Abstract
To identify the cortical sites where 5-hydroxytryptamine2A (5-HT2A) serotonin receptors respond to the action of hallucinogens and atypical antipsychotic drugs, we have examined the cellular and subcellular distribution of these receptors in the cerebral cortex of macaque monkeys (with a focus on prefrontal areas) by using light and electron microscopic immunocytochemical techniques. 5-HT2A receptor immunoreactivity was detected in all cortical layers, among which layers II and III and layers V and VI were intensely stained, and layer IV was weakly labeled. The majority of the receptor-labeled cells were pyramidal neurons and the most intense immunolabeling was consistently confined to their parallelly aligned proximal apical dendrites that formed two intensely stained bands above and below layer IV. In double-label experiments, 5-HT2A label was found in calbindin D28k-positive, nonphosphorylated-neurofilament-positive, and immuno-negative pyramidal cells, suggesting that probably all pyramidal cells express 5-HT2A receptors. 5-HT2A label was also found in large- and medium-size interneurons, some of which were immuno-positive for calbindin. 5-HT2A receptor label was also associated with axon terminals. These findings reconcile the data on the receptor’s cortical physiology and localization by (i) establishing that 5-HT2A receptors are located postsynaptically and presynaptically, (ii) demonstrating that pyramidal neurons constitute the major 5-HT2A-receptor-expressing cells in the cortex, and (iii) supporting the view that the apical dendritic field proximal to the pyramidal cell soma is the “hot spot” for 5-HT2A-receptor-mediated physiological actions relevant to normal and “psychotic” functional states of the cerebral cortex.
Keywords: dorsolateral prefrontal cortex, macaque monkey, inhibitory interneuron, schizophrenia, clozapine
5-Hydroxytryptamine2A (5-HT2A) serotonin receptors are involved in the actions of hallucinogenic drugs and have been implicated in the pathogenesis and treatment of schizophrenia (1). A wide variety of serotonin receptors are involved in cognitive behaviors in normal and pathological conditions (for review, see ref. 2), but 5-HT2A receptors are in the focus of distinctive attention by the clinical and pharmaceutical research community, because of the receptor’s involvement in the actions of atypical antipsychotic drugs. An important feature of clozapine’s atypicality is that this antipsychotic drug is an effective 5-HT2A receptor antagonist (3–5). Clozapine administration decreases 5-HT2A mRNA and [3H]ketanserin binding in the frontal cortex, whereas 5-HT1A and 5-HT2C mRNAs remain unchanged (6). 5-HT2A receptors act via the G protein-mediated second messenger system and facilitate neuronal excitability by reducing outward potassium currents (1) and by stimulating phospholipase C-mediated phosphoinositide hydrolysis (7). Cortical 5-HT2A receptor concentrations predominate over 5-HT2B and 5-HT2C receptors, the other receptors in the 5-HT2 family (8, 9).
The features highlighting the importance of 5-HT2A receptors are contrasted by a disarray of data on its cortical and cellular distribution and by a lack of information on its subcellular localization (8–19). In the rat cortex, 5-HT2A receptors have been reported to be expressed only by pyramidal cells according to an autoradiographic study (17) or only by interneurons according to an immunocytochemical study (19), but a recent mRNA in situ hybridization study of the human cortex (11) and an immunocytochemical study of the rat cortex have demonstrated the receptor both in pyramidal and nonpyramidal cells (20). 5-HT2 agonist drugs directly activate pyramidal cells in the rat prefrontal cortex (21, 22), but only interneurons were found to be responsive in the rat piriform cortex (23–25). The light and electron microscopic 5-HT2A-receptor-immunocytochemical experiments presented herein have enabled us to identify the neuronal elements containing 5-HT2A receptor in the primate cortex at a level of resolution and specificity not possible in previous autoradiographic and in situ hybridization studies.
