Abstract
In obese Zucker diabetic fatty (ZDF) rats with mutant leptin receptors, pancreatic islets have an ≈50-fold increase in fat (TG), overproduce nitric oxide (NO), and lack a normal proinsulin mRNA response to fatty acids. We overexpressed the wild-type full-length “b” isoform of the leptin receptor (OB-Rb) in ZDF islets by perfusing ZDF pancreata with recombinant adenovirus containing the cDNA encoding OB-Rb. In cultured islets isolated from these animals, leptin lowered islet TG by 87% and completely blocked TG formation from free fatty acids. Overproduction of NO was reduced, and the preproinsulin mRNA response to free fatty acids was restored. This establishes defective leptin action as the proximate cause of lipotoxic diabetes in ZDF rats.
The mechanism by which long-standing obesity leads to non-insulin-dependent diabetes mellitus (NIDDM) remains obscure. The diabetes is preceded by an extended period of insulin resistance during which compensatory hyperinsulinemia maintains normoglycemia (1, 2); hyperglycemia appears only when insulin production fails to keep pace with progressively increasing insulin resistance. The cause of this failure is unknown (3).
Zucker diabetic fatty (ZDF) rats provide a model of adipogenic NIDDM with a similar pattern of antecedent insulin resistance followed by β cell failure (4). We have found that during the compensated prediabetic phase of the disease the triglyceride (TG) content of their pancreatic islets rises in parallel with the general increase in body fat (5). An islet TG content of 5–10 times normal is associated with β cell hyperplasia (6), increased preproinsulin gene expression (7), and hypersecretion of insulin (6). However, at an islet TG content of ≈50 times normal the foregoing compensatory changes recede to precompensatory levels (5, 7), and NIDDM appears. Experimental maneuvers that reduce the islet TG content prevent all the β cell abnormalities and the NIDDM (1, 8, 9), raising the possibility of a causal relationship between the fat overload in islets and the β cell dysfunction. Because nitric oxide (NO) overproduction has recently been identified as a mediator of β cell toxicity in fat-laden islets (10, 11), it is quite possible that the pathogenesis of β cell failure is entirely secondary to the overabundance of islet TG (TG overload, excess NO production and inability of β cells to compensate for insulin resistance).
The excessive fat accumulation in islets of ZDF rats is the consequence of a Gln269 → Pro substitution in all isoforms of the leptin receptor (OB-R) (12, 13) that abolishes the direct lipopenic action of leptin on tissues (14). To determine whether the chain of pathogenic events culminating in NIDDM can be reversed by restoring the responsiveness of their islets to leptin, we overexpressed wild-type OB-R in islets of OB-R-defective obese ZDF (fa/fa) rats. This restored the lipopenic action of leptin, reduced the exaggerated iNOS expression by islets, and enabled free fatty acids (FFA) to up-regulate proinsulin gene expression. It therefore establishes that, at least in this rodent model of adipogenic diabetes, fat overload secondary to leptin resistance is the cause of the diabetogenic phenotype.
MATERIALS AND METHODS
Construction of a Recombinant Adenovirus Containing the OB-Rb cDNA.
A recombinant adenovirus was constructed containing the cDNA encoding the long form of the leptin receptor (OB-Rb) that includes the 304-aa intracellular domain containing consensus JAK/STAT binding motifs. Total RNA was extracted from hypothalamus of normal Wistar rats, reverse-transcribed by using oligo(dT)18, and PCR-amplified by using oligonucleotides encompassing the C-terminal portion of OB-Rb (amino acids 711-1162) (the sequences of the primers are 5′-TTCTGGCCATCAATTCCATCGGTGC-3′ and 5′-GTCGACTTACACAGTTAAGTCACACATCTT-3′). The fidelity of the resulting 1.35-Kb PCR fragment was confirmed by complete sequence analysis. This fragment was digested with EcoRV and SalI and subcloned into an EcoRV/SalI-digested expression vector, pACCMV-Ob-Rf, containing a short isoform of the leptin receptor lacking the long C-terminal intracellular domain (Ob-Rf; see ref. 15 for details). The ligation of the PCR-amplified fragment encoding the Ob-Rb C-terminal region to the DNA encoding the N-terminal domain common to all OB-R isoforms resulted in an intact, full-length OB-Rb receptor construct. This full-length construct was then ligated into the adenovirus vector pACCMV pLpA (16) and verified by sequence analysis. The preparation of a recombinant adenovirus containing the OB-Rb cDNA under control of the CMV promoter (AdCMV-OB-Rb) was then carried out by previously described methods (17).
