Abstract
Leptin has profound effects on feeding, metabolism, and neuroendocrine status. Evidence indicates that the hypothalamus coordinates these responses, though the specific brain pathways engaged by leptin remain obscure. The paraventricular nucleus of the hypothalamus (PVH) regulates pituitary gland function and feeding, and innervates autonomic preganglionic neurons, making it a candidate to regulate many of the responses to leptin. The subparaventricular zone, an anterior hypothalamic region receiving dense innervation from the suprachiasmatic nucleus, is thought to integrate circadian and metabolic information. We investigated the distribution of neurons in the rat brain activated by leptin administration that also project to the PVH or the subparaventricular zone by coupling immunohistochemistry for Fos with retrograde transport of cholera toxin-b. Intravenous leptin characteristically activated several cell groups including the ventromedial hypothalamic nucleus, the dorsomedial hypothalamic nucleus (DMH), and the PVH. When tracer injections were centered in the subparaventricular zone, many double-labeled cells were observed in the dorsomedial subdivision of the ventromedial hypothalamic nucleus. This projection may provide an anatomic substrate for integration of metabolic and circadian information to regulate the hypothalamo-pituitary axis. When cholera toxin-b injections were centered in the PVH, many double-labeled cells were found within the caudal DMH. Hence, activation of specific neuroendocrine and autonomic elements of the PVH may be triggered by leptin-activated afferents arising in the DMH. Our results demonstrate that a discrete set of hypothalamic pathways may underlie leptin’s autonomic, endocrine, and behavioral effects.
Leptin, produced by white adipose tissue, affects feeding, thermogenesis, and neuroendocrine status (1). Absence or resistance to leptin causes obesity, diabetes, reproductive failure, and other neuroendocrine abnormalities (reviewed in refs. 2 and 3). Replacement with exogenous leptin causes ob/ob mice to reduce food intake and normalize body mass (4–6). During starvation, a physiological state of leptin deficiency, similar changes of the reproductive, thyroid, and hypothalamo-pituitary-adrenal axes occur (2, 7–9). The behavioral and neuroendocrine effects of leptin are thought to be mediated via the hypothalamus, but the central pathways involved in leptin signaling have not been determined. The paraventricular nucleus of the hypothalamus (PVH) is ideally positioned to regulate responses in the face of changing energy availability. The PVH contains neuroendocrine parvicellular neurons that project to the median eminence and regulate the secretion of thyroid-stimulating hormone and corticotropin (ACTH) (10, 11). Lesions of the PVH induce hyperphagia and obesity (12) and injections of neuropeptide Y (NPY), which is found in terminals that innervate PVH neurons, increases food intake (13). Finally, the PVH contains parvicellular neurons that directly innervate parasympathetic and sympathetic preganglionic neurons in the medulla and spinal cord (10, 11), giving the hypothalamus a direct input to the autonomic nervous system. Thus, the PVH is a candidate to act as a final common pathway in regulating the endocrine and autonomic responses to circulating leptin.
We previously reported that i.v. leptin activates parvicellular subdivisions of the PVH that project to autonomic preganglionic neurons (14). However, the PVH does not contain high levels of leptin receptors, which are found in the highest densities in the ventrobasal hypothalamus (15–17), suggesting that leptin activation of the PVH may be caused by innervation from leptin responsive neurons. Thus, we investigated the origins of leptin-activated afferents to the PVH by using expression of Fos protein as a marker of cellular activation (18, 19) coupled with retrograde transport of the tracer cholera toxin-b (CTb) from the PVH.
MATERIALS AND METHODS
Animals and Leptin Administration.
Adult male pathogen free Sprague–Dawley rats (250–350 g; Taconic Farms) were housed in a light-controlled (12 hr on/12 hr off; lights on at 7 a.m.) and temperature-controlled environment (21.5–22.5°C). The animals and procedures used were approved by the Harvard Medial School and Beth Israel Deaconess Medical Center Institutional Animal Care and Use Committees. As previously described, i.v. catheters were surgically implanted (14, 20, 21) and 3–5 nl of CTb (1% in saline; List Biological Laboratories, Campbell, CA) was injected (20–22) into the vicinity of the PVH. Five to seven days later, rats were injected with recombinant murine leptin (0.1 or 1.0 mg/kg; Eli Lilly) followed by pyrogen-free saline (PFS; Sigma) or PFS alone (0.25 ml total volume/rat). All injections were given between 11 a.m. and 12 p.m. Groups consisted of: i.v. leptin 0.1 mg/kg (n = 7); i.v. leptin 1.0 mg/kg (n = 17), and i.v. PFS (n = 4).