MATERIALS AND METHODS
Four adult rhesus monkeys (Macaca mulatta), housed and treated in accordance with institutional guidelines, were deeply anesthetized with Nembutal (100 mg/kg, given intravenously) and perfused transcardially with normal saline followed by 4% paraformaldehyde/0.1% glutaraldehyde prepared in 0.1 M sodium phosphate buffer (PB, pH 7.4). Coronal blocks containing frontal, parietal, temporal, and occipital cortical regions were cut at 50 μm on a cryostat and Vibratome. The sections were treated for 1 h with a blocking serum (5% normal goat serum/1% albumin/0.1% lysine/0.1% glycine in PB; also used in all antibody dilutions), incubated for 2 days at 4°C with 5-HT2A receptor monoclonal antibodies raised in mouse (1:2,000 dilution; clone G186–1117; PharMingen), and processed by the avidin-biotin method using horse anti-mouse biotinylated antibodies (1:250 dilution; 1 h) and the Vectastain ABC Elite reagent (1:100 dilution, 1 h; Vector Laboratories). The immunoperoxidase reaction was visualized by using (i) 0.05% diaminobenzidine (DAB; Sigma) and 0.01% hydrogen peroxide in PB (resulting in a brown reaction product), (ii) DAB intensified with nickel ammonium sulfate (dark blue-to-black Ni-DAB reaction) (26), (iii) Vector SG substrate kit yielding a bluish-grey stain, or (iv) Vector VIP substrate kit resulting in purple color. Regardless of the chromogen used, the distribution and density of the immunostaining was identical. For light microscopic experiments, 0.3% Triton X-100 detergent was added to the antiserum solutions and the staining of some sections was intensified with 0.01% osmium tetroxide. Cortical areas and layers were delineated according to the nomenclature of Brodmann (27) and Walker (28) on adjacent immunoreacted sections counterstained with cresyl violet. The sections were photographed with a Zeiss Aristoplan microscope. Prefrontal (area 46) sections from each monkey were postfixed in 1% osmium tetroxide in PB for 45 min, flat-embedded in Durcupan ACM (Fluka), cut serially into ultrathin sections on an Ultramicrotome (Reichert), stained with uranyl acetate and lead citrate, and examined with a JEOL 1010 transmission electron microscope. Because the nonphosphorylated neurofilament protein SMI-32 and the calcium-binding protein calbindin D28k (CB) are excellent markers of pyramidal cell subgroups (29–31), selected 5-HT2A-immunoreacted prefrontal sections were further incubated with either SMI-32 (1:5,000 dilution; Sternberger–Meyer, Jarrettsville, MD) or CB (1:10,000 dilution; Sigma) mouse antibodies for 2 days at 4°C, treated with biotinylated anti-mouse antiserum (1:250 dilution; 1 h) and the ABC Elite reagent (1:100 dilution; 1 h), and reacted with a chromogen of different color than the one used in the first immunostaining. When one of the primary antisera was omitted, only single staining was observed; when both were omitted, no staining was detected. The specificity and use of the CB and SMI-32 antisera have been described (29, 32). The 5-HT2A receptor antibody was produced by PharMingen by using a recombinant fusion protein between glutathione S-transferase (GST) and human 5-HT2A receptor (amino acids 1–72) as immunogen. The hybridomas were screened by PharMingen by using ELISA, immunocytochemistry of frozen rat brain sections, and Western blot analysis: the 5-HT2A antibody recognizes the receptor as a 55-kDa band. The specificity of the band was verified by competition with a 5-HT2A receptor fusion protein and lack of competition with an irrelevant fusion protein. The supernatant was also tested against GST protein to rule out reactivity to this portion of the immunogen. The antibody was purified from hybridoma culture supernatant by protein G affinity chromatography. The antibody has specifically shown to react with human, monkey, and rat 5-HT2A receptor.
RESULTS
5-HT2A receptor immunoreactivity was observed throughout the anterior (area 24) and posterior (area 23) areas of the cingulate cortex; prefrontal cortical areas 9, 11, 12, and 46; motor cortex (areas 4 and 6); temporal cortical areas 21, 22, and 41; insular cortex; and primary and secondary visual cortex (areas 17 and 18). Throughout the cortical sheet, the receptor labeling featured a weakly stained band located in layer IV flanked by two intensely labeled bands in layers II and III and layers V and VI (Fig. 1A). Receptor immunoreactivity was restricted to neurons. In all cortical regions, pyramidal cells represented the majority of immunoreacted cells, characterized by intensely labeled apical dendrites emanating from moderately stained somata. Receptor-labeled nonpyramidal cells and fine processes were also consistently observed (Fig. 1 B–D). In area 46 of the prefrontal cortex, 5-HT2A labeled pyramidal cells were densely distributed throughout layers II, III, V, and VI (Fig. 1B). Apparently the majority (if not all) of pyramidal neurons expressed 5-HT2A label. The receptor label was consistently found to be most intense in the proximal part of their apical dendrites (Fig. 1 B–D). Perikarya and distal dendritic branches were labeled with moderate intensity (Fig. 1 C and D). Dendritic spines were devoid of the immunolabel completely or occasionally labeled weakly. 5-HT2A label was also detected in large- and medium-size (12–30 μm in diameter) nonpyramidal neurons (Figs. 1C and 2A) through layers II–VI and very rarely in layer I, but the majority of the labeled interneurons was located in infragranular layers. The label was strongest in proximal dendrites.
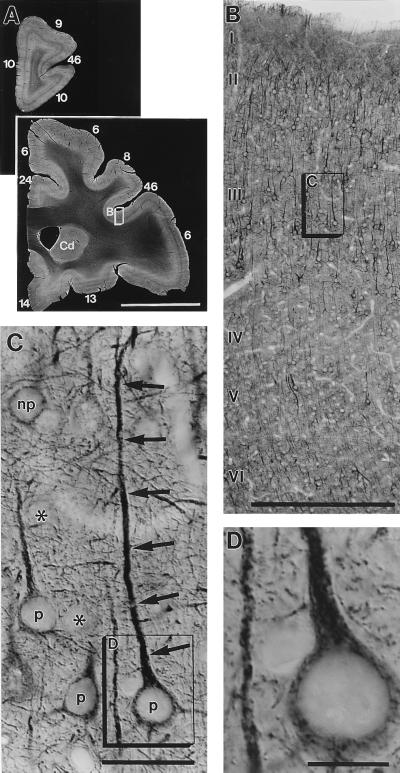
Figure 1.