Pancreas Perfusion, Isolation, and Culture of Islets.
Pancreata of 7- to 8-week-old male ZDF (fa/fa) rats were perfused with recombinant adenovirus containing either the OB-Rb cDNA (AdCMV-OB-Rb) or the β-galactosidase cDNA (AdCMV-β-gal) (1 × 1012 pfu) in Krebs–Ringer bicarbonate buffer containing 3.5% Dextran T70, 1% BSA, 5.6 mM glucose, and 5 mM each of sodium pyruvate, sodium glutamate, and sodium fumarate. Efficient adenovirus-gene transfer to islets of ZDF rats is best achieved by pancreas perfusion, in as much as simple incubation of islets of this type with the virus in vitro is ineffective (K. Koyama, R. J. Noel, R.H.U., and C.B.N., unpublished data). Pancreatic islets were then isolated and maintained in suspension culture in 60-mm Petri dishes at 37°C in a humidified atmosphere of 5% CO2 and 95% air, as described previously (14). The culture medium consisted of RPMI 1640 supplemented with 10% fetal bovine serum, 1% of penicillin and streptomycin, and 8 mM glucose either with or without 1 mM of long-chain fatty acids (2:1 oleate:palmitate).
TG Content of Islets.
After culture, 100–200 islets were counted, washed twice with PBS (pH 7.4), and sonicated in a high-salt buffer (2 M NaCl/2 mM EDTA/50 mM sodium phosphate buffer, pH 7.4). The homogenate was aliquoted and its TG concentration was then measured by using a Triglyceride (GPO-Trinder) kit (Sigma) as described previously (14).
Reverse Transcription–PCR (RT-PCR).
Total RNA from culture of 100–200 islets [isolated from ZDF (fa/fa) rats] perfused with either adenovirus AdCMV-OB-Rb or AdCMV-β-Gal was extracted by using TRIzol Reagent (Life Technologies, Gaithersburg, MD). After treating with RNase-free Dnase I (CLONTECH), reverse transcription was carried out by using 1 μg of total RNA. First-strand cDNA was PCR-amplified by using specific oligonucleotide pairs for the b isoform of the rat leptin receptor, OB-Rb. The sequences of upstream and downstream primers are 5′-CTAGCAACTCCTGGGAGATAGAGG and 5′-TTACACAGTTAAGTCACACATCTT, respectively. As a control for RNA quality and quantity, β-actin mRNA was amplified from all samples by using oligonucleotides as described (15). The PCR primers previously used for acyl CoA carboxylase (ACC), acyl CoA oxidase (ACO), glycerol-phosphate-acyl transferase (GPAT), iNOS, and preproinsulin cDNAs were again employed (7, 10, 18). The PCR for leptin receptor and actin was performed as previously described (15). For remaining genes, the conditions of PCR were as follows: denaturation for 45 sec at 92°C, annealing for 45 sec at 55°C, and elongation for 1 min at 72°C with either 25 cycles (ACO and GPAT), 50 cycles (ACC), 22 cycles (proinsulin), or 35 cycles (iNOS). The PCR products were subjected to electrophoresis on 1.2% agarose gel and quantified by Southern blot analyses by using gene-specific 32P-labeled probes.
Immunoblotting.
