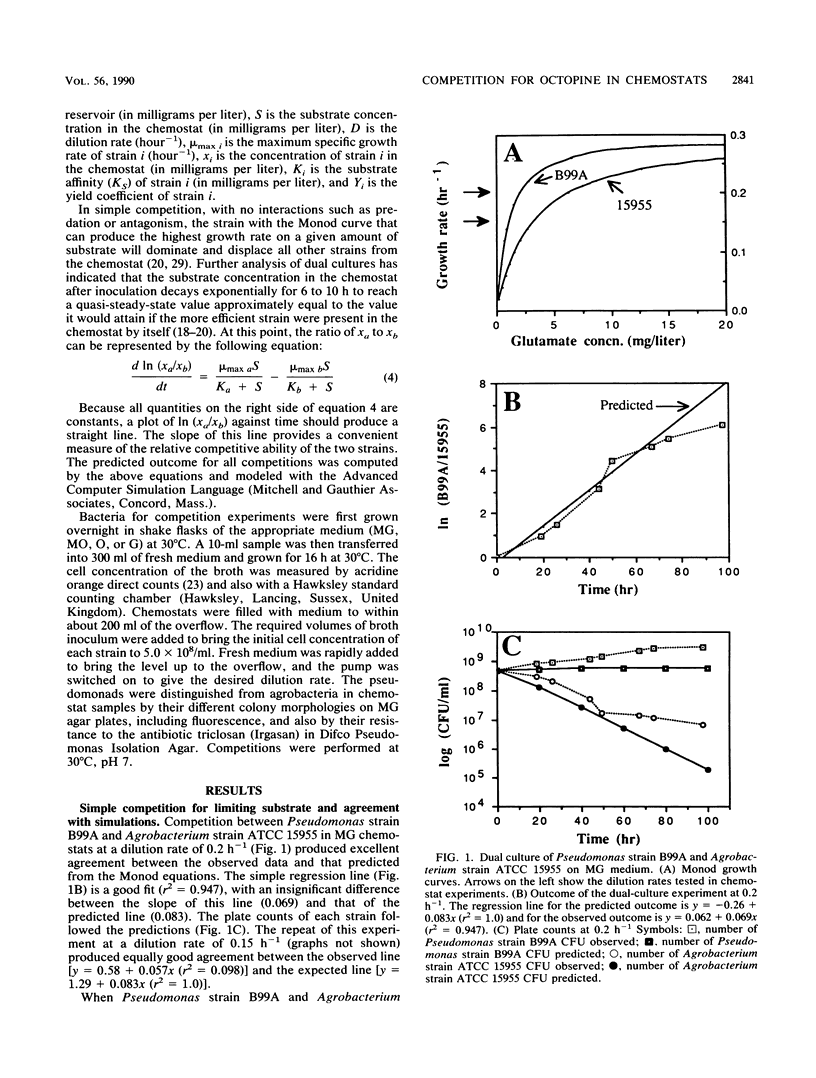
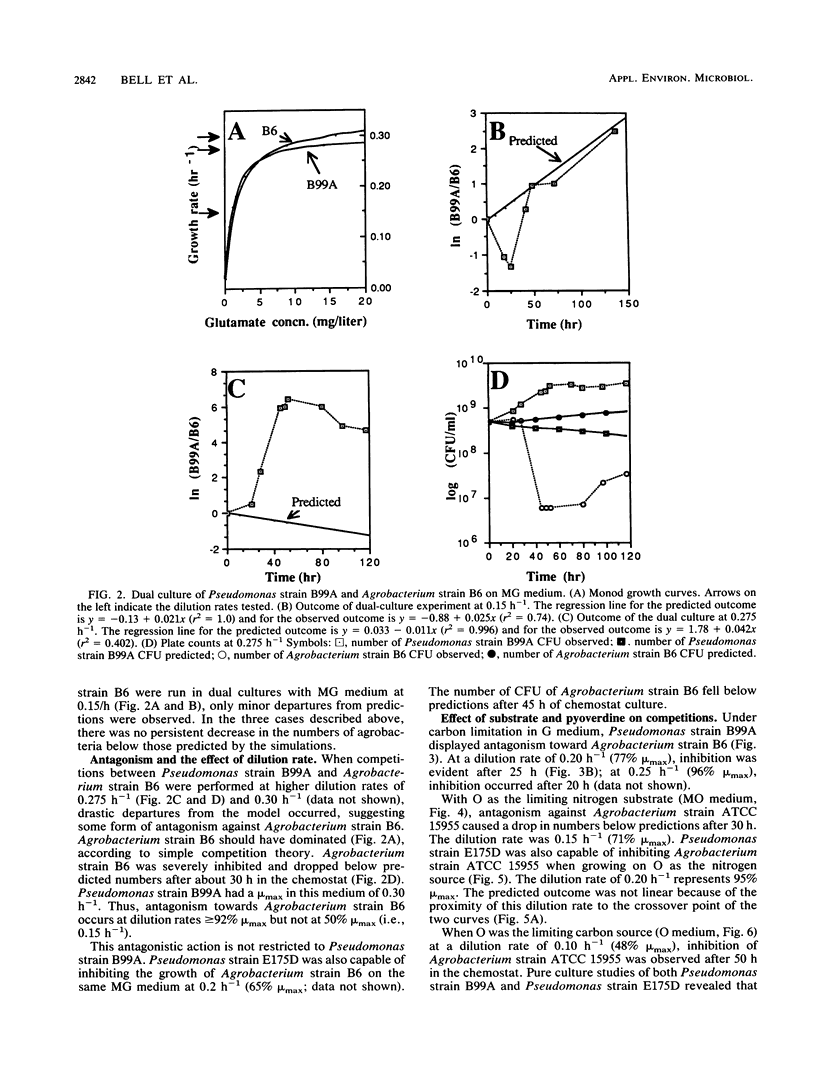
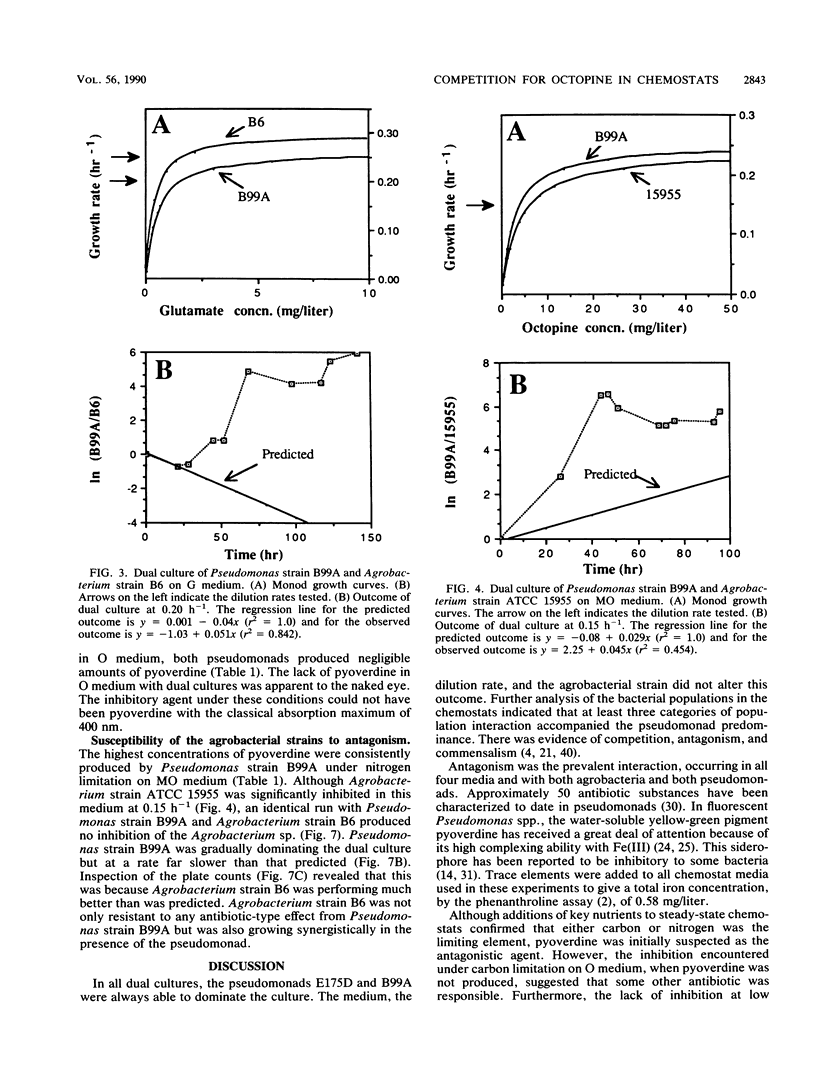
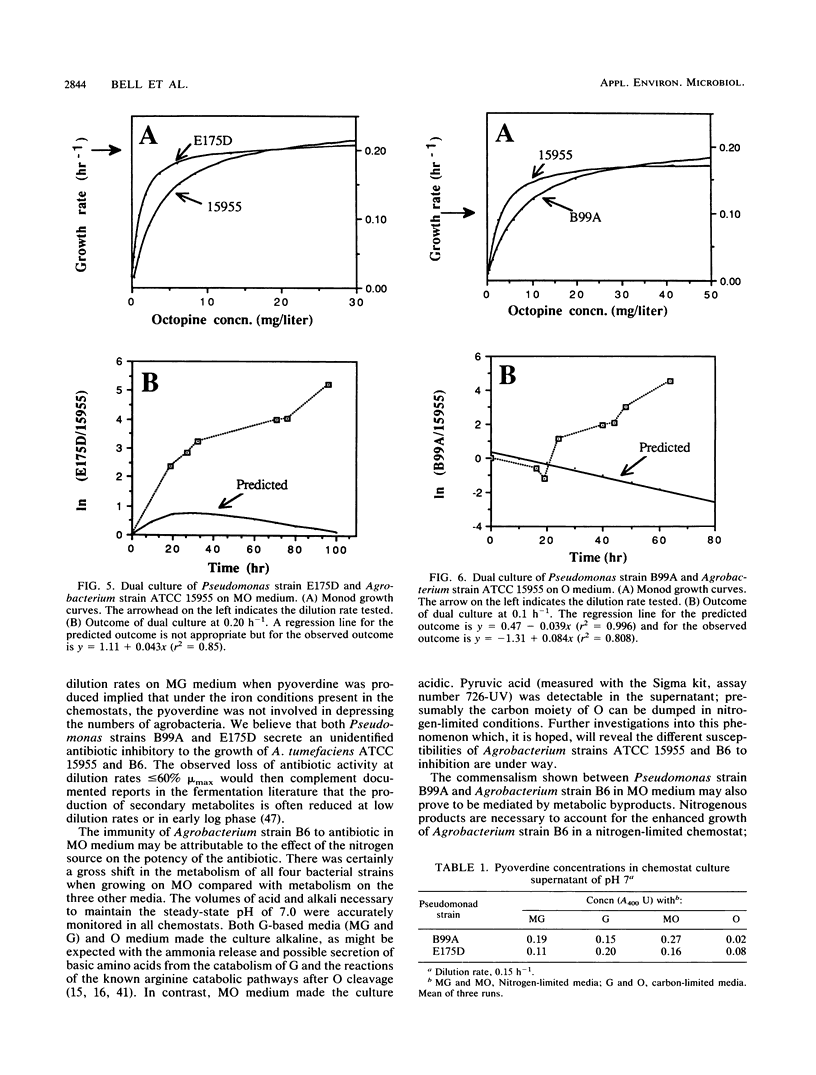
Abstract
The ability of two octopine-catabolizing Pseudomonas spp. and two virulent octopine-type Agrobacterium tumefaciens to compete for substrates has been examined in chemostats. In dual cultures with octopine or glutamate as the limiting carbon or nitrogen source, Pseudomonas fluorescens B99A and E175D always dominated over A. tumefaciens B6 or ATCC 15955. The growth dynamics of each strain in pure culture indicated that some form of antagonism was occurring in dual culture to permit the predominance of the pseudomonads under certain conditions. Although both pseudomonads fluoresce, pyoverdine was not responsible for the observed inhibition. An unidentified antibiotic secreted by both pseudomonads is believed to be responsible. A. tumefaciens B6 grew synergistically in the presence of P. fluorescens B99A with octopine as the limiting nitrogen source. This behavior of Agrobacterium strain B6 may help overcome its grossly inefficient use of octopine as previously reported. The ability of these two pseudomonads to outcompete the agrobacteria under all conditions tested raises the possibility that under field conditions, infectious agrobacteria may be succeeded by opine-catabolizing pseudomonads around crown gall tumors and in the rhizosphere.