(A) Dark-field photographs show the distribution of 5-HT2A receptor immunoreactivity in two representative sections of the prefrontal cortex of an adult macaque monkey. Cortical areas are numbered according to Walker’s nomenclature. Cd, caudate nucleus. Note that receptor labeling is weak in a stripe denoting layer IV throughout the cortical sheet. The framed cortical region, enlarged in B, exemplifies the receptor immunoreactivity in area 46 of the prefrontal cortex. 5-HT2A labeling is found in most (if not all) pyramidal neurons throughout cortical layers II and III and layers V and VI, including their dendritic branches in layer I. Receptor labeling is weak in layer IV because only moderately labeled interneurons and some en passant apical dendrites of layer V pyramids are present in this layer. The boxed area in layer III (enlarged in C and D) demonstrates receptor-labeled pyramidal cells (p), unlabeled (asterisks) and labeled nonpyramidal (np) cells, and receptor-positive fine processes (their ultrastructure is shown in Fig. 3). Note that the immunoreaction is strongest in the apical dendrites (arrows) of pyramidal cells and relatively weak in perikarya. [Bars = 1 cm (A), 0.5 mm (B), 50 μm (C), and 20 μm (D).]
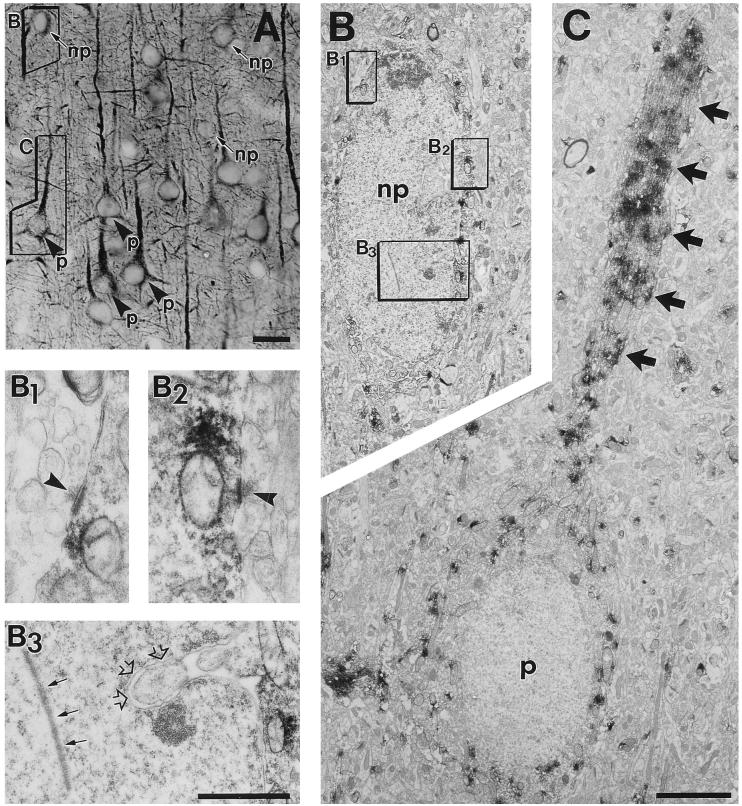
Figure 2.
Cellular (A) and subcellular (B and C) localization of 5-HT2A receptor immunoreactivity in pyramidal (p) and nonpyramidal (np) neurons, detected by correlated light and electron microscopy in layer III of prefrontal area 46. (A) The framed nonpyramidal and pyramidal cells were selected for electron microscopic examination and enlargements are shown in B and C. (B) Labeled medium-size nonpyramidal neuron (np) exhibiting intranuclear rod (B3, small arrows), nuclear infolding (B3, open arrows), and axosomatic asymmetric synapses (B1 and B2, arrowheads), three typical features of cortical interneurons. (C) The receptor label concentration is high in the pyramidal cell apical dendrite (arrows) and low in the perikaryal cytoplasm. [Bars = 25 μm (A), 5 μm (B and C), and 0.5 μm (B1–B3).]
Electron microscopic analysis confirmed the presence of 5-HT2A receptor label in the cytoplasm of pyramidal (Fig. 2C) and nonpyramidal neurons (Fig. 2B), substantiated that the receptor label is strongest in their proximal large-diameter dendrites (Fig. 2C), and revealed 5-HT2A label in axons. Somata (Fig. 2 B and C) and thin (distal) dendrites (Fig. 3 A–C) were moderately stained. Dendritic spines were rarely and weakly labeled (Fig. 3 A–C). The nucleus, mitochondria, and Golgi apparatus of labeled cells was immunonegative. Labeled nonpyramidal neurons (Fig. 2B) typically exhibited large- or medium-size cell body, infolded nucleus with an intranuclear rod (Fig. 2B3), and asymmetric axosomatic synapses (Fig. 2B1–2). A minor group of axon terminals (Fig. 3 B–E) forming asymmetric synapses (Fig. 3E) or containing dense-core vesicles (Fig. 3F) was also immunolabeled.