Histology and Immunohistochemistry.
Two hours after leptin or pyrogen-free saline administration, anesthetized rats were perfused transcardially with 0.9% saline followed by 500 ml of 10% neutral buffered formalin (Sigma). The brains were removed, postfixed, and submerged in 20% sucrose for 2–3 nights, and five series of coronal sections were cut at 30 μm. Sections were processed for double-label immunohistochemistry as reported previously from our laboratory (14, 21, 22). Fos rabbit primary antiserum (Ab-5, Oncogene Science; 1:150,000) was used overnight at room temperature followed by biotinylated goat anti-rabbit IgG (Vector; 1:600) for 2 hr at room temperature. Both antibodies were diluted in 3% normal donkey serum (Jackson Laboratories) and 0.25% Triton X-100 in PBS containing 0.02% sodium azide (PDT). Sections then were reacted with avidin-biotin complex (Vector Elite Kit; 1:200 in PBS) for 1 hr, rinsed, and incubated in 0.04% diaminobenzidine tetrahydrochloride (Sigma), 0.02% nickel sulfate, and 0.02% cobalt chloride (Fisher Scientific), and 0.01% hydrogen peroxide dissolved in PBS for 6–10 min.
Tissue sections were reexposed to 0.3% hydrogen peroxide, rinsed in PBS, incubated in PDT for 2 hr, and exposed to CTb primary antiserum raised in goat (1:100,000 in PDT; List Biological Laboratories) overnight at room temperature. The sections were incubated in biotinylated donkey anti-goat antisera (1:1,000 in PDT; Jackson Laboratories) for 2 hr, and incubated in avidin-biotin complex and diaminobenzidine tetrahydrochloride solution as described for Fos except that the cobalt chloride and nickel sulfate were omitted from the diaminobenzidine tetrahydrochloride solution, yielding a brown cytoplasmic reaction product. In addition, an adjacent series of sections from seven cases (R1118, R1119, R1122, R1124, R1175, R1178, and R1180) were stained for Fos and CTb as described above except that the CTb was visualized via immunofluoresence with a Cy-3-conjugated streptavidin rather than the avidin-biotin complex solution.
Control experiments resulted in no specific staining that included incubation of the tissue in antisera that had been preadsorbed with the Fos peptide (peptide-2, Oncogene; 15 μM) and with brain sections from c-fos knockout mice. In addition, negative controls were generated by omission of each primary antiserum.
The tissue sections were mounted onto subbed slides, dehydrated in alcohol, cleared in xylene, coverslipped with Permaslip, and analyzed with a Zeiss Axioplan light microscope. Photomicrographs were produced by capturing images with a digital camera (Kodak, DCS) mounted directly on the microscope and a Apple Macintosh Power PC computer. Image editing software (Adobe Photoshop and Specular Collage) was used to combine photomicrographs into plates, and figures were printed on a dye sublimation printer (Kodak 8600).
Immunoreactive cells (Fos, CTb, or Fos+CTb) in the dorsomedial division of the ventromedial hypothalamic nucleus (VMH) and the caudal subdivision of the dorsomedial hypothalamic nucleus (DMH) were counted by using a grid reticule and a 20× objective (14, 20) in seven cases treated with i.v. leptin (1.0 mg/kg; see Tables 1 and 2). These data were not corrected for double-counting, e. g., by the method of Abercrombie (23) or by using a stereological technique (24). However, because the objects we were counting (nuclei and retrogradely labeled cells) did not change in size, and section thickness did not vary between groups, any systematic error should be identical for all groups. Hence, as all double-label studies are inherently qualitative our results are meant to provide relative data on expression of Fos-like immunoreactivity (Fos-IR) and CTb-like immunoreactivity (CTb-IR), but are not meant to be accurate estimates of absolute cell counts.
Table 1.