Approximately 50 islets isolated from ZDF (fa/fa) pancreata that had been perfused with AdCMV-OB-Rb or AdCMV-β-Gal were cultured for 3 days, and total islet proteins were directly denatured in boiled SDS/PAGE sample buffer, followed by resolution with 7.5% SDS/PAGE. After electrophoresis, the proteins were transferred onto polyvinylidene difluoride membrane (Millipore). The membrane was then treated with anti-leptin receptor antibody K-20 (Santa Cruz Biotechnology). After washing the blot, bound primary antibody was detected by reaction with anti-goat IgG antibody-peroxidase conjugate. The antibody complexes were visualized with a Super Signal-CL kit (Pierce) as described (19).
Nitrite Determination.
Two hundred and fifty microliters of culture medium was incubated with an equal volume of Griess reagent (1% sulfanilamide in 0.1 mol/1 M HCl and 0.1% naphthyl ethylenediamine dihydrochloride) for 10 min at room temperature and NO was determined as nitrite from the absorbance at 550 nm by using sodium nitrite as standard (20). Nitrite could not be measured in β-gal-expressing islets because of interference with optical density.
RESULTS
Overexpression of OB-Rb WT in Islets.
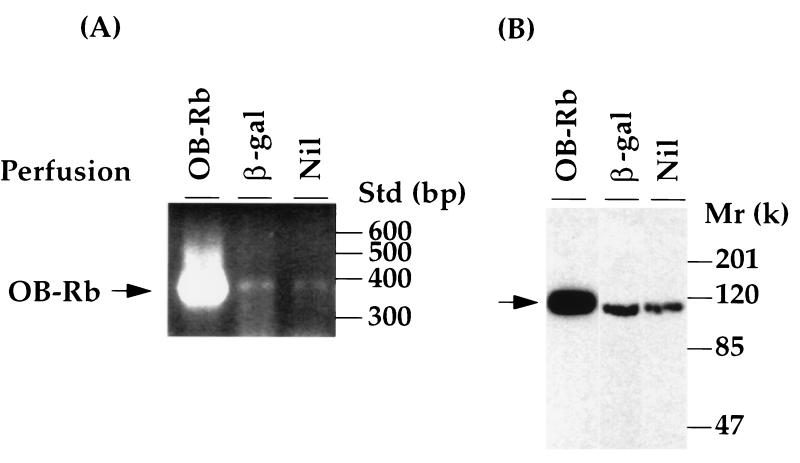
In islets isolated from ZDF (fa/fa) pancreata perfused with recombinant adenovirus AdCMV-OB-Rb and cultured for 3 days, OB-Rb mRNA appeared within 24 h and reached extremely high levels by 72 h (Fig. 1A), at which time immunoblotting revealed an abundance of OB-Rb protein. [OB-Rb is the full-length isoform expressed in abundance in hypothalamus but also present in islets (21). It is the only isoform for which biologic activity has been established (22)]. In control islets from pancreata perfused with control adenovirus AdCMV-β-gal or unperfused, the endogenous mutant OB-R mRNA was barely detectable by RT-PCR at 50 cycles, and the endogenous mutant protein in these controls was present at approximately 20% of that of the AdCMV-OB-Rb-perfused islets (Fig. 1B).
Figure 1.
Overexpression of the recombinant b isoform of rat leptin receptor (OB-Rb) in the islets of ZDF fa/fa rats. (A) RT-PCR assays to detect mRNA of the receptor in ZDF islets isolated after perfusion of the pancreata with recombinant adenovirus carrying either OB-Rb cDNA (OB-Rb) or E. coli lacZ gene (β-gal), or without perfusion (Nil) and cultured for 3 days. The PCR products specific for OB-Rb are indicated on the left. The DNA size standard (Std) is shown on the right. (B) Immunoblotting analysis of OB-Rb with a polyclonal antibody to the extracellular domain of the receptor by using total lysates from cultured islets as described in A. Positions of protein markers are shown on the right.
Effect of OB-Rb Overexpression on Leptin-Induced Lipopenia in Islets.
