Abstract
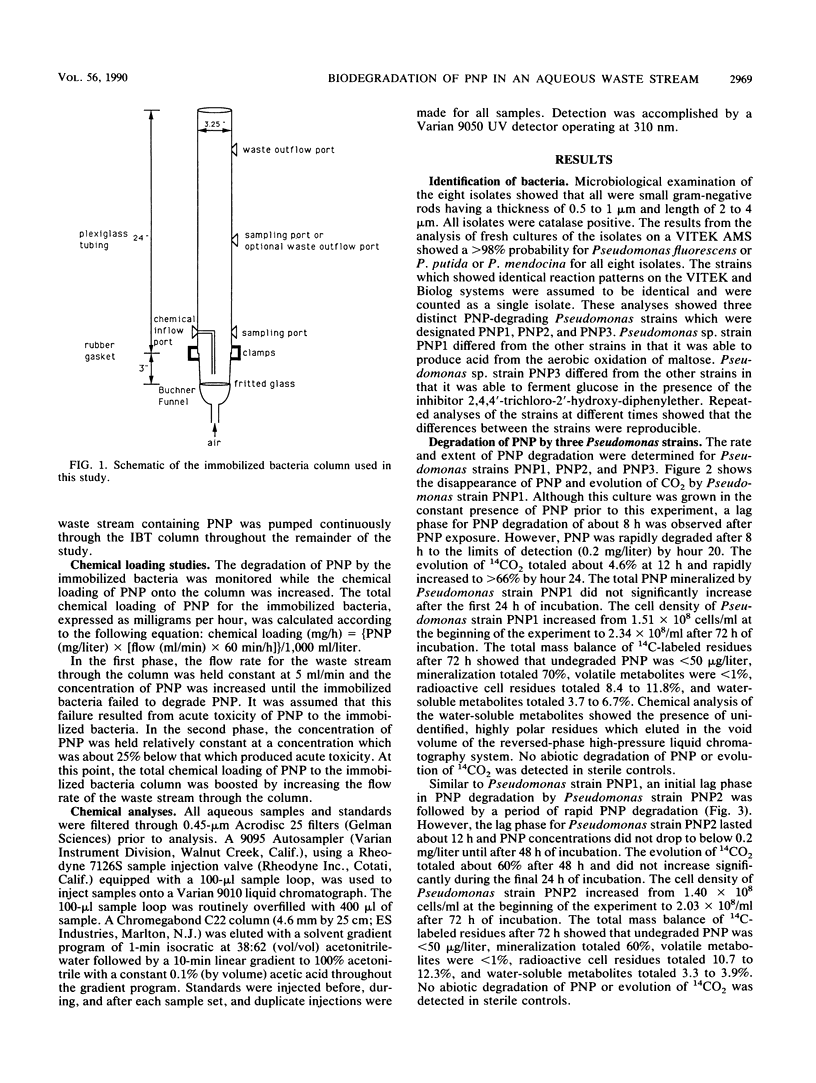
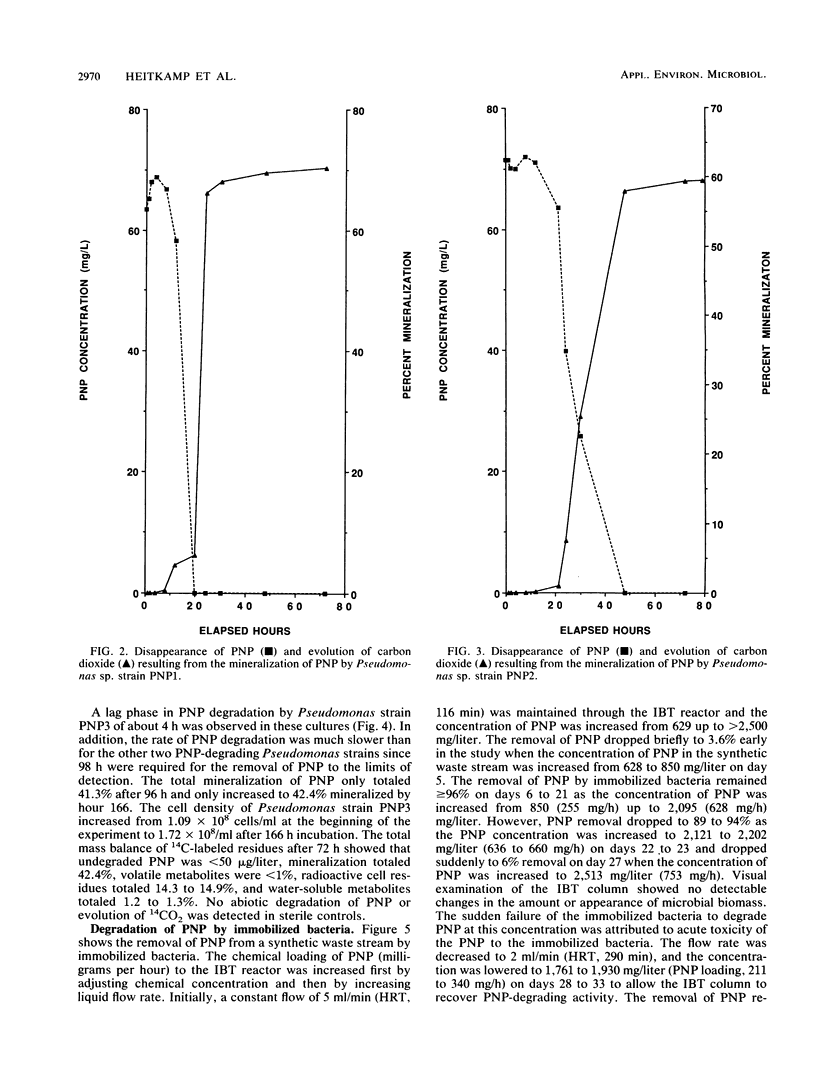
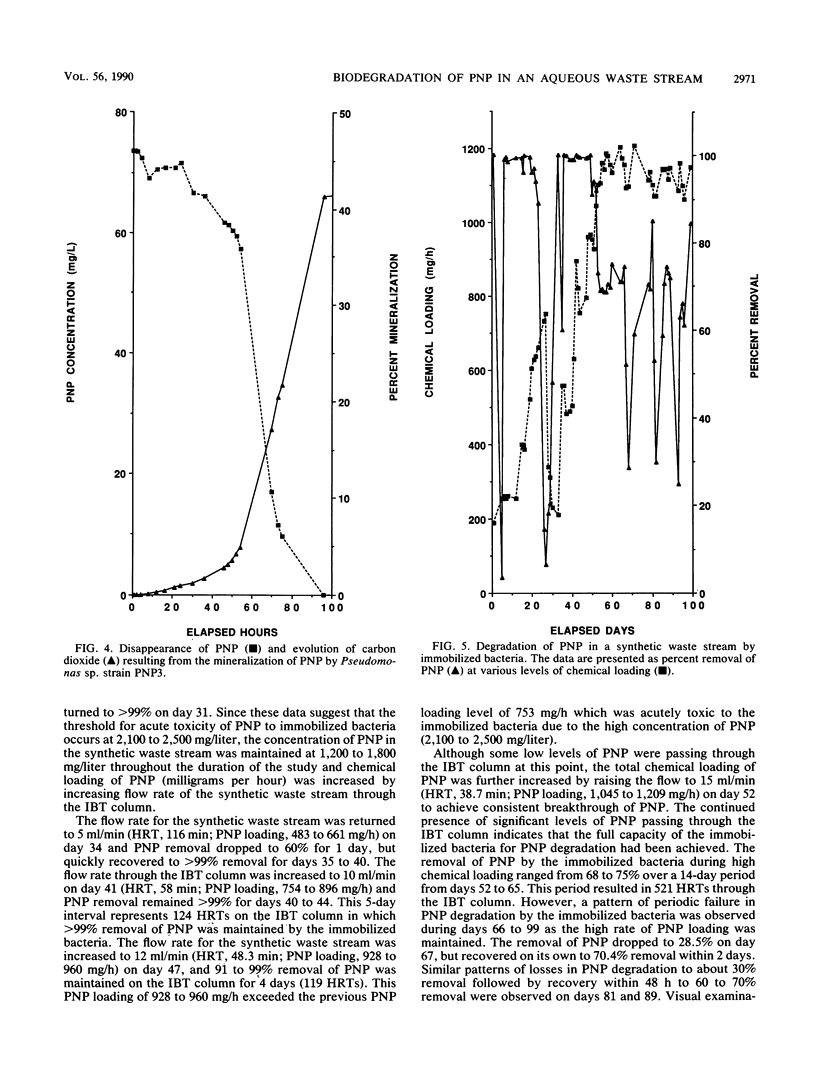
Microbiological analyses of activated sludge reactors after repeated exposure to 100 mg of p-nitrophenol (PNP) per liter resulted in the isolation of three Pseudomonas species able to utilize PNP as a sole source of carbon and energy. Cell suspensions of the three Pseudomonas sp., designated PNP1, PNP2, and PNP3, mineralized 70, 60, and 45% of a 70-mg/liter dose of PNP in 24, 48, and 96 h, respectively. Mass-balance analyses of PNP residues for all three cultures showed that undegraded PNP was less than 1% (less than 50 micrograms); volatile metabolites, less than 1%; cell residues, 8.4 to 14.9%; and water-soluble metabolites, 1.2 to 6.7%. A mixed culture of all three PNP-degrading Pseudomonas sp. was immobilized by adsorption onto diatomaceous earth biocarrier in a 1.75-liter Plexiglas column. The column was aerated and exposed to a synthetic waste stream containing 629 to 2,513 mg of PNP per liter at flow rates of 2 to 15 ml/min. Chemical loading studies showed that the threshold concentration for acute toxicity of PNP to the immobilized bacteria was 2,100 to 2,500 mg/liter. Further studies at PNP concentrations of 1,200 to 1,800 mg/liter showed that greater than 99 and 91 to 99% removal of PNP was achieved by immobilized bacteria at flow rates of 10 and 12 ml/min, respectively. These values represent hydraulic retention times of 48 to 58 min and PNP removal rates of 0.99 to 1.1 mg/h per g of biocarrier at 25 degrees C under optimal conditions. This study shows the successful use of immobilized bacteria technology to remove high concentrations of PNP from aqueous waste streams.