PVH injections
| Region | Total Fos | Total CTb | Double-labeled (Fos + CTb) | % Doubled-labeled (Fos + CTb/Total CTb) |
|---|---|---|---|---|
| dmVMH | 88.3 ± 12.3 | 119.6 ± 11.6 | 7.7 ± 1.9 | 6.7 ± 2.0 |
| cDMH | 197.3 ± 2.0* | 142.0 ± 33.1 | 75.3 ± 17.8* | 53.3 ± 2.8* |
Values represent estimates of mean counts of cells ± SEM. Data were analyzed by ANOVA and differences between groups by ANOVA and Scheffe F-test. AHA, anterior hypothalamic area; cDMH, caudal dorsomedial hypothalamic nucleus; dmVMH, dorsomedial division of the ventromedial hypothalamic nucleus; PVH, paraventricular hypothalamic nucleus.
*P < 0.05 compared to VMH. n = 3.
Table 2.
AHA/subparaventricular injections
| Region | Total Fos | Total CTb | Double-labeled (Fos + CTb) | % Double-labeled (Fos + CTb/Total CTb) |
|---|---|---|---|---|
| dmVMH | 121.7 ± 2.3 | 110.3 ± 6.8 | 43.5 ± 4.9 | 39.2 ± 2.2 |
| cDMH | 275.0 ± 30.5* | 20.3 ± 0.8* | 6.5 ± 0.6* | 32.2 ± 3.5 |
Values represent estimates of mean counts of cells ± SEM. Data were analyzed by ANOVA and differences between groups by ANOVA and Scheffe F-test. AHA, anterior hypothalamic area; cDMH, caudal dorsomedial hypothalamic nucleus; dmVHM, dorsomedial division of the ventromedial hypothalamic nucleus; PVH, paraventricular hypothalamic nucleus.
*P < 0.05 compared to VMH. n = 4.
RESULTS
Similar to previous findings, i.v. leptin induced Fos-IR in several nuclear groups including the VMH, DMH, and the parvicellular subdivisions of the PVH (14). CTb-IR was observed exclusively in the cytoplasmic compartment whereas Fos-IR was found in the nucleus. Only cells that had a clearly distinguishable black nucleus surrounded by brown granular cytoplasmic staining were considered double-labeled. Retrogradely labeled cells with an unstained nucleus or no nucleus in the plane of section were recorded as singly labeled neurons. To confirm these observations, some of the cases were stained by using immunofluoresence for CTb and peroxidase methods for Fos. This procedure resulted in black nuclei in cells containing Fos-IR and red fluorescence in retrogradely labeled cells under the rhodamine filter cube. Both techniques resulted in similar staining patterns and identified two populations of leptin-activated neurons projecting to distinct sites in the rat hypothalamus.
Distribution of Retrogradely Labeled Cells.
In five cases (R1175, R1176, R1177, R1178, and R1180) injections were centered in the PVH with very little leakage outside its boundaries. The distribution of cells containing CTb-IR (retrogradely labeled neurons) after these five injections were very similar to those previously reported (21, 25–27). Characteristically, retrogradely labeled cells were seen in cell groups including the subfornical organ, median preoptic nucleus, anteroventral periventricular nucleus, ventromedial preoptic area, dorsomedial hypothalamic nucleus, ventromedial hypothalamic nucleus, lateral parabrachial subnuclei, ventrolateral medulla, and nucleus of the solitary tract.
In five cases (R1118, R1119, R1121, R1122, and R1124), injections were centered in the rostral anterior hypothalamic area just lateral and ventral to the PVH excluding the PVH boundaries. These injections included the subparaventricular region defined by Watts and Swanson (28, 29). Unlike PVH injections, these cases did not have retrogradely labeled cells within the subfornical organ or in the ventrolateral medulla. Instead these cases contained large numbers of retrogradely labeled cells in the VMH, suprachiasmatic nucleus, ventral division of the lateral septum, bed nucleus of the stria terminalis, and subiculum.
Distribution of Fos-IR.
