Abstract
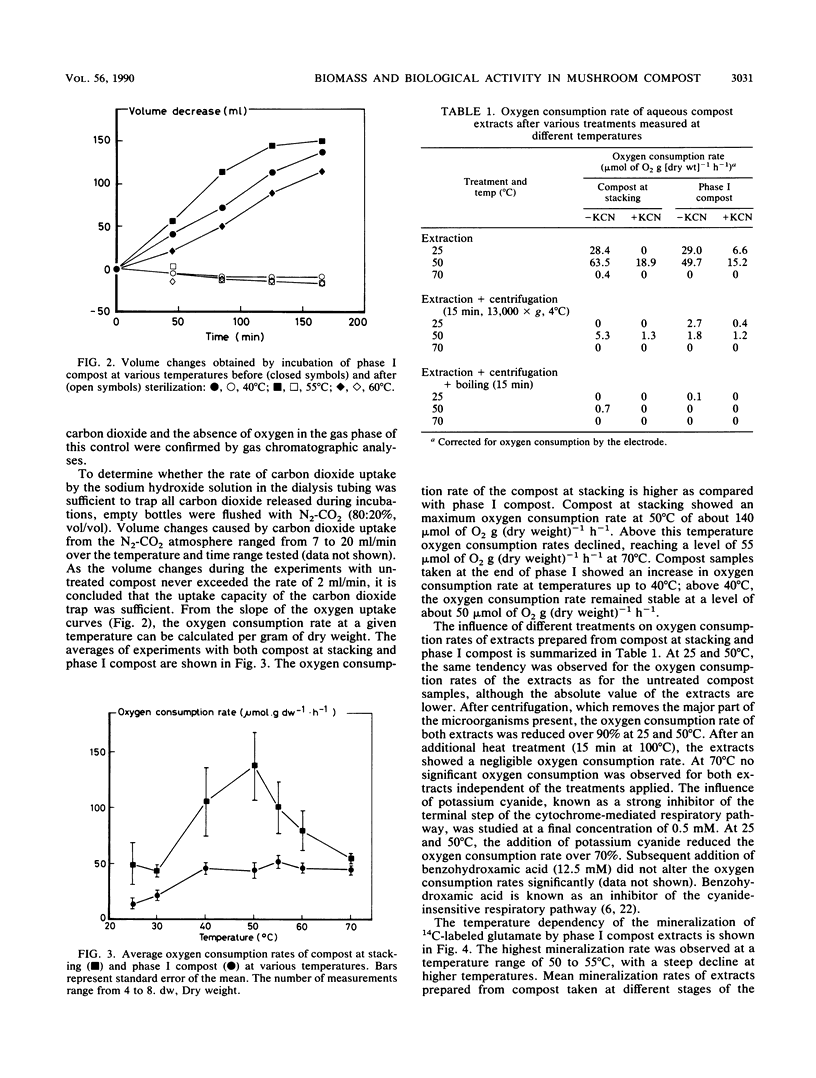
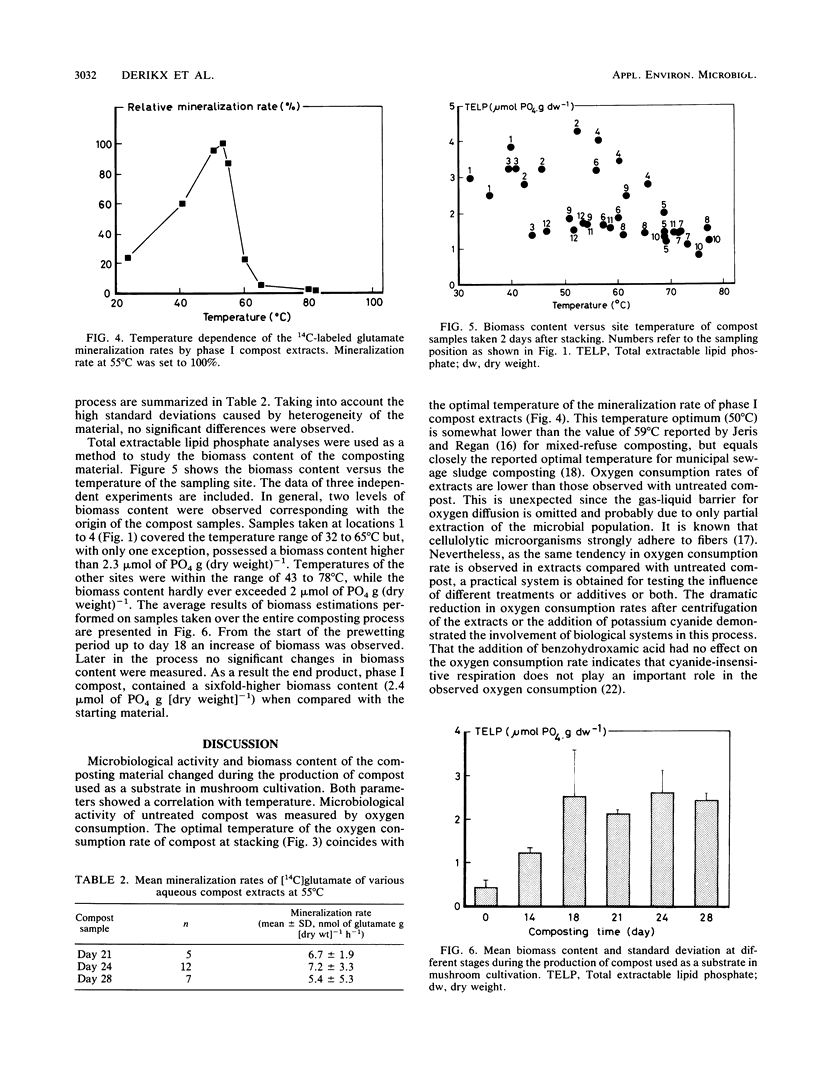
The production of a suitable substrate for the cultivation of the common white button mushroom, Agaricus bisporus, is referred to as composting. High microbiological activity causes temperatures of the composting material to rise as high as 80°C. At stacking, an optimal oxygen consumption rate of 140 μmol of O2 h−1 g (dry weight)−1 was found in the compost at 50°C, whereas the oxygen consumption rate of the end product was lower at all temperatures tested. No significant differences were observed between biomass content and mineralization rate of 14C-labeled glutamate of the two composts. Biomass content was shown to be a major function of both temperature and the sampling site position in the stack. On the basis of the results reported here, a minimal composting time of 3.3 days for the phase I process was calculated. Further suggestions are made to reduce the time necessary for the production of a substrate for A. bisporus considerably.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Barlaz M. A., Schaefer D. M., Ham R. K. Bacterial population development and chemical characteristics of refuse decomposition in a simulated sanitary landfill. Appl Environ Microbiol. 1989 Jan;55(1):55–65. doi: 10.1128/aem.55.1.55-65.