Abstract
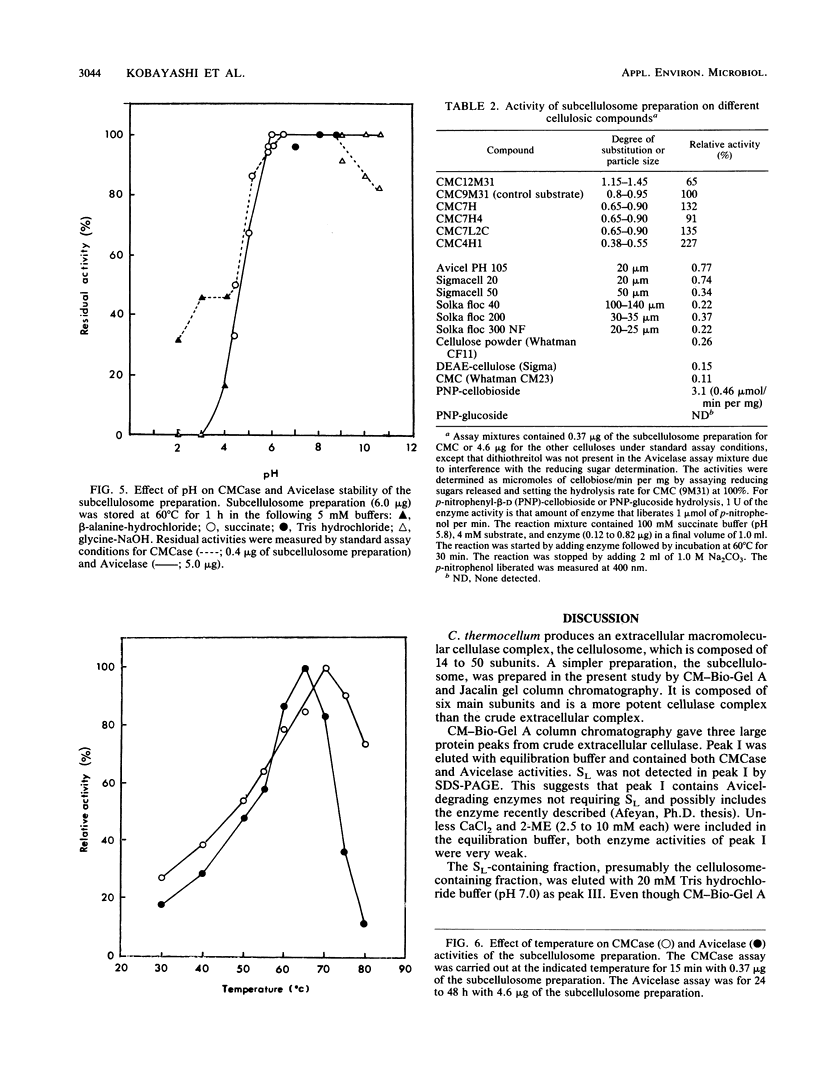
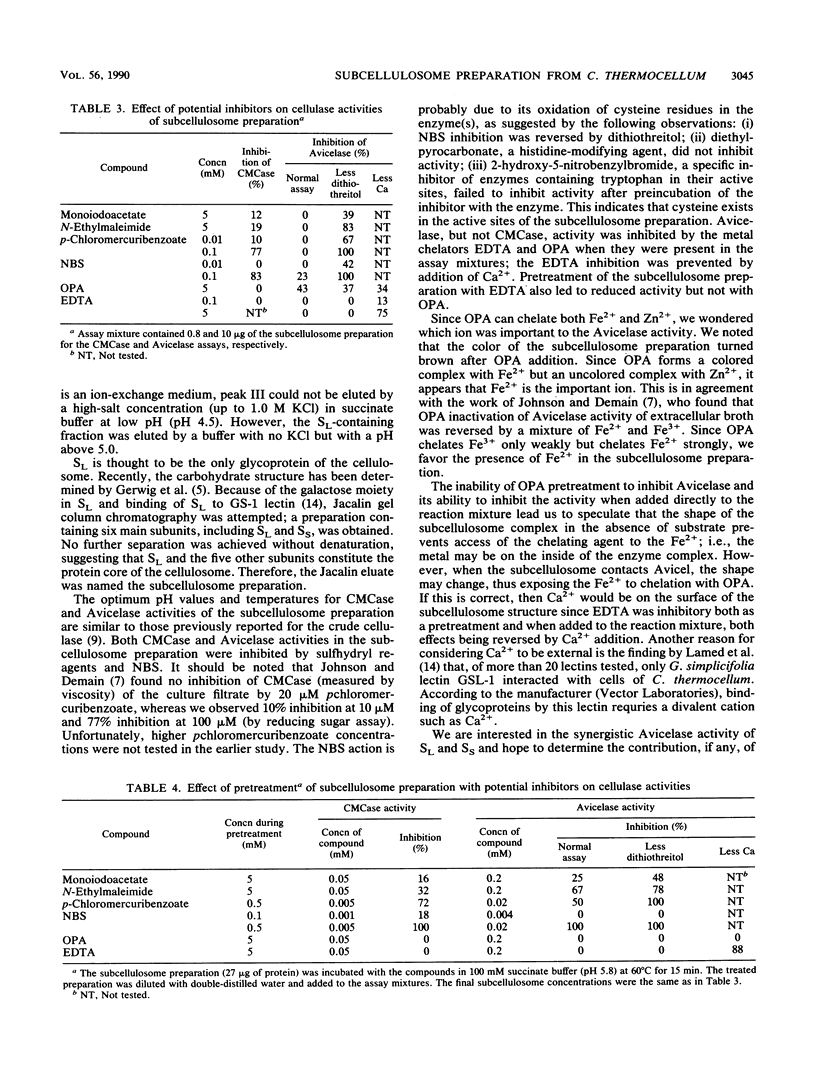
We have prepared a much simpler cellulase preparation than that of cellulosomes from the extracellular broth of Clostridium thermocellum. This "subcellulosome" preparation from C. thermocellum was obtained by column chromatography on CM-Bio-Gel A and then on a lectin-affinity material (Jacalin). The subcellulosome preparation is a macromolecular complex, composed of six main protein subunits (molecular weight, 210,000 to 58,000) revealed on sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The specific activities of carboxymethylcellulase (CMCase) and Avicelase are 15- and 8-fold-higher, respectively, than those of crude extracellular cellulase. We could not further fractionate this preparation without denaturing it. The optimum pH and temperature of the subcellulosome preparation are 5.5 to 7.0 and 70 degrees C for CMCase and 5.5 to 7.0 and 65 degrees C for Avicelase. The subcellulosome preparation acted on various types of carboxymethyl cellulose, cellulose, and p-nitrophenyl-beta-D-cellobioside but not on p-nitrophenyl-beta-D-glucoside. Sulfhydryl reagents and N-bromosuccinimide inhibited both CMCase and Avicelase activities, whereas EDTA and o-phenanthroline inhibited Avicelase activity only.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Coughlan M. P., Hon-Nami K., Hon-Nami H., Ljungdahl L. G., Paulin J. J., Rigsby W. E. The cellulolytic enzyme complex of Clostridium thermocellum is very large. Biochem Biophys Res Commun. 1985 Jul 31;130(2):904–909. doi: 10.1016/0006-291x(85)90502-9. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Gerwig G. J., de Waard P., Kamerling J. P., Vliegenthart J. F., Morgenstern E., Lamed R., Bayer E. A. Novel O-linked carbohydrate chains in the cellulase complex (cellulosome) of Clostridium thermocellum. 3-O-Methyl-N-acetylglucosamine as a constituent of a glycoprotein. J Biol Chem. 1989 Jan 15;264(2):1027–1035. [PubMed] [Google Scholar]