Intravenous injections of leptin (0.1 and 1.0 mg/kg) induced a dose-dependent pattern of Fos-IR as recently described (14). Briefly, after the 1.0 mg/kg dose substantial collections of neurons containing Fos-IR were present in the dorsomedial VMH, DMH, the ventral and dorsal parvicellular subdivisions of the PVH, the ventral premamillary nucleus, and the superior lateral parabrachial subnucleus. The Fos-IR in the DMH was characteristically observed in the caudal portion of the ventral subdivision defined by Swanson (30) or Paxinos and Watson (31). The lower dose of leptin (0.1 mg/kg) consistently induced Fos-IR in the caudal DMH and was seen in all animals. Other regions including the PVH, parabrachial nucleus, and ventral premamillary nucleus showed much more variability after the lower dose of leptin.
Distribution of Double-Labeled Cells.
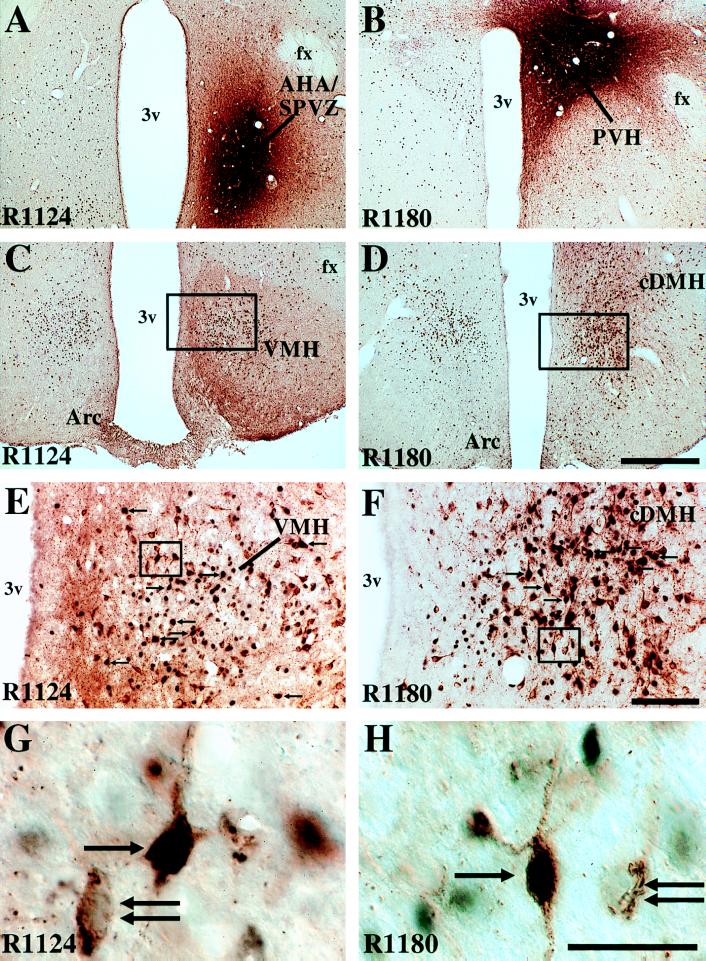
Intravenous leptin.Three cases (R1175, R1178, and R1180) had CTb injections centered in the PVH and had a typical distribution of Fos-IR after i.v. leptin (1.0 mg/kg). In these cases, many double-labeled neurons (containing Fos-IR and CTb-IR) were observed in one brain region, the caudal DMH (Fig. 1 D, F, and H; Table 1). These neurons were observed ventral to the compact formation of the DMH. Small numbers of double-labeled cells were found in the dorsomedial VMH (Table 1), although large numbers of singly labeled cells containing either Fos-IR or CTb-IR were found. A few double-labeled cells were observed in the paraventricular thalamic nucleus, lateral septum, rostral bed nucleus of the stria terminalis surrounding the anterior commissure, medial preoptic nucleus, suprachiasmatic nucleus, anterior perifornical area, anterior hypothalamic nucleus, ventral premammillary nucleus, medial amygdala, posterior ventrolateral periaqueductal gray matter, and medial pretectal area. In one of the cases (R1175) a few double-labeled cells were found in the lateral aspects of the arcuate nucleus of the hypothalamus. Within the brainstem, the typical retrograde labeling patterns from the PVH were seen in the ventrolateral medulla and nucleus of the solitary tract. However, no double-labeled cells were observed even though Fos-IR was found within the commissural subdivision of the nucleus of the solitary tract (32).