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finstein M. S., Morris M. L. Microbiology of municipal solid waste composting. Adv Appl Microbiol. 1975;19:113–151. doi: 10.1016/s0065-2164(08)70427-1. [DOI] [PubMed] [Google Scholar]
- Hutten T. J., De Jong M. H., Peeters B. P., van der Drift C., Vogels G. D. Coenzyme M derivatives and their effects on methane formation from carbon dioxide and methanol by cell extracts of Methanosarcina barkeri. J Bacteriol. 1981 Jan;145(1):27–34. doi: 10.1128/jb.145.1.27-34.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinley V. L., Vestal J. R. Biokinetic analyses of adaptation and succession: microbial activity in composting municipal sewage sludge. Appl Environ Microbiol. 1984 May;47(5):933–941. doi: 10.1128/aem.47.5.933-941.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHULZE K. L. Continuous thermophilic composting. Appl Microbiol. 1962 Mar;10:108–122. doi: 10.1128/am.10.2.108-122.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonbaum G. R., Bonner W. D., Jr, Storey B. T., Bahr J. T. Specific inhibition of the cyanide-insensitive respiratory pathway in plant mitochondria by hydroxamic acids. Plant Physiol. 1971 Jan;47(1):124–128. doi: 10.1104/pp.47.1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]