- Johnson E. A., Sakajoh M., Halliwell G., Madia A., Demain A. L. Saccharification of Complex Cellulosic Substrates by the Cellulase System from Clostridium thermocellum. Appl Environ Microbiol. 1982 May;43(5):1125–1132. doi: 10.1128/aem.43.5.1125-1132.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadam S. K., Demain A. L. Addition of cloned beta-glucosidase enhances the degradation of crystalline cellulose by the Clostridium thermocellum cellulose complex. Biochem Biophys Res Commun. 1989 Jun 15;161(2):706–711. doi: 10.1016/0006-291x(89)92657-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamed R., Naimark J., Morgenstern E., Bayer E. A. Specialized cell surface structures in cellulolytic bacteria. J Bacteriol. 1987 Aug;169(8):3792–3800. doi: 10.1128/jb.169.8.3792-3800.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamed R., Setter E., Bayer E. A. Characterization of a cellulose-binding, cellulase-containing complex in Clostridium thermocellum. J Bacteriol. 1983 Nov;156(2):828–836. doi: 10.1128/jb.156.2.828-836.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer F., Coughlan M. P., Mori Y., Ljungdahl L. G. Macromolecular Organization of the Cellulolytic Enzyme Complex of Clostridium thermocellum as Revealed by Electron Microscopy. Appl Environ Microbiol. 1987 Dec;53(12):2785–2792. doi: 10.1128/aem.53.12.2785-2792.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REESE E. T., SIU R. G. H., LEVINSON H. S. The biological degradation of soluble cellulose derivatives and its relationship to the mechanism of cellulose hydrolysis. J Bacteriol. 1950 Apr;59(4):485–497. doi: 10.1128/jb.59.4.485-497.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacco M., Millet J., Aubert J. P. Cloning and expression in Saccharomyces cerevisiae of a cellulase gene from Clostridium thermocellum. Ann Microbiol (Paris) 1984 May-Jun;135A(3):485–488. doi: 10.1016/s0769-2609(84)80088-5. [DOI] [PubMed] [Google Scholar]
- Schwarz W. H., Schimming S., Rücknagel K. P., Burgschwaiger S., Kreil G., Staudenbauer W. L. Nucleotide sequence of the celC gene encoding endoglucanase C of Clostridium thermocellum. Gene. 1988;63(1):23–30. doi: 10.1016/0378-1119(88)90542-2. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]