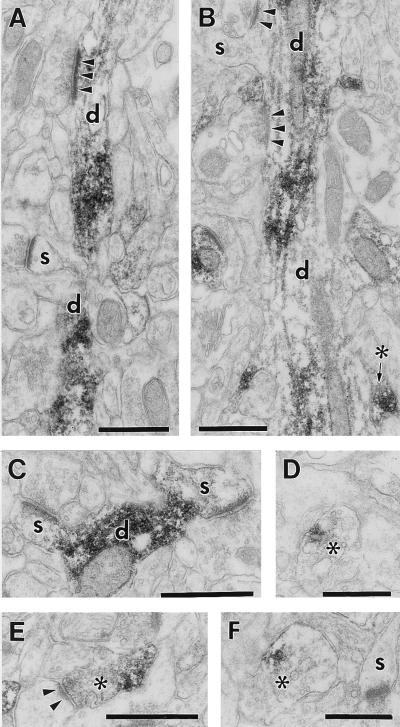
Figure 3.
Postsynaptic (A–C) and presynaptic (B, D–F) localization of 5-HT2A receptor immunoreactivity in area 46 of the prefrontal cortex. (A and B) 5-HT2A label is localized to dendritic shafts (d), but dendritic spines (s) are generally immunonegative. Unlabeled axons form asymmetric synapses (arrowheads) on these dendrites. (C) A rare example of two weakly labeled dendritic spines (s). (B, D–F) Immunolabeled axon terminals (asterisks) forming asymmetric synapse (E, arrowheads) or containing dense core vesicle (F). 5-HT2A label is restricted to a portion of the axoplasm. (Bars = 0.5 μm.)
In 5-HT2A/CB and 5-HT2A/SMI-32 double-label experiments, both CB-positive supragranular and CB-negative infragranular pyramids (Fig. 4 B–E) and both SMI-32-positive (Fig. 4 F and G) and SMI-32-negative pyramidal cells contained 5-HT2A label. The double-label patterns accented the intracellular segmentation of the receptor label. Note in Fig. 4 B–G that the color of the 5-HT2A label dominates in the proximal apical dendrites but that CB and SMI-32 labels highlight the cell bodies. The presence of SMI-32 label in distal dendrites (Fig. 4G) indicates that the weak 5HT2A receptor label in the same cell segment is not due to a possibly inadequate antibody penetration, affirming that the intradendritic segmentation of 5-HT2A receptor label is a true feature of pyramidal cells.
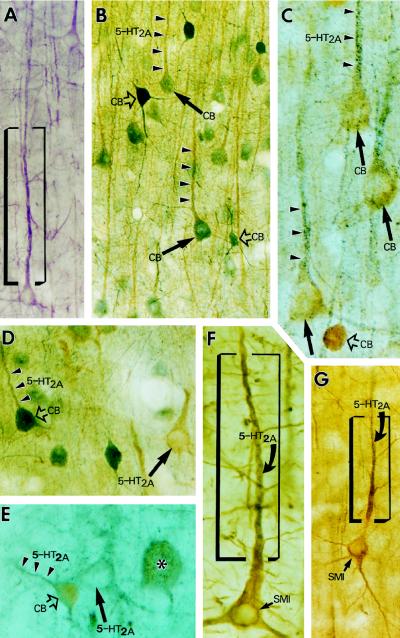
Figure 4.
Color light micrographs demonstrate the accumulation of 5-HT2A receptor immunoreactivity in the proximal dendrites of pyramidal and nonpyramidal neurons in prefrontal area 46. The neurons were neurochemically identified by using 5-HT2A/CB (B–E) or 5-HT2A/SMI-32 (F–G) double immunocytochemistry. Arrowheads point to dendrites, arrows point to pyramidal cell somata, and open arrows point to nonpyramidal cell bodies. (A) 5-HT2A-positive pyramidal cell in layer III (stained purple with the Vector VIP staining kit). (B and C) Two 5-HT2A/CB double-stained sections from cortical layer III demonstrate that 5-HT2A immunoreactivity (arrowheads) is colocalized with CB immunolabel (arrows) in pyramidal neurons. (B) 5-HT2A label is light brown and CB label is bluish gray. (C) Double labeling is “reverse,” 5-HT2A label is bluish gray, and CB label is light brown. (D and E) Two micrographs from layer V demonstrate that large- (asterisk) and medium-size interneurons (open arrows) colocalize 5-HT2A receptor and CB and show that 5-HT2A receptor is also present in CB-negative pyramidal cells (arrows), typical of the infragranular layers. (D) 5-HT2A, light brown; CB, bluish gray. (E) 5-HT2A, bluish gray; CB, light brown. (F and G) 5-HT2A/SMI-32 double-labeled pyramidal cells from layer III. Note that the gray color of 5-HT2A label dominates only in the proximal apical dendrites (within the frame), whereas thinner dendritic branches and the cell body are overshadowed by the brown-colored SMI-32 staining.
