Abstract
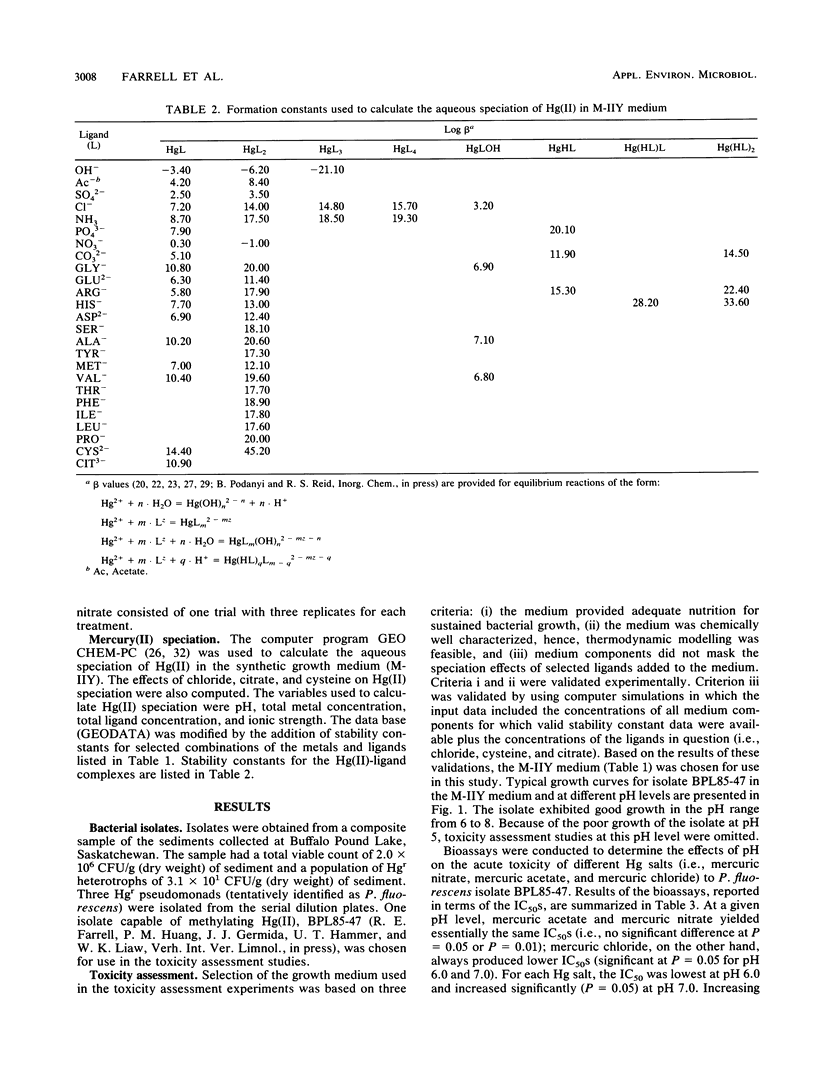
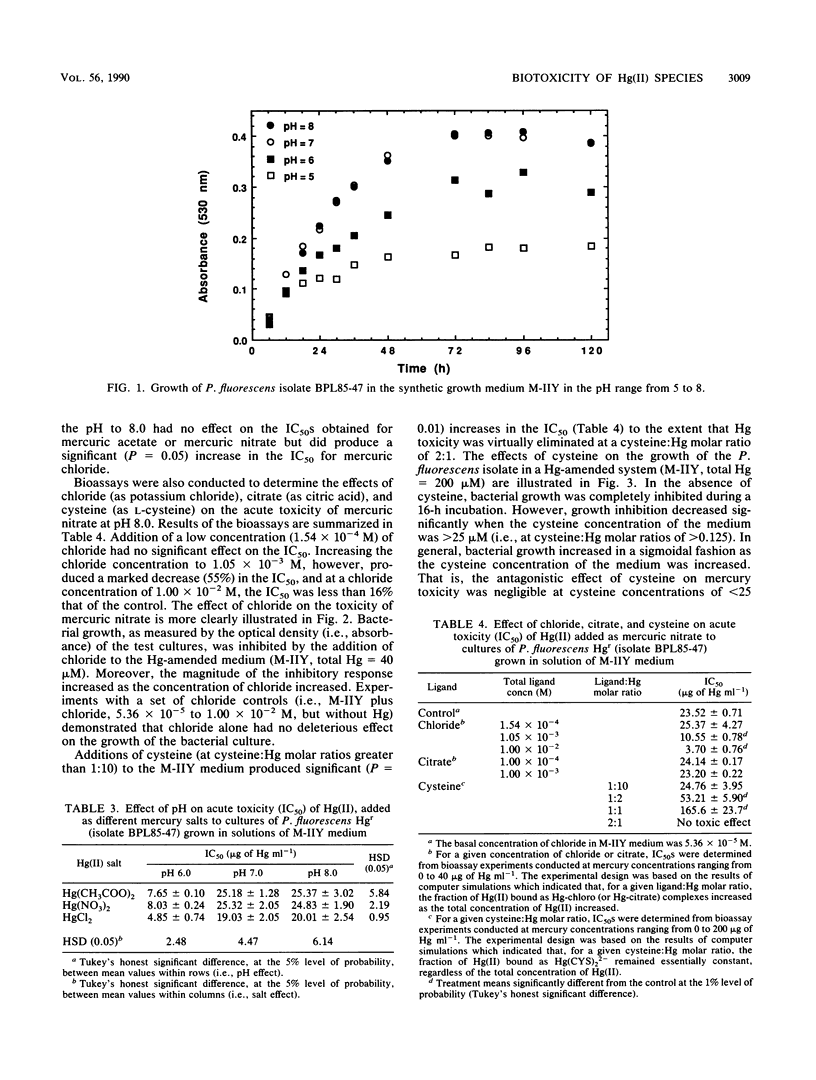
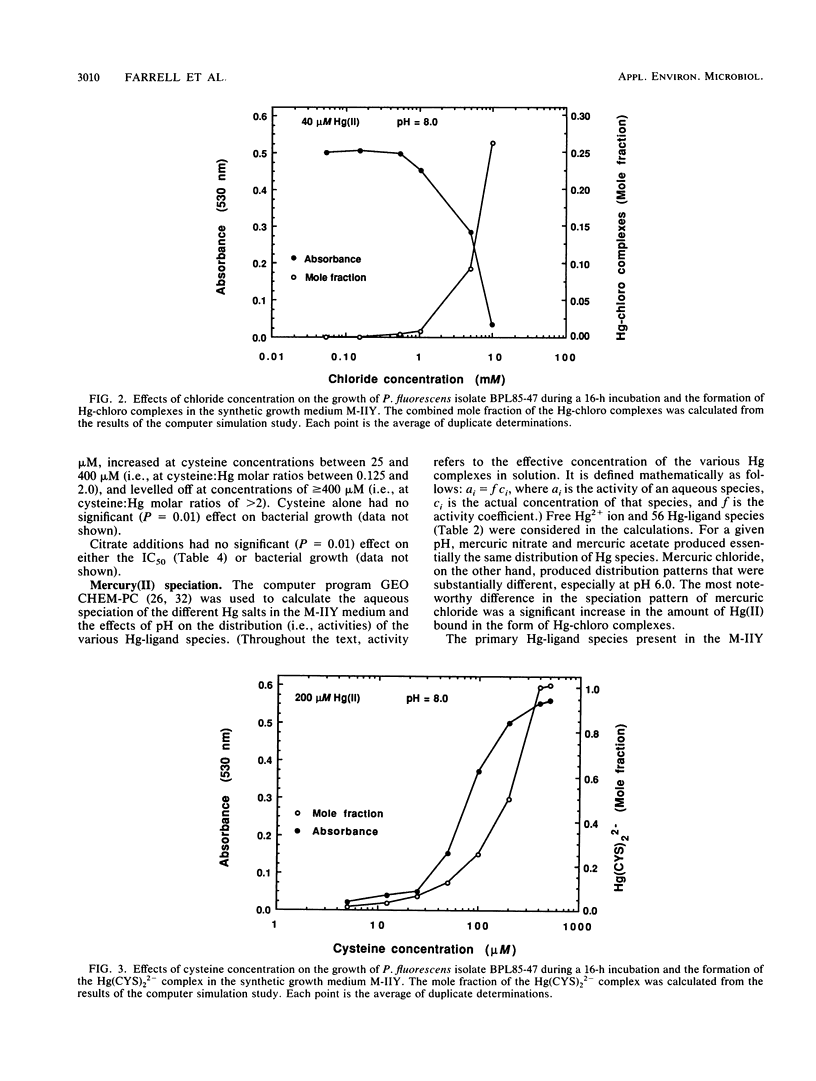
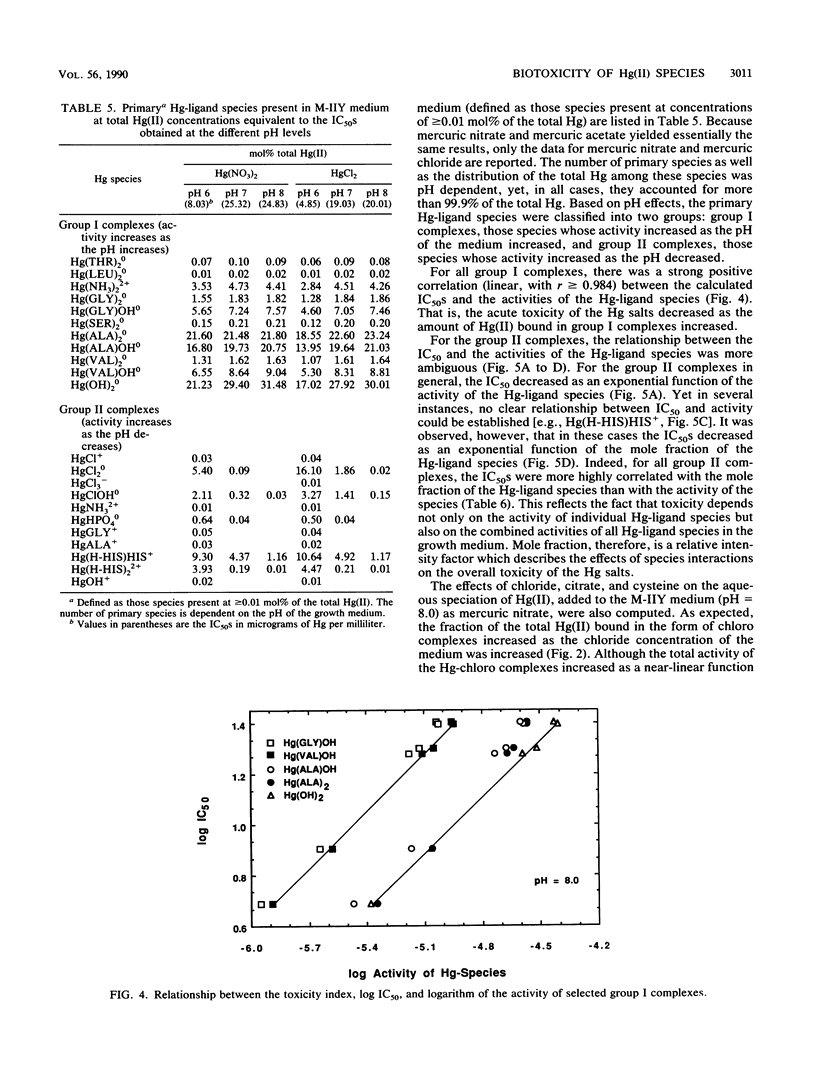
Integration of physicochemical procedures for studying mercury(II) speciation with microbiological procedures for studying the effects of mercury on bacterial growth allows evaluation of ionic factors (e.g., pH and ligand species and concentration) which affect biotoxicity. A Pseudomonas fluorescens strain capable of methylating inorganic Hg(II) was isolated from sediment samples collected at Buffalo Pound Lake in Saskatchewan, Canada. The effect of pH and ligand species on the toxic response (i.e., 50% inhibitory concentration [IC50]) of the P. fluorescens isolated to mercury were determined and related to the aqueous speciation of Hg(II). It was determined that the toxicities of different mercury salts were influenced by the nature of the co-ion. At a given pH level, mercuric acetate and mercuric nitrate yielded essentially the same IC50s; mercuric chloride, on the other hand, always produced lower IC50s. For each Hg salt, toxicity