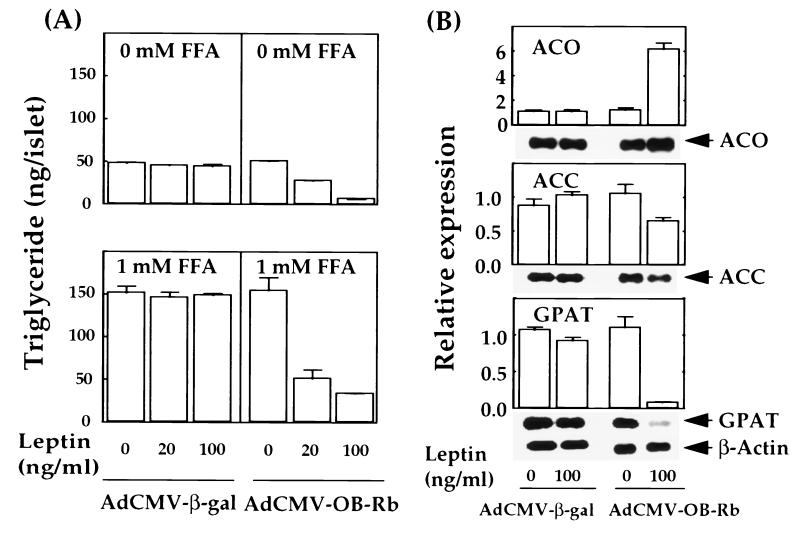
In contrast to islets from normal rats, in which leptin reduces TG content and blocks esterification of FFA, islets from ZDF rats are resistant to the lipopenic action of leptin (14). These lipopenic effects are attributed to leptin-induced up-regulation of expression of enzymes of FFA oxidation, carnitine palmitoyltransferase-1 (CPT-1) and acyl CoA oxidase (ACO), and down-regulation of enzymes of FFA synthesis and esterification, acetyl CoA carboxylase (ACC) and glycerol-phosphate-acyl transferase (GPAT). These changes in mRNA cannot be induced by leptin in the fat-laden islets of ZDF rats homozygous for the OB-R mutation (18). However, in ZDF islets treated to overexpress the wild-type OB-Rb, a full lipopenic response to leptin was observed (Fig. 2A). Islet TG declined to 50% and 13% of the control level with 20 and 100 ng/ml of leptin, respectively, and TG formation in the presence of 1 mM FFA was blocked. Leptin had no effect on these parameters in islets from ZDF rats perfused with AdCMV-β-gal or in islets from unperfused pancreata (Fig. 2A).
Figure 2.
(A) The effects of overexpression of the recombinant leptin receptor on triglyceride content in cultured ZDF islets perfused with either AdCMV-OB-Rb or AdCMV-β-gal without or with the addition of FFA (1 mM) and various amounts of leptin (0–100 ng/ml) in the culture medium. (B) The effects of overproduction of the recombinant leptin receptor on the gene expression of several enzymes involved in FFA metabolism. RT-PCR assays were employed to estimate the transcript levels of genes encoding ACO, ACC, GPAT, and β-actin in cultured ZDF islets perfused with either AdCMV-OB-Rb or AdCMV-β-gal in the presence or absence of leptin (100 ng/ml) in the medium. The PCR products detected by Southern blot analysis with labeled oligo probes specific for the coding region of each gene are displayed together with quantitative results of the RT-PCR assays. Data represent four independent experiments in which signal intensities of the PCR products from transcripts of each gene were normalized to those of β-actin, and the resulting values are taken as relative expression of the corresponding genes. Error bars indicate standard deviations.
The AdCMV-OB-Rb treatment of ZDF islets also restored leptin-mediated control of the expression of three of the enzymes of FFA metabolism. ACC mRNA declined very slightly but significantly (P < 0.05), whereas ACO mRNA rose 5-fold and GPAT mRNA declined to 7% of normal (Fig. 2B).