Figure 1.
Photomicrographs demonstrating the distribution of Fos-like immunoreactivity (black nuclei) and CTb-IR (retrogradely labeled cells; brown cytoplasm), 2 hr after i.v. leptin (1.0 mg/kg) and injections of CTb into the anterior hypothalamic area/subparaventricular zone (AHA/SPVZ; A, case R1124) or the paraventricular hypothalamic nucleus (PVH; B, case R1180). After injections of CTb into the AHA/SPVZ, many double-labeled cells are observed in the dorsomedial division of the ventromedial hypothalamic nucleus (VMH; C, E, and G). After injections of CTb into the PVH, many double-labeled cells are observed in the caudal dorsomedial hypothalamic nucleus (cDMH; D, F, and H). Double-labeled cells are denoted by arrows, and single-labeled CTb cells by double arrows. (A–D) Bar = 500 μm. (E and F) Bar = 100 μm. (G and H) Bar = 25 μm). Arc, arcuate nucleus of the hypothalamus; fx, fornix; 3v, third ventricle.
Injections into the subparaventricular zone demonstrated a distinct pattern of double-labeled cells. Four such cases (R1118, R1119, R1122, and R1124) had a typical distribution of Fos-IR after i.v. leptin (1.0 mg/kg) but very few retrogradely or double-labeled neurons were observed in the DMH. However, many double-labeled neurons were observed in the dorsomedial subdivision of the VMH (Fig. 1 C, E, and G; Table 2), including several neurons in the contralateral VMH. Several double-labeled cells also were observed in the suprachiasmatic nucleus, ventral lateral septum, parabrachial nucleus, and ventral premammillary nucleus.
Intravenous saline.
Saline injections resulted in a restricted distribution of double-labeled neurons. One case (R1177) included the PVH and demonstrated a typical retrograde pattern. A few double-labeled neurons were present in the lateral septum, parastrial nucleus, posterior hypothalamus, and paraventricular thalamic nucleus. Several double-labeled cells were found in the suprachiasmatic nucleus (bilaterally) and anterior hypothalamic nucleus. Many retrogradely labeled cells were seen in the DMH and VMH, but very few double-labeled cells were observed as i.v. saline did not result in Fos expression in either group. Two cases (R1117 and R1121) that received i.v. saline had CTb injection sites in the subparaventricular zone. Several double-labeled cells were observed in the suprachiasmatic nucleus and lateral septum. Scattered double-labeled cells were observed in the medial preoptic area, lateral hypothalamic area, retrochiasmatic area, pretectal area, and paraventricular thalamic nucleus. Many retrogradely labeled cells were seen in the VMH, but very few double-labeled cells were observed.
DISCUSSION
We have identified two leptin-activated cell groups innervating distinct targets in the rat brain. These leptin-sensitive pathways may represent anatomic substrates for leptin to affect the autonomic nervous system and to regulate the hypothalamo-pituitary axis. An i.v. dose of leptin of 1.0 mg/kg induced Fos-IR in normally fed, intact male rats. Although little has been reported regarding the effects of different doses of leptin in rats, the 1.0 mg/kg dose previously has been shown to blunt starvation-induced changes in ACTH, corticosterone, thyroxine, hypothalamic NPY mRNA, and estrus delay in mice (7).
Our results confirm previous findings that leptin activates nuclear groups in the rat brain thought to be involved in regulation of neuroendocrine function and energy balance (14, 33). However, as in all Fos studies it must be noted that absence of Fos-IR within an area does not exclude a particular nuclear group’s participation in a physiological response, because inhibitory responses may not be associated with Fos expression (34, 35). Moreover, firing rates of neurons may change sufficiently to produce relevant physiological responses without activating intracellular pathways that induce Fos-IR. It also should be noted that fewer than half of the Fos immunoreactive cells in the VMH or caudal DMH were double-labeled after i.v. leptin (see Tables 1 and 2). Undoubtedly, neurons within the DMH and VMH, as well as other cell groups whose participation may be critical to elicit responses to leptin (e.g., NPY neurons in the arcuate nucleus of the hypothalamus), are not accounted for in our experimental paradigms. Nonetheless, we can clearly identify two specific pathways that are activated by leptin and may be involved in producing its effects.
