Abstract
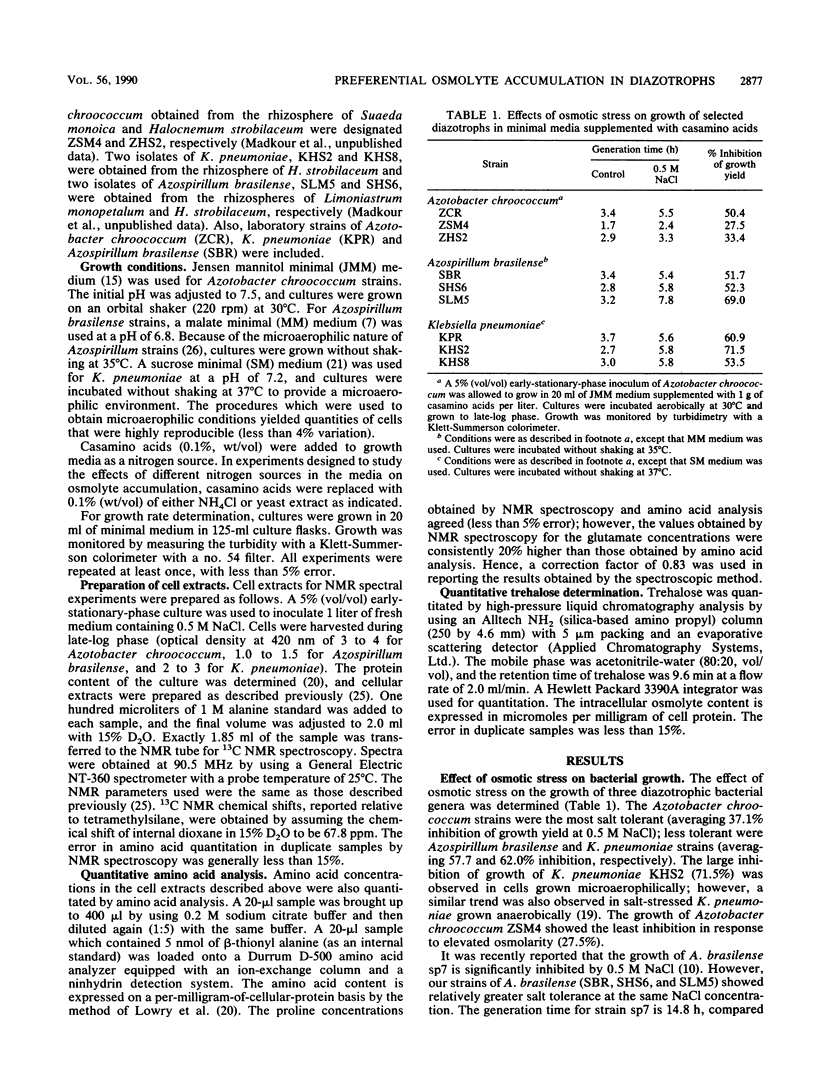
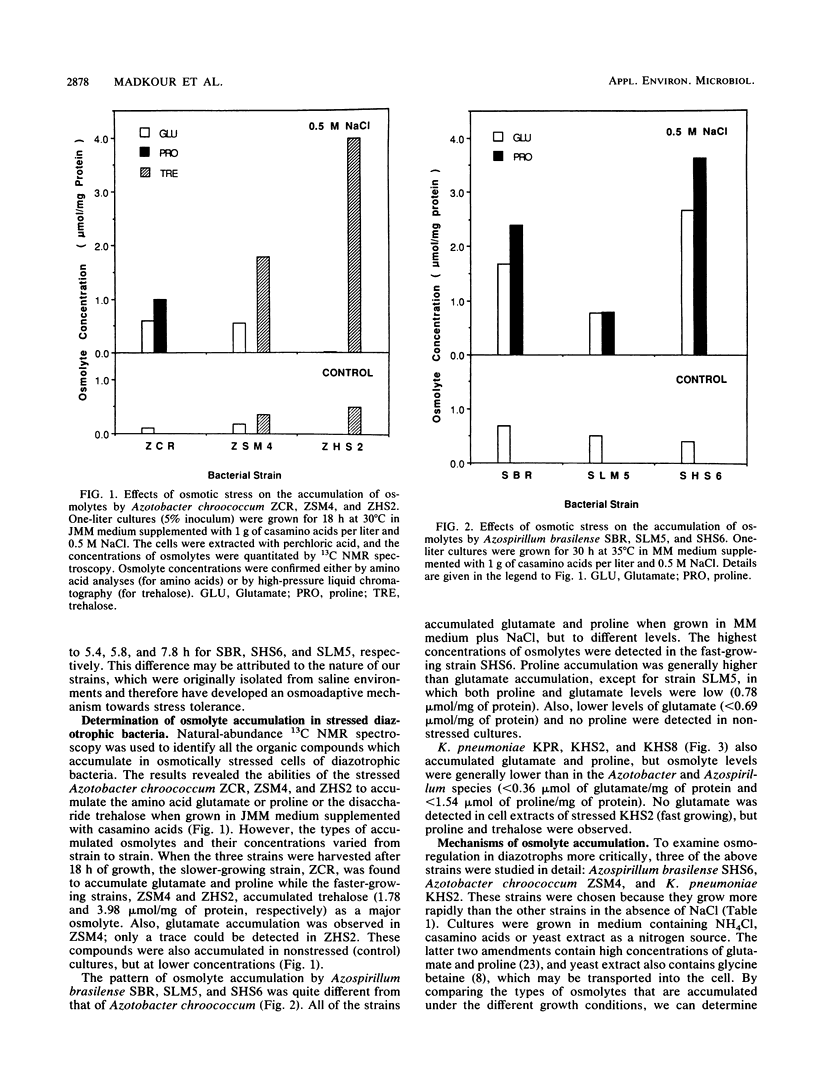
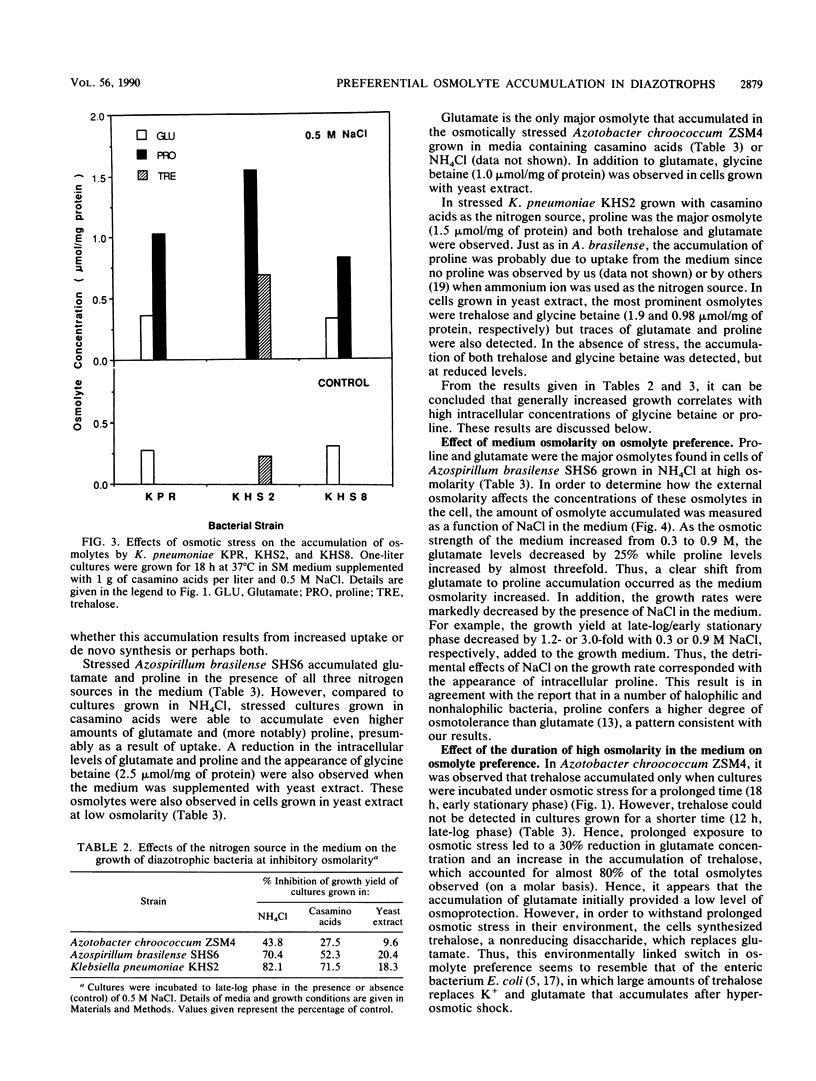
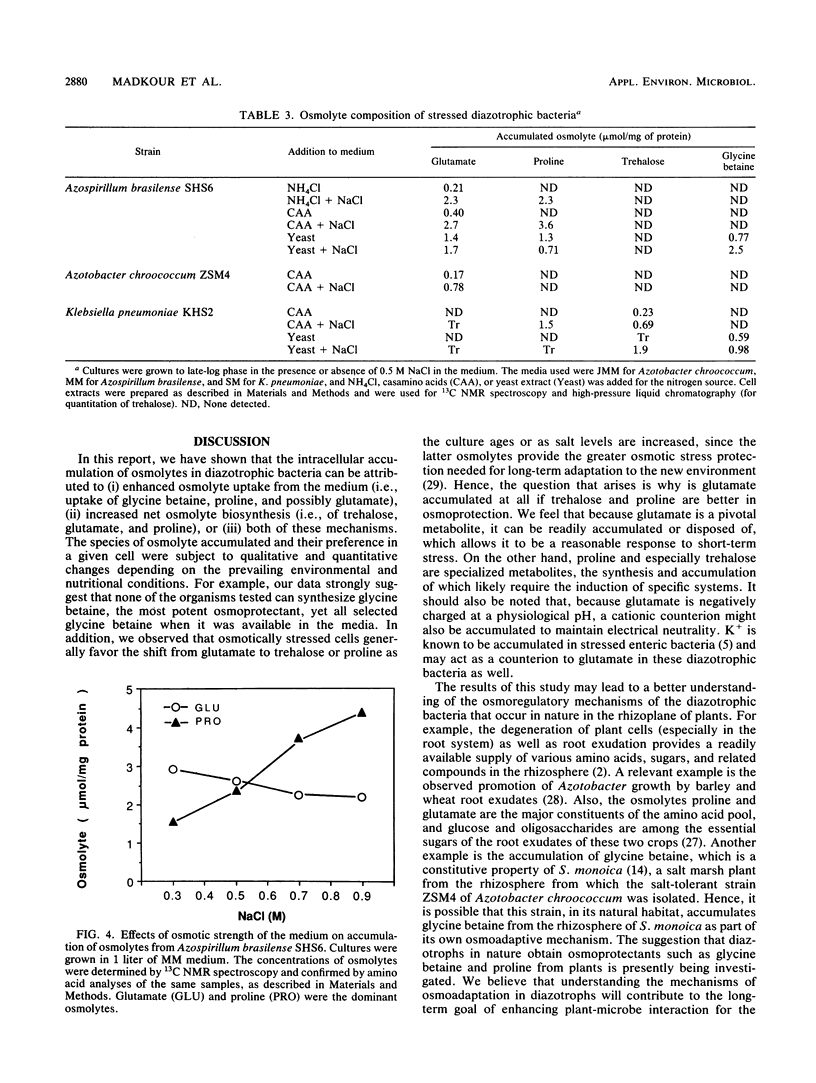
A common cellular mechanism of osmotic-stress adaptation is the intracellular accumulation of organic solutes (osmolytes). We investigated the mechanism of osmotic adaptation in the diazotrophic bacteria Azotobacter chroococcum, Azospirillum brasilense, and Klebsiella pneumoniae, which are adversely affected by high osmotic strength (i.e., soil salinity and/or drought). We used natural-abundance 13C nuclear magnetic resonance spectroscopy to identify all the osmolytes accumulating in these strains during osmotic stress generated by 0.5 M NaCl. Evidence is presented for the accumulation of trehalose and glutamate in Azotobacter chroococcum ZSM4, proline and glutamate in Azospirillum brasilense SHS6, and trehalose and proline in K. pneumoniae. Glycine betaine was accumulated in all strains grown in culture media containing yeast extract as the sole nitrogen source. Alternative nitrogen sources (e.g., NH4Cl or casamino acids) in the culture medium did not result in measurable glycine betaine accumulation. We suggest that the mechanism of osmotic adaptation in these organisms entails the accumulation of osmolytes in hyperosmotically stressed cells resulting from either enhanced uptake from the medium (of glycine betaine, proline, and glutamate) or increased net biosynthesis (of trehalose, proline, and glutamate) or both. The preferred osmolyte in Azotobacter chroococcum ZSM4 shifted from glutamate to trehalose as a consequence of a prolonged osmotic stress. Also, the dominant osmolyte in Azospirillum brasilense SHS6 shifted from glutamate to proline accumulation as the osmotic strength of the medium increased.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Csonka L. N. Physiological and genetic responses of bacteria to osmotic stress. Microbiol Rev. 1989 Mar;53(1):121–147. doi: 10.1128/mr.53.1.121-147.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaever H. M., Styrvold O. B., Kaasen I., Strøm A. R. Biochemical and genetic characterization of osmoregulatory trehalose synthesis in Escherichia coli. J Bacteriol. 1988 Jun;170(6):2841–2849. doi: 10.1128/jb.170.6.2841-2849.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Larsen P. I., Sydnes L. K., Landfald B., Strøm A. R. Osmoregulation in Escherichia coli by accumulation of organic osmolytes: betaines, glutamic acid, and trehalose. Arch Microbiol. 1987 Feb;147(1):1–7. doi: 10.1007/BF00492896. [DOI] [PubMed] [Google Scholar]
- Le Rudulier D., Bernard T., Goas G., Hamelin J. Osmoregulation in Klebsiella pneumoniae: enhancement of anaerobic growth and nitrogen fixation under stress by proline betaine, gamma-butyrobetaine, and other related compounds. Can J Microbiol. 1984 Mar;30(3):299–305. doi: 10.1139/m84-045. [DOI] [PubMed] [Google Scholar]
- Le Rudulier D., Yang S. S., Csonka L. N. Nitrogen fixation in Klebsiella pneumoniae during osmotic stress. Effect of exogenous proline or a proline overproducing plasmid. Biochim Biophys Acta. 1982 Nov 24;719(2):273–283. doi: 10.1016/0304-4165(82)90099-x. [DOI] [PubMed] [Google Scholar]
- MacNeil T., Brill W. J., Howe M. M. Bacteriophage mu-induced deletions in a plasmid containing the nif (N2 fixation) genes of Klebsiella pneumoniae. J Bacteriol. 1978 Jun;134(3):821–829. doi: 10.1128/jb.134.3.821-829.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan R. A. Amino acids and growth factors in vitamin-free casamino acids. Mycologia. 1971 Nov-Dec;63(6):1231–1234. [PubMed] [Google Scholar]
- Smith L. T., Pocard J. A., Bernard T., Le Rudulier D. Osmotic control of glycine betaine biosynthesis and degradation in Rhizobium meliloti. J Bacteriol. 1988 Jul;170(7):3142–3149. doi: 10.1128/jb.170.7.3142-3149.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L. T., Smith G. M. An osmoregulated dipeptide in stressed Rhizobium meliloti. J Bacteriol. 1989 Sep;171(9):4714–4717. doi: 10.1128/jb.171.9.4714-4717.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancey P. H., Clark M. E., Hand S. C., Bowlus R. D., Somero G. N. Living with water stress: evolution of osmolyte systems. Science. 1982 Sep 24;217(4566):1214–1222. doi: 10.1126/science.7112124. [DOI] [PubMed] [Google Scholar]