was greatest at pH 6.0 and decreased significantly (P = 0.05) at pH 7.0. Increasing the pH to 8.0 had no effect on the toxicity of mercuric acetate or mercuric nitrate but significantly (P = 0.05) reduced the toxicity of mercuric chloride. The aqueous speciation of Hg(II) in the synthetic growth medium M-IIY (a minimal salts medium amended to contain 0.1% yeast extract and 0.1% glycerol) was calculated by using the computer program GEOCHEM-PC with a modified data base. Results of the speciation calculations indicated that complexes of Hg(II) with histidine [Hg(H-HIS)HIS+ and Hg(H-HIS)2(2+)], chloride (HgCl+, HgCl2(0), HgClOH0, and HgCl3-), phosphate (HgHPO4(0), ammonia (HgNH3(2+), glycine [Hg(GLY)+], alanine [Hg(ALA)+], and hydroxyl ion (HgOH+) were the Hg species primarily responsible for toxicity in the M-IIY medium. The toxicity of mercuric nitrate at pH 8.0 was unaffected by the addition of citrate, enhanced by the addition of chloride, and reduced by the addition of cysteine. In the chloride-amended system, HgCl+, HgCl2(0), and HgClOH0 were the species primarily responsible for observed increases in toxicity. In the cysteine-amended system, formation of Hg(CYS)2(2-) was responsible for detoxification effects that were observed. The formation of Hg-citrate complexes was insignificant and had no effect on Hg toxicity.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babich H., Stotzky G. Differential toxicities of mercury to bacteria and bacteriophages in sea and in lake water. Can J Microbiol. 1979 Nov;25(11):1252–1257. doi: 10.1139/m79-197. [DOI] [PubMed] [Google Scholar]
- Babich H., Stotzky G. Environmental factors that influence the toxicity of heavy metal and gaseous pollutants to microorganisms. Crit Rev Microbiol. 1980;8(2):99–145. doi: 10.3109/10408418009081123. [DOI] [PubMed] [Google Scholar]
- Babich H., Stotzky G. Toxicity of zinc to fungi, bacteria, and coliphages: influence of chloride ions. Appl Environ Microbiol. 1978 Dec;36(6):906–914. doi: 10.1128/aem.36.6.906-914.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAN E. C. MORPHOLOGICAL ABERRATION OF ARTHROBACTER GLOBIFORMIS CELLS DUE TO BIOTIN DEFICIENCY. J Bacteriol. 1964 Mar;87:641–651. doi: 10.1128/jb.87.3.641-651.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furutani A., Rudd J. W. Measurement of mercury methylation in lake water and sediment samples. Appl Environ Microbiol. 1980 Oct;40(4):770–776. doi: 10.1128/aem.40.4.770-776.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuzzi R. Toxicity of mercury to phytoplankton. Nature. 1972 May 5;237(5349):38–40. doi: 10.1038/237038a0. [DOI] [PubMed] [Google Scholar]
- Rai L. C., Gaur J. P., Kumar H. D. Protective effects of certain environmental factors on the toxicity of zinc, mercury, and methylmercury to Chlorella vulgaris. Environ Res. 1981 Aug;25(2):250–259. doi: 10.1016/0013-9351(81)90026-8. [DOI] [PubMed] [Google Scholar]
- Reid R. S., Podányi B. A proton NMR study of the glycine-mercury(II) system in aqueous solution. J Inorg Biochem. 1988 Mar;32(3):183–195. doi: 10.1016/0162-0134(88)80026-6. [DOI] [PubMed] [Google Scholar]
- Westö G. Methylmercury as percentage of total mercury in flesh and viscera of salmon and sea trout of various ages. Science. 1973 Aug 10;181(4099):567–568. doi: 10.1126/science.181.4099.567. [DOI] [PubMed] [Google Scholar]



