Abstract
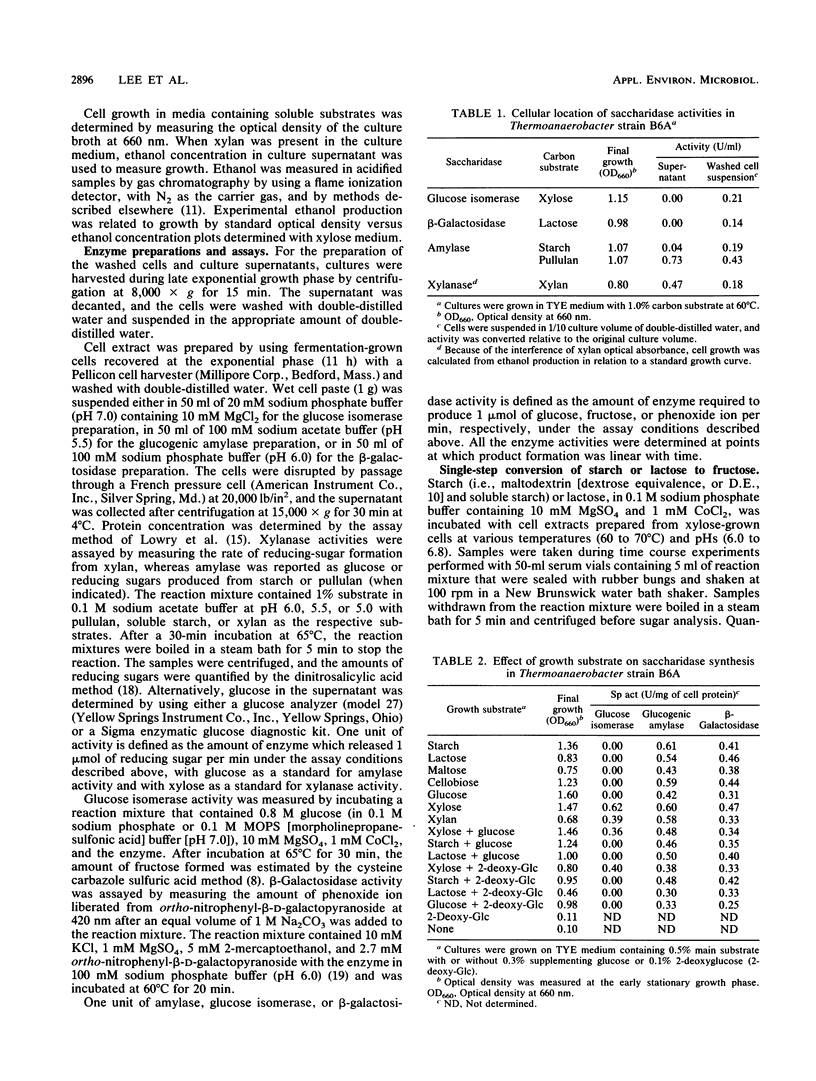
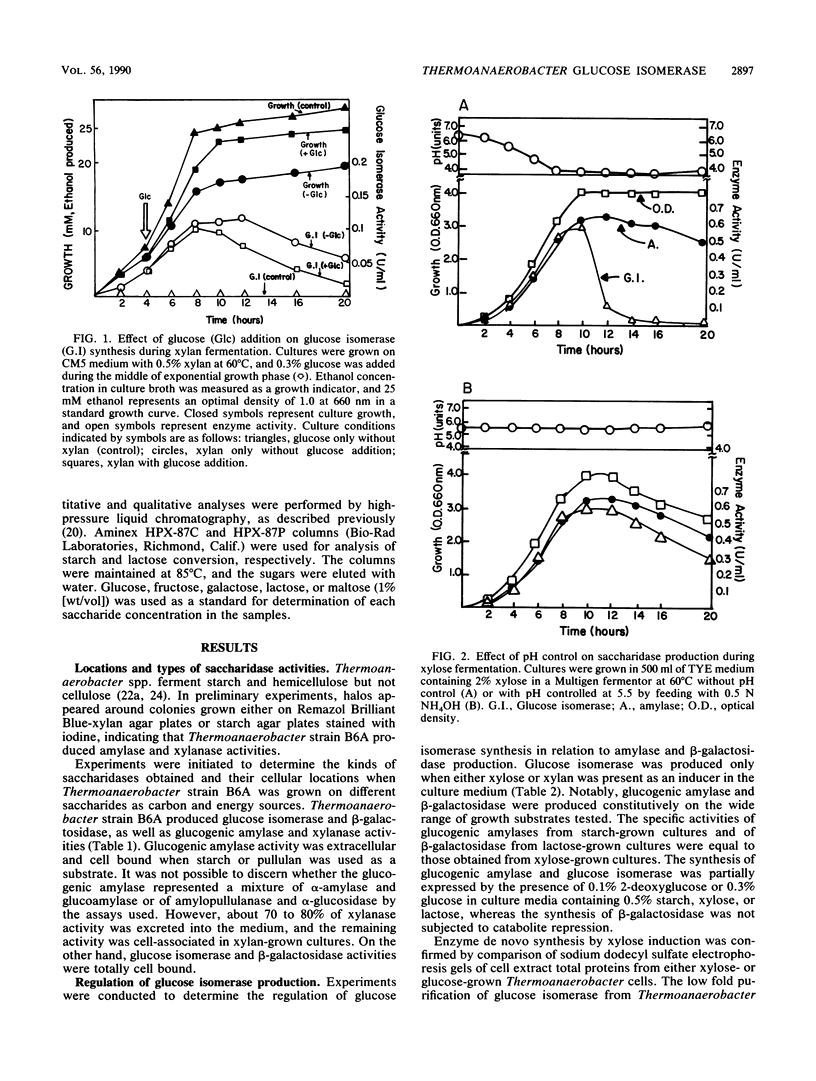
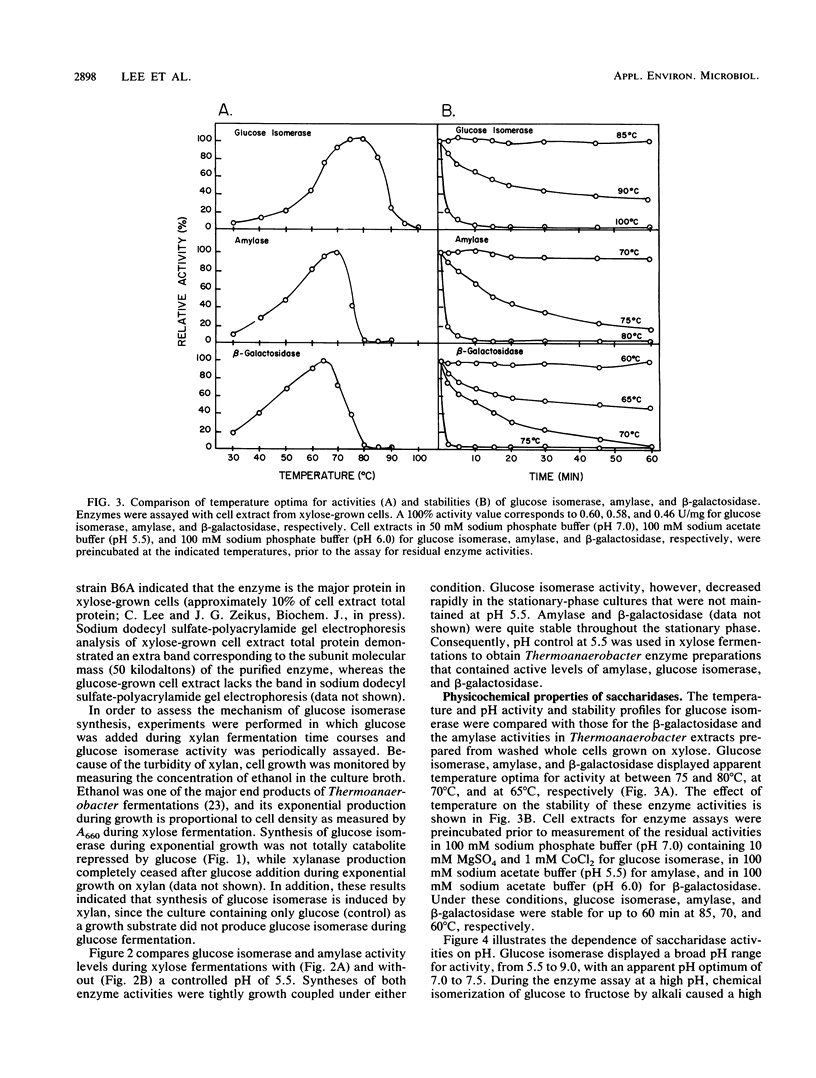
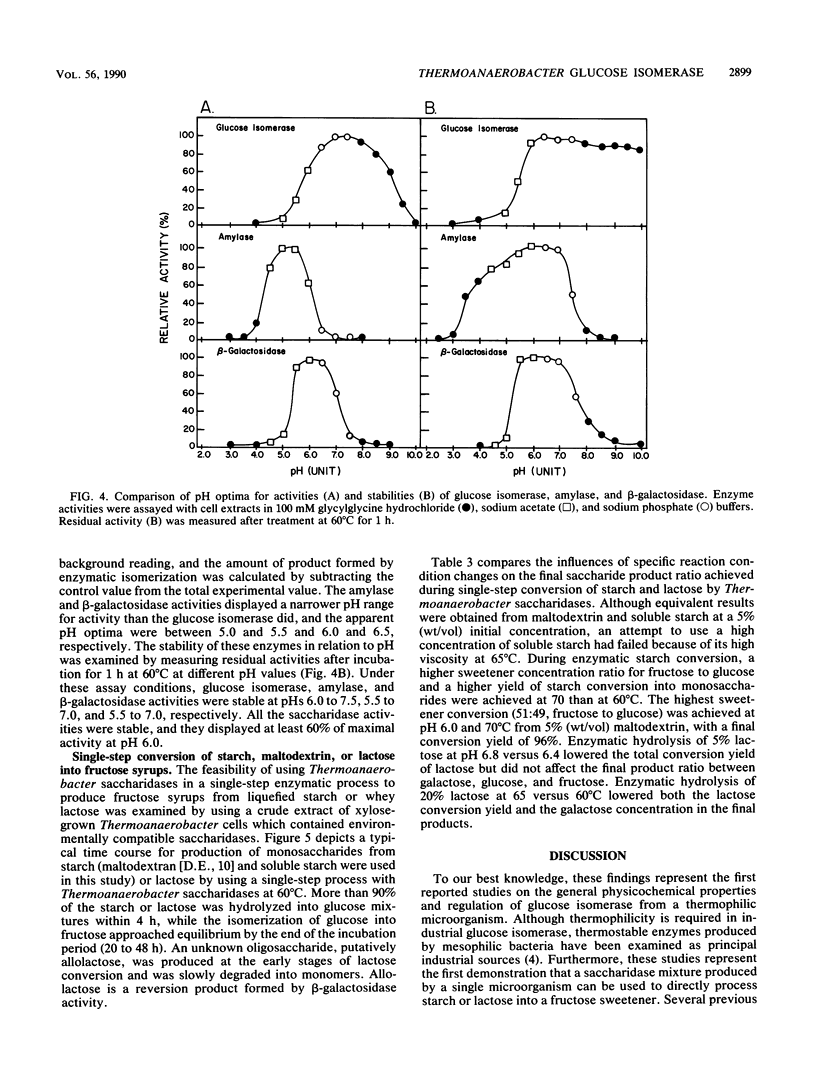
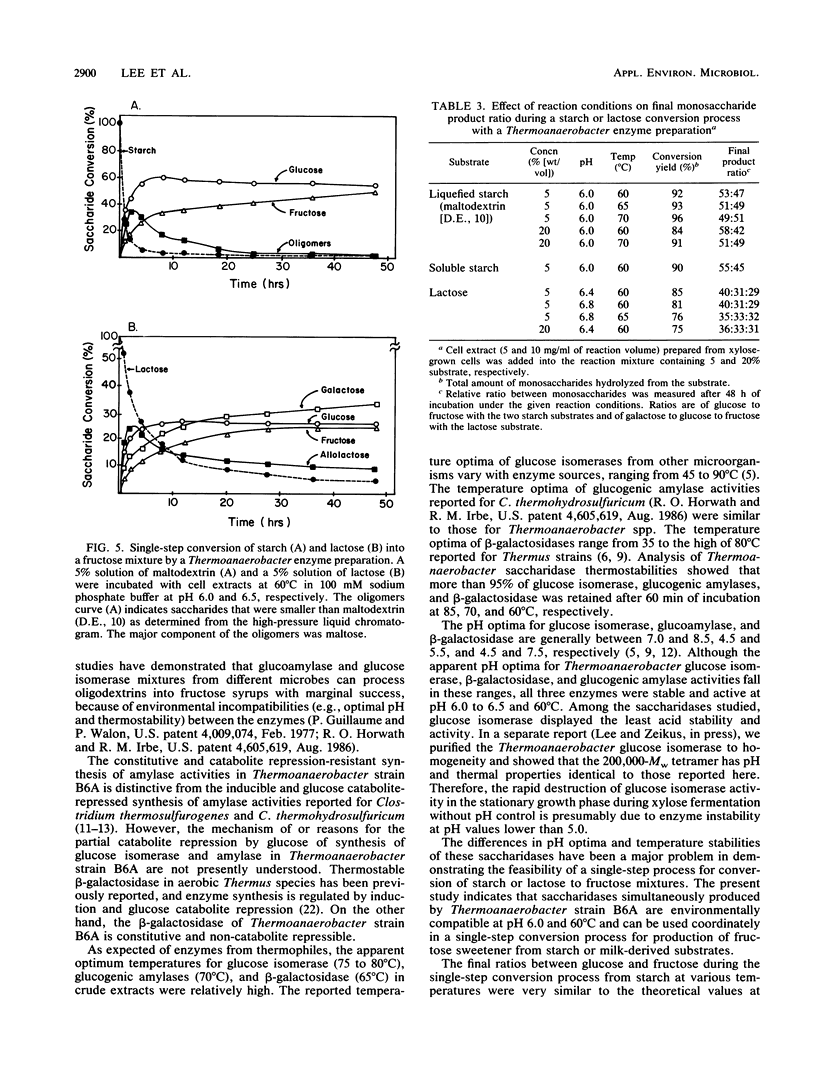
Regulation of glucose isomerase synthesis was studied in Thermoanaerobacter strain B6A, which fermented a wide variety of carbohydrates including glucose, xylose, lactose, starch, and xylan. Glucogenic amylase activities and β-galactosidase were produced constitutively, whereas the synthesis of glucose isomerase was induced by either xylose or xylan. Production of these saccharidase activities was not significantly repressed by the presence of glucose or 2-deoxyglucose in the growth media. Glucose isomerase production was optimized by controlling the culture pH at 5.5 during xylose fermentation. The apparent temperature and pH optima for these cell-bound saccharidase activities were as follows: glucose isomerase, 80°C, pH 7.0 to 7.5; glucogenic amylase, 70°C, pH 5.0 to 5.5; and β-galactosidase, 60°C, pH 6.0 to 6.5 Glucose isomerase, glucogenic amylase, and β-galactosidase were produced in xylose-grown cells that