaciens indicated that autoxidation of Fe(II) was not responsible for the absence of Fe(III) reduction. Inhibition of cytochrome oxidase with CN− resulted in a high rate of Fe(III) reduction in the presence of dissolved O2, which suggested that respiratory control mechanisms did not involve inhibition of Fe(III) reductase activities or Fe(III) transport by molecular oxygen. Decreasing the intracellular ATP concentrations by using an uncoupler, 2,4-dinitrophenol, did not increase Fe(III) reduction, indicating that the reduction rate was not controlled by the energy status of the cell. Control of electron transport at branch points could account for the observed pattern of respiration in the presence of the competing electron acceptors Fe(III) and O2.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold R. G., DiChristina T. J., Hoffmann M. R. Inhibitor studies of dissimilative Fe(III) reduction by Pseudomonas sp. strain 200 ("Pseudomonas ferrireductans") Appl Environ Microbiol. 1986 Aug;52(2):281–289. doi: 10.1128/aem.52.2.281-289.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter P. Spectrophotometric determination of serum iron at the submicrogram level with a new reagent (ferrozine). Anal Biochem. 1971 Apr;40(2):450–458. doi: 10.1016/0003-2697(71)90405-2. [DOI] [PubMed] [Google Scholar]
- Harrison D. E. The regulation of respiration rate in growing bacteria. Adv Microb Physiol. 1976;14(11):243–313. doi: 10.1016/s0065-2911(08)60229-5. [DOI] [PubMed] [Google Scholar]
- Lovley D. R., Phillips E. J. Competitive mechanisms for inhibition of sulfate reduction and methane production in the zone of ferric iron reduction in sediments. Appl Environ Microbiol. 1987 Nov;53(11):2636–2641. doi: 10.1128/aem.53.11.2636-2641.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovley D. R., Phillips E. J. Novel mode of microbial energy metabolism: organic carbon oxidation coupled to dissimilatory reduction of iron or manganese. Appl Environ Microbiol. 1988 Jun;54(6):1472–1480. doi: 10.1128/aem.54.6.1472-1480.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovley D. R., Phillips E. J. Organic matter mineralization with reduction of ferric iron in anaerobic sediments. Appl Environ Microbiol. 1986 Apr;51(4):683–689. doi: 10.1128/aem.51.4.683-689.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovley D. R., Phillips E. J. Rapid assay for microbially reducible ferric iron in aquatic sediments. Appl Environ Microbiol. 1987 Jul;53(7):1536–1540. doi: 10.1128/aem.53.7.1536-1540.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers C. R., Nealson K. H. Bacterial manganese reduction and growth with manganese oxide as the sole electron acceptor. Science. 1988 Jun 3;240(4857):1319–1321. doi: 10.1126/science.240.4857.1319. [DOI] [PubMed] [Google Scholar]
- Obuekwe C. O., Westlake D. W., Cook F. D. Effect of nitrate on reduction of ferric iron by a bacterium isolated from crude oil. Can J Microbiol. 1981 Jul;27(7):692–697. doi: 10.1139/m81-107. [DOI] [PubMed] [Google Scholar]
- Obuekwe C. O., Westlake D. W., Cook F. D., William Costerton J. Surface changes in mild steel coupons from the action of corrosion-causing bacteria. Appl Environ Microbiol. 1981 Mar;41(3):766–774. doi: 10.1128/aem.41.3.766-774.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen J. Reduction of ferric iron in anaerobic, marine sediment and interaction with reduction of nitrate and sulfate. Appl Environ Microbiol. 1982 Feb;43(2):319–324. doi: 10.1128/aem.43.2.319-324.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]