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell C. R. Growth of Agrobacterium tumefaciens under octopine limitation in chemostats. Appl Environ Microbiol. 1990 Jun;56(6):1775–1781. doi: 10.1128/aem.56.6.1775-1781.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell C. R., Moore L. W., Canfield M. L. Growth of Octopine-Catabolizing Pseudomonas spp. under Octopine Limitation in Chemostats and Their Potential To Compete with Agrobacterium tumefaciens. Appl Environ Microbiol. 1990 Sep;56(9):2834–2839. doi: 10.1128/aem.56.9.2834-2839.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouzar H., Moore L. W. Isolation of different agrobacterium biovars from a natural oak savanna and tallgrass prairie. Appl Environ Microbiol. 1987 Apr;53(4):717–721. doi: 10.1128/aem.53.4.717-721.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo A., Mora J. Ammonium assimilation in Rhizobium phaseoli by the glutamine synthetase-glutamate synthase pathway. J Bacteriol. 1988 Feb;170(2):980–984. doi: 10.1128/jb.170.2.980-984.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C. M., Macdonald-Brown D. S., Stanley S. O. The mechanisms of nitrogen assimilation in pseudomonads. Antonie Van Leeuwenhoek. 1973;39(1):89–98. doi: 10.1007/BF02578844. [DOI] [PubMed] [Google Scholar]