were active and stable at 60 to 70°C and pH 6.0 to 6.5. Under single-step process conditions, these saccharidase activities in whole cells or cell extracts converted starch or lactose directly into fructose mixtures. A total of 96% of initial liquefied starch was converted into a 49:51 mixture of glucose and fructose, whereas 85% of initial lactose was converted into a 40:31:29 mixture of galactose, glucose, and fructose.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DISCHE Z., BORENFREUND E. A new spectrophotometric method for the detection and determination of keto sugars and trioses. J Biol Chem. 1951 Oct;192(2):583–587. [PubMed] [Google Scholar]
- Goodman R. E., Pederson D. M. beta-Galactosidase from Bacillus stearothermophilus. Can J Microbiol. 1976 Jun;22(6):817–825. doi: 10.1139/m76-118. [DOI] [PubMed] [Google Scholar]
- Hyun H. H., Shen G. J., Zeikus J. G. Differential amylosaccharide metabolism of Clostridium thermosulfurogenes and Clostridium thermohydrosulfuricum. J Bacteriol. 1985 Dec;164(3):1153–1161. doi: 10.1128/jb.164.3.1153-1161.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyun H. H., Zeikus J. G. General Biochemical Characterization of Thermostable Pullulanase and Glucoamylase from Clostridium thermohydrosulfuricum. Appl Environ Microbiol. 1985 May;49(5):1168–1173. doi: 10.1128/aem.49.5.1168-1173.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyun H. H., Zeikus J. G. Regulation and genetic enhancement of glucoamylase and pullulanase production in Clostridium thermohydrosulfuricum. J Bacteriol. 1985 Dec;164(3):1146–1152. doi: 10.1128/jb.164.3.1146-1152.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Matsumoto K., Kimura H., Noguchi Y., Uzuka Y., Honda I. [Experimental studies on minocycline intravenous infusion (author's transl)]. Jpn J Antibiot. 1974 Jun;27(3):302–306. [PubMed] [Google Scholar]
- Melasniemi H. Characterization of alpha-amylase and pullulanase activities of Clostridium thermohydrosulfuricum. Evidence for a novel thermostable amylase. Biochem J. 1987 Aug 15;246(1):193–197. doi: 10.1042/bj2460193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha B. C., Lamed R., Lee C. Y., Mathupala S. P., Zeikus J. G. Characterization of an endo-Acting Amylopullulanase from Thermoanaerobacter Strain B6A. Appl Environ Microbiol. 1990 Apr;56(4):881–886. doi: 10.1128/aem.56.4.881-886.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez S., Quinto C. M. D-Glucose isomerase: constitutive and catabolite repression-resistant mutants of Streptomyces phaeochromogenes. Appl Microbiol. 1975 Nov;30(5):750–754. doi: 10.1128/am.30.5.750-754.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich J. T., McFeters G. A., Temple K. L. Induction and characterization of -galactosidase in an extreme thermophile. J Bacteriol. 1972 May;110(2):691–698. doi: 10.1128/jb.110.2.691-698.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weimer P. J., Wagner L. W., Knowlton S., Ng T. K. Thermophilic anaerobic bacteria which ferment hemicellulose: characterization of organisms and identification of plasmids. Arch Microbiol. 1984 May;138(1):31–36. doi: 10.1007/BF00425403. [DOI] [PubMed] [Google Scholar]
- Zeikus J. G., Ben-Bassat A., Hegge P. W. Microbiology of methanogenesis in thermal, volcanic environments. J Bacteriol. 1980 Jul;143(1):432–440. doi: 10.1128/jb.143.1.432-440.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]