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aelion C. M., Swindoll C. M., Pfaender F. K. Adaptation to and biodegradation of xenobiotic compounds by microbial communities from a pristine aquifer. Appl Environ Microbiol. 1987 Sep;53(9):2212–2217. doi: 10.1128/aem.53.9.2212-2217.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd S. A., Shelton D. R., Berry D., Tiedje J. M. Anaerobic biodegradation of phenolic compounds in digested sludge. Appl Environ Microbiol. 1983 Jul;46(1):50–54. doi: 10.1128/aem.46.1.50-54.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S. H., Alexander M. Kinetics of mineralization of phenols in lake water. Appl Environ Microbiol. 1986 May;51(5):891–897. doi: 10.1128/aem.51.5.891-897.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEADBETTER E. R., FOSTER J. W. Studies on some methane-utilizing bacteria. Arch Mikrobiol. 1958;30(1):91–118. doi: 10.1007/BF00509229. [DOI] [PubMed] [Google Scholar]
- Paris D. F., Wolfe N. L., Steen W. C. Structure-activity relationships in microbial transformation of phenols. Appl Environ Microbiol. 1982 Jul;44(1):153–158. doi: 10.1128/aem.44.1.153-158.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scow K. M., Simkins S., Alexander M. Kinetics of mineralization of organic compounds at low concentrations in soil. Appl Environ Microbiol. 1986 May;51(5):1028–1035. doi: 10.1128/aem.51.5.1028-1035.