DISCUSSION
Postsynaptic Localization of 5-HT2A Receptor.
The findings presented herein clearly demonstrate that the bulk of 5-HT2A-receptor-expressing cells in the primate cortex are pyramidal neurons, and the receptor label is most intense in their apical dendrites, in agreement with recent immunocytochemical findings in the rat cortex (20). Although previous studies using autoradiographic detection of receptor binding (14) or in situ hybridization histochemistry have inferred that pyramidal neurons contribute to the expression of 5-HT2A receptor (8, 11, 17), the fact that, in the present study, neither CB- and SMI-32-positive pyramidal cells nor pyramids lacking these markers were seen without 5-HT2A label indicates the absence of any major 5-HT2A-receptor-lacking pyramidal cell class and suggests the nonselective presence of 5-HT2A receptor in pyramidal cells.
In cortical interneurons, 5-HT2A receptor expression is selective: large- and medium-size interneurons were labeled, whereas many small- and medium-size interneurons were unlabeled. The predominance of 5-HT2A-expressing interneurons in infragranular layers is in agreement with the preservation of 5-HT2A receptor binding sites after intrastriatal injection of volkensin, which destroys layer V pyramidal neurons (33). The presence of 5-HT2A receptor in interneurons of the rat cortex has been indicated by immunocytochemical (19, 34) and electrophysiological studies (23–25). The failure of some of the earlier studies (e.g., ref. 17) to detect 5-HT2A label (autoradiography silver grains) over nonpyramidal cell bodies can be explained by the weak perikaryal receptor label (see Fig. 4 B–E). However, the failure of immunocytochemical studies (19, 34) to detect 5-HT2 receptor in pyramidal cells is harder to reconcile with the rest of the literature and the present data: the antiserum may have recognized an epitope or receptor subunit expressed in interneurons and not pyramidal cells.
Presynaptic Localization of 5-HT2A Receptor.
The presence of the receptor label in a minor group of asymmetric synapse-forming cortical axons suggests that 5-HT2A receptors may presynaptically modulate excitatory neurotransmission in a discrete cortical axonal system. The presynaptic serotonergic modulation of glutamatergic cortical neurotransmission has been implied by the reduction of serotonin-induced excitatory postsynaptic potentials by an inhibitory metabotropic glutamate receptor agonist (22). The candidates for these axons include specific corticocortical or thalamocortical pathways that form asymmetric synapses and use glutamate (35, 36). Alternatively, we cannot exlude serotonergic axons as a possible source, because they can also form asymmetric synapses in the primate cortex (37, 38), and dense-core vesicles—markers of cortical monoaminergic axons—are present in some of the 5-HT2A-labeled boutons.
Laminar Localization of 5-HT2A Receptor, a Consequence of Intracellular Segmentation.
It has long been recognized that 5-HT2 receptors have a laminar pattern in the cortex (9, 13). The finding that virtually all pyramidal cells exhibit 5-HT2A receptor label makes it unlikely that the laminar pattern would be based on an alternation of 5-HT2A-lacking and 5-HT2A-containing pyramidal cells. Instead, it appears to be a consequence of the intracellular segmentation of 5-HT2A receptor concentration within pyramidal cells. This intracellular segmentation of strong proximal apical dendritic label and faint perikaryal and distal dendritic label is inevitably manifested in a laminar pattern. 5-HT2A receptor concentration is highest in layers III and V because these layers contain most apical dendrites, and low in layer IV because this lamina lacks pyramidal cells and harbors only some en passant apical dendrites and moderately labeled interneurons. Similar to the present data, 5-HT2A receptor binding sites and mRNA signals in the rat and human cortex (8, 11, 39) are concentrated to two bands associated with layers III and V.
Accumulation of 5-HT2A Receptors in Proximal Apical Dendrites.
The disproportionately high concentration of 5-HT2A receptor labeling in the proximal apical dendritic segment may indicate that the accumulated protein is undergoing transport to other segments of the dendritic tree, but may as well suggest a unique receptivity of this segment to actions mediated by 5-HT2A ligands. Because receptor and ligand concentrations determine physiological effectiveness (40), it is remarkable that serotonin concentrations also appear to be high in the microenvironment of these segments: a dense band of 5-HT2 receptors in upper layer V has been found in register with a dense plexus of fine 5-HT axons (14), and dendritic shafts and not spines have been shown to be the most common targets of serotonin-immunolabeled cortical synaptic boutons (37). Consistent with this is the lack of labeling in dendritic spines, suggesting that this segment is not involved in 5-HT2A-mediated neurotransmission. Finally, the “key” physiological finding that microiontophoresis of serotonin only to hot spots near the border of layers IV and V within the apical (but not basilar!) dendritic field of pyramidal cells induced rapid excitatory postsynaptic currents in layer V pyramids (22) affirms the conclusion that the most potent 5-HT2A-receptor-mediated actions are confined to the hot spots of the proximal apical dendritic shafts.