Effect of OB-Rb Overexpression on FFA-Induced Increase of Preproinsulin mRNA.
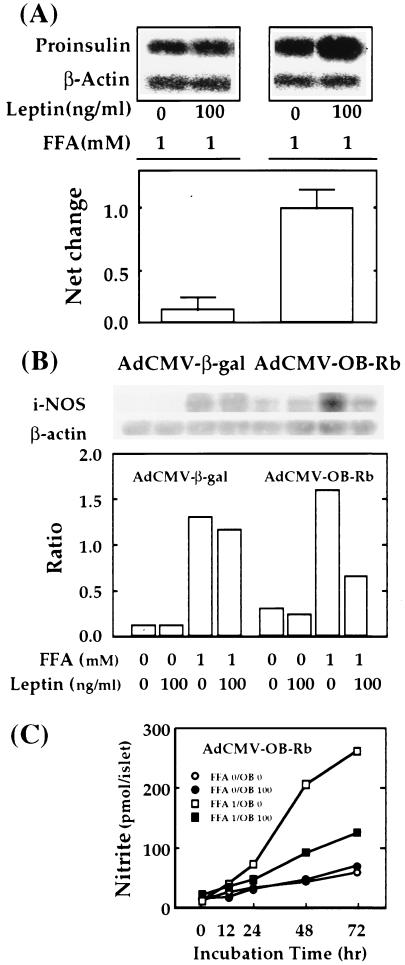
In normal islets, 1 mM FFA increases preproinsulin mRNA (7) and insulin production (6). This response must play a major role in β cell compensation for the insulin resistance of obesity. Because it does not occur in fat-laden compensated prediabetic islets (6), we have suggested that this unresponsiveness of prediabetic ZDF to FFA accounts for their ultimate inability to prevent hyperglycemia (6). If this is, in fact, the case, the expression of normal OB-Rb in the ZDF islets should, in the presence of leptin, repair the pathogenic condition. We therefore cultured AdCMV-OB-Rb-treated ZDF islets with 100 ng/ml of leptin for 2 days and then added 1 mM FFA for 1 day. A 9-fold net increase in the response of the preproinsulin/β-actin mRNA ratio was observed compared with AdCMV-β-gal-treated controls (Fig. 3A).
Figure 3.
(A) The effects of overproduction of the recombinant leptin receptor on FFA-induced up-regulation of proinsulin mRNA in cultured ZDF islets perfused with either AdCMV-OB-Rb or AdCMV-β-gal with or without 2-day treatment with leptin (100 ng/ml) followed by 1-day incubation of FFA (1 mM) in the medium. RT-PCR was performed as described in the Methods, and PCR products were detected by Southern blot analysis with specific probes (Upper). The signal intensities of proinsulin PCR products were first normalized to those of β-actin products, and the differences of normalized intensities between samples from islets with and without leptin treatment are expressed as net change in proinsulin expression (Lower). The results suggest that the normal up-regulation of proinsulin gene expression by FFA can be restored by reducing islet fat content through leptin action. (B) Effects of overexpression of the recombinant leptin receptor on FFA-induced accumulation of iNOS mRNA in islets of ZDF rats. Islets isolated from ZDF rats were perfused with either AdCMV-OB-Rb or AdCMV-β-gal and cultured for 3 days with or without FFA (1 mM) in the presence or absence of leptin (100 ng/ml). RT-PCR for iNOS and β-actin genes was performed as described in the Methods, and representative PCR products detected by the Southern blot analysis with specific probes are shown. (C) Effects of overexpression of the recombinant leptin receptor on FFA-induced NO formation. Data represent the mean of two independent experiments.
Effect of OB-Rb Overexpression on iNOS mRNA and NO Production.