- Buyer J. S., Leong J. Iron transport-mediated antagonism between plant growth-promoting and plant-deleterious Pseudomonas strains. J Biol Chem. 1986 Jan 15;261(2):791–794. [PubMed] [Google Scholar]
- Dessaux Y., Petit A., Tempé J., Demarez M., Legrain C., Wiame J. M. Arginine catabolism in Agrobacterium strains: role of the Ti plasmid. J Bacteriol. 1986 Apr;166(1):44–50. doi: 10.1128/jb.166.1.44-50.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykhuizen D. E., Hartl D. L. Selection in chemostats. Microbiol Rev. 1983 Jun;47(2):150–168. doi: 10.1128/mr.47.2.150-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykhuizen D., Hartl D. L. Selective neutrality of 6PGD allozymes in E. coli and the effects of genetic background. Genetics. 1980 Dec;96(4):801–817. doi: 10.1093/genetics/96.4.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrickson A. G., Stephanopoulos G. Microbial competition. Science. 1981 Aug 28;213(4511):972–979. doi: 10.1126/science.7268409. [DOI] [PubMed] [Google Scholar]
- Hobbie J. E., Daley R. J., Jasper S. Use of nuclepore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol. 1977 May;33(5):1225–1228. doi: 10.1128/aem.33.5.1225-1228.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leisinger T., Margraff R. Secondary metabolites of the fluorescent pseudomonads. Microbiol Rev. 1979 Sep;43(3):422–442. doi: 10.1128/mr.43.3.422-442.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POWELL E. O. Criteria for the growth of contaminants and mutants in continuous culture. J Gen Microbiol. 1958 Feb;18(1):259–268. doi: 10.1099/00221287-18-1-259. [DOI] [PubMed] [Google Scholar]
- Panagopoulos C. G., Psallidas P. G. Characteristics of Greek Isolates of Agrobacterium tumefaciens (E. F. Smith & Townsend) Conn. J Appl Bacteriol. 1973 Jun;36(2):233–240. doi: 10.1111/j.1365-2672.1973.tb04096.x. [DOI] [PubMed] [Google Scholar]
- Schell J., Van Montagu M., De Beuckeleer M., De Block M., Depicker A., De Wilde M., Engler G., Genetello C., Hernalsteens J. P., Holsters M. Interactions and DNA transfer between Agrobacterium tumefaciens, the Ti-plasmid and the plant host. Proc R Soc Lond B Biol Sci. 1979 Apr 11;204(1155):251–266. doi: 10.1098/rspb.1979.0026. [DOI] [PubMed] [Google Scholar]
- Tremblay G., Gagliardo R., Chilton W. S., Dion P. Diversity among Opine-Utilizing Bacteria: Identification of Coryneform Isolates. Appl Environ Microbiol. 1987 Jul;53(7):1519–1524. doi: 10.1128/aem.53.7.1519-1524.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]