5-HT2A-Receptor-Rich Apical Dendrites, a Possible Site for Switching Pyramidal Cell Firing from Normal to “Psychotic.”
It is postulated that 5-HT2A receptors accumulated in the apical dendritic “bottle-neck” segment of pyramidal cells have a strategic role in controlling the rate of dendritic currents that can pass through the apical dendrite and reach the soma. The manipulation of ion channels along the pyramidal cell apical dendrite can amplify or gate this information flow (41–43), and 5-HT2A receptors are able to modulate these ion channels; their activation in the microenvironment of apical dendrites enhances a subthreshold tetrodotoxin-sensitive inward current (22), and this current underlies a postsynaptic amplification of excitatory postsynaptic potentials impinging upon apical dendrites (41, 44). We suggest that this apical dendritic gating mechanism may play an important role in working memory processes and the related behavioral phenomenon called latent inhibition. Working memory reflects a capacity of neurons in association cortices to keep information “on line” (45), whereas latent inhibition is a capacity of all vertebrates to attenuate the rate at which a stimulus can evoke a conditioned response, if the prior exposure of that stimulus has been without consequence for the human or animal subject (46–48). Both phenomena are associated with temporal processing of information that, under normal circumstances, could prevent sensory overload and distractibility; and both phenomena are impaired in schizophrenic patients (for review see refs. 49 and 50). The entire pyramidal dendritic tree is bombarded by sensory and intrinsic inputs but, probably in part due to the gating mechanism of apical dendritic ion channels, only a fraction of these stimuli reaches the soma and results in cell firing during normal behavioral states. A nominal 5-HT2A receptor activation seems beneficial for working memory because 5-HT2A agonists increase activity for preferred directions and reduce activity for nonpreferred directions in pyramidal cells in monkeys performing spatial delayed-response task (G. V. Williams, S. G. Rao, and P.S.G.-R., unpublished data). However, excessive 5-HT2A receptor activation may be disruptive. For example, 5-HT2A agonists impair latent inhibition in rats (51). We suggest that the gating mechanisms of apical dendritic ion channels may be dysfunctional in psychotic behavioral states occurring in the acute “positive” phase of schizophrenia or induced by 5-HT2A agonist drugs (e.g. the powerful “recreational drug 3,4-methylenedioxymethamphetamine, also known as “Ecstasy”). Our hypothesis infers that the hallucinogenic-drug-induced state and the acute schizophrenic condition both are concomitant with the inability of pyramidal cells to switch to a resting firing mode after or in the absence of a specific stimulus. An excessive rate of dendritic currents reaching the soma may underlie the powerful hallucinogenic effect of 5-HT2A agonists. Similarly, hyperactive pyramidal cells may produce the “positive” hallucination-like symptoms observed in acute schizophrenia due to a dysfunctional 5-HT2A receptor system in their apical dendrites. A compromised protein kinase C-mediated 5HT2A signal transduction cascade in the pyramidal cells (17) may send erroneous signals to the apical dendritic ion channels. Willins and Roth recently have shown that the administration of the partial 5-HT2A antagonist drug, clozapine, to the rat cortex down-regulates apical dendritic (but not perikaryal) 5-HT2A receptors (personal communications). Clozapine’s capability to deplete 5-HT2A receptors from apical dendrites or to slow the transport of the receptor from the soma to the dendritic tree may normalize the gating function of apical dendritic ion channels, and this effect may specifically contribute to the drug’s clinical effectiveness in the treament of schizophrenia.
Whether pyramidal cell firing switches from normal to hyperactive, as proposed, is at present unknown. However, findings from in vivo recordings in the prefrontal cortex of monkeys in this laboratory indicate that serotonin facilitates excitatory neurotransmission in pyramidal neurons engaged in information processing (G. V. Williams, S. G. Rao, and P.S.G.-R., unpublished data). The present subcellular localization of 5HT2A receptors in proximal apical dendrites of pyramidal cells provides a starting point for identification of factors such as under- or overexpressed or genetically mutated proteins at this site in schizophrenic patients and animal subjects with experimentally induced “psychotic” symptoms.
Acknowledgments
This work is dedicated to Prof. Sandor Koch. We thank Drs. George Aghajanian and Graham Willams for critical comments on the manuscript and Ms. Klara Szigeti for excellent technical assistance. This work is supported by a grant from the National Alliance for Research on Schizophrenia and Depression (R.L.J.) and National Institutes of Health Grant MH 44866 (P.S.G.).