It has been proposed that the FFA-induced dysfunction and β-cytotoxicity in fat-laden islets of ZDF rats is mediated by exaggerated production of NO (10). If so, the reduction of TG should be accompanied by a significant decline in FFA-induced iNOS mRNA and NO production. Compared with control islets from pancreata perfused with AdCMV-β-gal and cultured with 100 ng/ml of leptin, in which the FFA-induced increase in iNOS/actin mRNA ratio remained high, in islets overexpressing OB-Rb, leptin treatment (100 ng/ml) caused a 51% decline in the FFA-induced iNOS ratio (Fig. 3B) and a 49% reduction in the NO response to 1 mM FFA (Fig. 3C).
DISCUSSION
The study provides evidence that the β cell phenotype in ZDF rats is the result of the lack of a functional leptin receptor. Overexpression of the wild-type OB-Rb, the long isoform of leptin receptors, restored to leptin-unresponsive islets of ZDF rats the normal lipopenic action of leptin. This was associated with a 15-fold decrease in expression of GPAT, an enzyme of FFA esterification, and a 5-fold increase in ACO, an enzyme of oxidation. These responses were lacking in control ZDF islets. Thus, leptin action regulates the expression of enzymes involved in lipid metabolism in islets.
The study also indicates that the diabetogenic dysfunction of ZDF rat islets is secondary to their excessive fat content. The failure of ZDF β cells to mount an increase in preproinsulin mRNA when exposed to FFA was corrected by leptin in ZDF islets overexpressing OB-Rb. This strongly supports the hypothesis that islet fat overload can result in inability of β cells to increase insulin production.
Finally, the excessive NO response was significantly decreased by leptin treatment of OB-Rb-overexpressing ZDF islets, supporting the premise (1, 23) that the cytotoxicity is secondary to the accumulation of fat. The exaggerated induction by FFA of iNOS in ZDF islets is believed to be responsible for the loss of 50–75% of their β cells (4), because it can be completely prevented by iNOS inhibitors, such as nicotinamide and aminoguanidine (10).
In summary, the findings indicate, first, that leptin directly prevents overaccumulation of fat in OB-Rb-expressing tissues, and second, that FFA-induced overproduction of NO and the associated defect in preproinsulin gene expression can be reversed by depleting islet fat. The latter suggests a mechanism by which obesity causes NIDDM and a therapeutic strategy for treating human adipogenic diabetes by using leptinomimetic drugs that lower islet fat content, thereby blocking the putative lipotoxic mechanism by which obesity leads to diabetes.
Acknowledgments
We thank Kay McCorkle and Tagan Ferguson for outstanding technical support and Sharryn Harris for secretarial assistance. This work was supported by National Institutes of Health (DK02700-37), NIH/Juvenile Diabetes Foundation International Diabetes Interdisciplinary Research Program, and Veterans Administration Institutional Research Support Grant SMI821-109. We thank Daniel W. Foster, M.D., and Chris Rhodes, Ph.D., for critical review of the paper.
ABBREVIATIONS
- OB-Rb
leptin receptor b isoform
- TG
triglyceride
- FFA
free fatty acid
- ZDF
Zucker diabetic fatty
- NIDDM
non-insulin-dependent diabetes mellitus
References
- 1.Unger R H. Diabetes. 1995;44:863–870. doi: 10.2337/diab.44.8.863. [DOI] [PubMed] [Google Scholar]