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spain J. C., Pritchard P. H., Bourquin A. W. Effects of adaptation on biodegradation rates in sediment/water cores from estuarine and freshwater environments. Appl Environ Microbiol. 1980 Oct;40(4):726–734. doi: 10.1128/aem.40.4.726-734.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spain J. C., Van Veld P. A. Adaptation of natural microbial communities to degradation of xenobiotic compounds: effects of concentration, exposure time, inoculum, and chemical structure. Appl Environ Microbiol. 1983 Feb;45(2):428–435. doi: 10.1128/aem.45.2.428-435.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spain J. C., Van Veld P. A., Monti C. A., Pritchard P. H., Cripe C. R. Comparison of p-Nitrophenol Biodegradation in Field and Laboratory Test Systems. Appl Environ Microbiol. 1984 Nov;48(5):944–950. doi: 10.1128/aem.48.5.944-950.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swindoll C. M., Aelion C. M., Pfaender F. K. Influence of inorganic and organic nutrients on aerobic biodegradation and on the adaptation response of subsurface microbial communities. Appl Environ Microbiol. 1988 Jan;54(1):212–217. doi: 10.1128/aem.54.1.212-217.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggins B. A., Alexander M. Role of chemical concentration and second carbon sources in acclimation of microbial communities for biodegradation. Appl Environ Microbiol. 1988 Nov;54(11):2803–2807. doi: 10.1128/aem.54.11.2803-2807.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]