ABBREVIATIONS
- 5-HT2A serotonin receptor
5-hydroxytryptamine2A serotonin receptor
- CB
calcium-binding protein calbindin D28k
References
- 1.Aghajanian G K. In: Psychopharmacology: The Fourth Generation of Progress. Bloom F E, Kupfer D J, editors. New York: Raven; 1995. pp. 451–460. [Google Scholar]
- 2.Buhot M-C. Curr Opin Neurobiol. 1997;7:243–254. doi: 10.1016/s0959-4388(97)80013-x. [DOI] [PubMed] [Google Scholar]
- 3.Deutch A Y, Moghaddam B, Innis R B, Krystal J H, Aghajanian G K, Bunney B S, Charney D S. Schizophr Res. 1991;4:121–156. doi: 10.1016/0920-9964(91)90030-u. [DOI] [PubMed] [Google Scholar]
- 4.Meltzer H Y, Nash J F. Pharmacol Rev. 1991;43:587–604. [PubMed] [Google Scholar]
- 5.Schmidt C J, Sorensen S M, Kehne J H, Carr A A, Palfreyman M G. Life Sci. 1995;56:2209–2222. doi: 10.1016/0024-3205(95)00210-w. [DOI] [PubMed] [Google Scholar]
- 6.Burnet P W, Chen C P, McGowan S, Franklin M, Harrison P J. Neuroscience. 1996;73:531–540. doi: 10.1016/0306-4522(96)00062-0. [DOI] [PubMed] [Google Scholar]
- 7.Sanders-Bush E, Conn P J. Psychopharmacol Bull. 1986;22:829–836. [PubMed] [Google Scholar]
- 8.Pompeiano M, Palacios J M, Mengod G. Mol Brain Res. 1994;23:163–178. doi: 10.1016/0169-328x(94)90223-2. [DOI] [PubMed] [Google Scholar]
- 9.Wright D E, Seroogy K B, Lundgren K H, Davis B M, Jennes L. J Comp Neurol. 1995;351:357–373. doi: 10.1002/cne.903510304. [DOI] [PubMed] [Google Scholar]
- 10.Pazos A, Cortes R, Palacios J M. Brain Res. 1985;346:231–249. doi: 10.1016/0006-8993(85)90857-1. [DOI] [PubMed] [Google Scholar]
- 11.Burnet P W, Eastwood S L, Lacey K, Harrison P J. Brain Res. 1995;676:157–168. doi: 10.1016/0006-8993(95)00104-x. [DOI] [PubMed] [Google Scholar]
- 12.Pasqualetti M, Nardi I, Ladinsky H, Marazziti D, Cassano G B. Brain Res Mol Brain Res. 1996;39:223–233. doi: 10.1016/0169-328x(96)00026-5. [DOI] [PubMed] [Google Scholar]
- 13.Slater P, Patel S. Eur J Pharmacol. 1983;92:297–298. doi: 10.1016/0014-2999(83)90303-5. [DOI] [PubMed] [Google Scholar]
- 14.Blue M E, Yagaloff K A, Mamounas L A, Hartig P R, Molliver M E. Brain Res. 1988;453:315–328. doi: 10.1016/0006-8993(88)90172-2. [DOI] [PubMed] [Google Scholar]
- 15.Schotte A, Leyson J E. Eur J Pharmacol. 1989;172:99–106. doi: 10.1016/0922-4106(89)90001-1. [DOI] [PubMed] [Google Scholar]
- 16.Appel N M, Mitchell W M, Garlick R K, Glennon R A, Teitler M, DeSouza E B. J Pharmacol Exp Ther. 1990;255:843–857. [PubMed] [Google Scholar]
- 17.Rahman S, McLean J H, Darby-King A, Paterno G, Reynolds J N, Neumann R S. Neuroscience. 1995;66:891–901. doi: 10.1016/0306-4522(95)00002-z. [DOI] [PubMed] [Google Scholar]
- 18.Lidow M S, Goldman-Rakic P S, Gallager D W, Rakic P. J Comp Neurol. 1989;280:27–42. doi: 10.1002/cne.902800104. [DOI] [PubMed] [Google Scholar]
- 19.Morilak D A, Garlow S J, Ciaranello R D. Neuroscience. 1993;54:701–717. doi: 10.1016/0306-4522(93)90241-7. [DOI] [PubMed] [Google Scholar]
- 20.Willins D L, Deutsch A Y, Roth B L. Synapse. 1997;27:79–82. doi: 10.1002/(SICI)1098-2396(199709)27:1<79::AID-SYN8>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 21.Araneda R, Andrade R. Neuroscience. 1991;40:399–412. doi: 10.1016/0306-4522(91)90128-b. [DOI] [PubMed] [Google Scholar]
- 22.Aghajanian G K, Marek G J. Neuropharmacology. 1997;36:589–599. doi: 10.1016/s0028-3908(97)00051-8. [DOI] [PubMed] [Google Scholar]
- 23.Sheldon P W, Aghajanian G K. Brain Res. 1990;506:62–69. doi: 10.1016/0006-8993(90)91199-q. [DOI] [PubMed] [Google Scholar]
- 24.Gellman R L, Aghajanian G K. Brain Res. 1993;600:63–73. doi: 10.1016/0006-8993(93)90402-9. [DOI] [PubMed] [Google Scholar]