- 2.DeFronzo R A, Bonadonna R C, Ferrannini E. Diabetes Care. 1992;15:318–368. doi: 10.2337/diacare.15.3.318. [DOI] [PubMed] [Google Scholar]
- 3.Harris M I. Diabetes Care. 1993;16:642–652. doi: 10.2337/diacare.16.4.642. [DOI] [PubMed] [Google Scholar]
- 4.Peterson R G, Shaw W N, Neel M, Little L A, Eichberg J. ILAR News. 1990;32:16–19. doi: 10.1093/ilar.32.3.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee Y H, Hirose H, Ohneda M, Johnson J H, McGarry J D, Unger R H. Proc Natl Acad Sci USA. 1994;91:10878–10882. doi: 10.1073/pnas.91.23.10878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hirose H, Lee Y H, Inman L R, Nagasawa Y, Johnson J H, Unger R H. J Biol Chem. 1996;271:5633–5637. doi: 10.1074/jbc.271.10.5633. [DOI] [PubMed] [Google Scholar]
- 7.Zhou Y-T, Shimabukuro M, Lee Y H, Koyama K, Trieu F, Unger R H. J Biol Chem. 1997;272:25648–25651. doi: 10.1074/jbc.272.41.25648. [DOI] [PubMed] [Google Scholar]
- 8.Ohneda M, Inman L R, Unger R H. Diabetologia. 1995;38:173–179. doi: 10.1007/BF00400091. [DOI] [PubMed] [Google Scholar]
- 9.McGarry J D. J Cell Biochem. 1994;555:29–38. doi: 10.1002/jcb.240550005. [DOI] [PubMed] [Google Scholar]
- 10.Shimabukuro M, Ohneda M, Lee Y H, Unger R H. J Clin Invest. 1997;100:290–295. doi: 10.1172/JCI119534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shimabukuro M, Koyama K, Lee Y H, Unger R H. J Clin Invest. 1997;100:1750–1754. doi: 10.1172/JCI119700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Phillips M S, Liu Q, Hammond H, Dugan V, Hey P, Caskey C T, Hess J F. Nat Genet. 1996;13:18–19. doi: 10.1038/ng0596-18. [DOI] [PubMed] [Google Scholar]
- 13.Iida M, Murakami T, Ishida K, Mizuno A, Kuwajima M, Shima K. Biochem Biophys Res Commun. 1996;224:597–604. doi: 10.1006/bbrc.1996.1070. [DOI] [PubMed] [Google Scholar]
- 14.Shimabukuro M, Koyama K, Chen G, Wang M-Y, Trieu F, Lee Y H, Newgard C B, Unger R H. Proc Natl Acad Sci USA. 1997;94:4637–4641. doi: 10.1073/pnas.94.9.4637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang M-Y, Zhou Y-T, Newgard C B, Unger R H. FEBS Lett. 1996;392:87–90. doi: 10.1016/0014-5793(96)00790-9. [DOI] [PubMed] [Google Scholar]
- 16.Becker T C, Noel R J, Baque S, Coats W S, Gomez-Foix A M, Alam T, Gerard R D, Newgard C B. Methods Cell Biol. 1994;43:161–189. doi: 10.1016/s0091-679x(08)60603-2. [DOI] [PubMed] [Google Scholar]
- 17.Gomez-Foix, Coats A M, Baque W S, Alam T, Gerard R D, Newgard C B. J Biol Chem. 1992;267:25129–25134. [PubMed] [Google Scholar]
- 18.Zhou Y T, Shimabukuro M, Koyama K, Lee Y, Wang M-Y, Trieu F, Newgard C B, Unger R H. Proc Natl Acad Sci USA. 1997;94:6386–6390. doi: 10.1073/pnas.94.12.6386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koyama K, Chen G, Wang M-Y, Lee Y H, Shimabukuro M, Newgard C B, Unger R H. Diabetes. 1997;46:1276–1280. doi: 10.2337/diab.46.8.1276. [DOI] [PubMed] [Google Scholar]
- 20.Green L C, Wagner D A, Glogowski J, Skipper P L, Wishnok J, Tannenbaum S R. Anal Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 21.Emilsson V, Liu Y-L, Cawthorne M A, Morton N M, Davenport M. Diabetes. 1997;46:313–316. doi: 10.2337/diab.46.2.313. [DOI] [PubMed] [Google Scholar]
- 22.Baumann H, Morella K K, White D W, Dembski M, Bailon P S, Kim H, Lai C-F, Tartaglia L A. Proc Natl Acad Sci USA. 1996;93:8374–8378. doi: 10.1073/pnas.93.16.8374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Unger R H. Trends Endocrinol Metab. 1997;6:276–282. doi: 10.1016/s1043-2760(97)00094-5. [DOI] [PubMed] [Google Scholar]