- 25.Marek G J, Aghajanian G K. J Pharmacol Exp Ther. 1996;278:1373–1382. [PubMed] [Google Scholar]
- 26.Hancock M B. J Histochem Cytochem. 1982;30:578–579. [Google Scholar]
- 27.Brodmann K. J Psychol Neurol. 1905;4:177–226. [Google Scholar]
- 28.Walker A E. J Comp Neurol. 1940;73:59–86. [Google Scholar]
- 29.Campbell M J, Morrison J H. J Comp Neurol. 1989;282:191–205. doi: 10.1002/cne.902820204. [DOI] [PubMed] [Google Scholar]
- 30.Hayes T L, Lewis D A. Cerebral Cortex. 1992;2:56–67. doi: 10.1093/cercor/2.1.56. [DOI] [PubMed] [Google Scholar]
- 31.Conde F, Lund J S, Jacobowitz D M, Baimbridge K G, Lewis D A. J Comp Neurol. 1994;341:95–116. doi: 10.1002/cne.903410109. [DOI] [PubMed] [Google Scholar]
- 32.Jakab R L, Goldman-Rakic P S, Leranth C. Cerebral Cortex. 1997;7:359–373. doi: 10.1093/cercor/7.4.359. [DOI] [PubMed] [Google Scholar]
- 33.Francis P T, Pangalos M N, Pearson R C A, Middlemiss D N, Stratmann G C, Bowen D M. J Pharmacol Exp Ther. 1992;261:1273–1281. [PubMed] [Google Scholar]
- 34.Morilak D A, Somogyi P, Lujan-Miras R, Ciaranello R D. Neuropsychopharmacology. 1994;11:157–166. doi: 10.1038/sj.npp.1380102. [DOI] [PubMed] [Google Scholar]
- 35.Sloper J J, Powell T P S. Philos Trans R Soc London Ser B. 1978;285:199–226. doi: 10.1098/rstb.1979.0005. [DOI] [PubMed] [Google Scholar]
- 36.Winfield D A, Rivera-Dominguez M, Powell T P S. Brain Res. 1982;231:19–32. doi: 10.1016/0006-8993(82)90004-x. [DOI] [PubMed] [Google Scholar]
- 37.Smiley J, Goldman-Rakic P S. J Comp Neurol. 1996;367:431–443. doi: 10.1002/(SICI)1096-9861(19960408)367:3<431::AID-CNE8>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 38.de Lima A D, Bloom F E, Morrison J H. J Comp Neurol. 1988;274:280–294. doi: 10.1002/cne.902740211. [DOI] [PubMed] [Google Scholar]
- 39.Pazos A, Probst A, Palacios J M. Neuroscience. 1987;21:123–139. doi: 10.1016/0306-4522(87)90327-7. [DOI] [PubMed] [Google Scholar]
- 40.Jakab R L, Hazrati L-N, Goldman-Rakic P S. J Comp Neurol. 1996;369:137–149. doi: 10.1002/(SICI)1096-9861(19960520)369:1<137::AID-CNE10>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 41.Schwindt P C, Crill W E. J Neurophysiol. 1997;78:187–198. doi: 10.1152/jn.1997.78.1.187. [DOI] [PubMed] [Google Scholar]
- 42.Midtgaard J. Trends Neurosci. 1994;17:166–173. doi: 10.1016/0166-2236(94)90095-7. [DOI] [PubMed] [Google Scholar]
- 43.Yuste R, Tank D W. Neuron. 1996;16:701–716. doi: 10.1016/s0896-6273(00)80091-4. [DOI] [PubMed] [Google Scholar]
- 44.Stuart G, Sakmann B. Neuron. 1995;15:1065–1076. doi: 10.1016/0896-6273(95)90095-0. [DOI] [PubMed] [Google Scholar]
- 45.Goldman-Rakic P S. In: Handbook of Physiology. Plum F, Mountcastle V, editors. Vol. 5. Bethesda, MD: Am. Physiol. Soc.; 1987. pp. 373–414. [Google Scholar]
- 46.Lubow R E. Psychol Bull. 1973;79:398–407. doi: 10.1037/h0034425. [DOI] [PubMed] [Google Scholar]
- 47.Lubow R E. Latent Inhibition and Conditioned Attention Theory. Cambridge, U.K.: Cambridge Univ. Press; 1989. [Google Scholar]
- 48.Mackintosh N J. Conditioning and Associative Learning. London: Oxford Univ. Press; 1983. [Google Scholar]
- 49.Goldman-Rakic P S, Seleman L D. Schizophrenia Bull. 1997;23:437–458. doi: 10.1093/schbul/23.3.437. [DOI] [PubMed] [Google Scholar]
- 50.Baruch I, Hemsley D R, Gray J A. J Nerv Ment Dis. 1988;176:598–606. doi: 10.1097/00005053-198810000-00004. [DOI] [PubMed] [Google Scholar]
- 51.Cassaday H J, Hodges H, Gray J A. J Psychopharmacol. 1993;7:63–71. doi: 10.1177/026988119300700110. [DOI] [PubMed] [Google Scholar]