The PVH and Circulating Leptin.
The PVH may be critical in responding to circulating leptin. The PVH has been implicated in the regulation of feeding and body weight as lesions of the PVH induce hyperphagia and obesity (12). In addition, injections of neuromodulators such as NPY into the PVH have profound effects on feeding behavior (13). The PVH also contains neuroendocrine parvicellular neurons that project to the median eminence and regulate secretion of hormones, including thyroid stimulating hormone and ACTH (see refs. 10 and 11). Interestingly, recent studies have demonstrated that leptin regulates many neuroendocrine systems, including the hypothalamo-pituitary-adrenal axis (7, 8, 15) and the thyroid axis (7, 9). Leptin also influences the autonomic nervous system as leptin administration affects body temperature, insulin levels, and brown adipose tissue norepinephrine turnover (see refs. 2, 3, 36, and 37). As reported previously, i.v. leptin induced Fos-IR within the ventral and dorsal parvicellular subnuclei (14), which are a source of descending axons to autonomic preganglionic neurons within the medulla and spinal cord (10, 11). These projections may give leptin-activated neurons in the hypothalamus a direct input to the autonomic nervous system. Taken together these findings suggest that the PVH acts as a final common pathway to the autonomic nervous system and pituitary gland in response to circulating leptin.
The DMH and Circulating Leptin.
After CTb injections into the PVH the largest number of double-labeled cells in the brain after i.v. leptin were seen in the caudal DMH. van Dijk and colleagues (33) found a similar pattern of Fos-IR after injections of leptin into the third ventricle. The functional significance of the DMH in the response to circulating leptin is still obscure. However, the DMH has been implicated in the regulation of a wide range of physiological processes. For example, DMH lesions result in changes in pancreatic nerve activity (38) and stimulation of the DMH causes hyperglycemia and increases in plasma catecholamines (39). These findings suggest a role for this nucleus in regulating insulin secretion, presumably through interactions with pancreatic parasympathetic and sympathetic preganglionic neurons. Additionally, lesions of the DMH cause alterations in feeding behavior and have complex effects on long-term growth and body composition (40). Recent physiological studies also have demonstrated a role for the DMH in regulating cardiovascular parameters, including heart rate, blood pressure, and respiratory rate (41).
These observations indicate that the DMH is involved in regulating the hypothalamic outflow to the autonomic nervous systems. Consistent with this hypothesis, DMH efferents innervate the PVH (10, 42, 43). Specifically, the DMH targets the parvicellular PVH subdivisions that directly innervate parasympathetic and sympathetic preganglionic neurons in the medulla and spinal cord. Furthermore, leptin receptors are found in the DMH (15, 16). Interestingly, mRNA for the long splice form variant of the leptin receptor is found in the caudal DMH in a pattern very similar pattern to the cells that are leptin-activated and project to the PVH (unpublished observations). Additionally, increased expression of NPY mRNA is seen in a similar region of the mouse caudal DMH in two models of the agouti obesity syndrome (44). Although, the functional significance of the leptin-activated projection from the DMH to the PVH remains to be established, our results demonstrate a population of leptin-sensitive cells that reside in the DMH and directly innervate the PVH. Therefore, circulating leptin may induce its autonomic effects by engaging this projection.
The VMH and Circulating Leptin.
In contrast to the DMH, very few double-labeled cells were found in the VMH after i.v. leptin and CTb injections into the PVH. However, when the CTb injections were centered in the subparaventricular zone, many double-labeled cells were seen in the dorsomedial VMH. The VMH has been reported to regulate feeding and metabolism as lesions of the VMH and surrounding fiber pathways result in hyperphagia, obesity, diabetes, and elevated levels of corticosteroids (12, 45, 46). Interestingly, the subparaventricular zone receives a very dense innervation from the suprachiasmatic nucleus, the circadian pacemaker of the mammalian brain (28, 47). The anatomic makeup of the region (inputs from the VMH and the suprachiasmatic nucleus) suggests that the subparaventricular zone is essential in integrating nutritional and circadian information into endocrine responses (28, 47–49).
Food restriction in the dark period results in changes in the circadian rhythms of corticosterone illustrating a link between nutritional signals and corticosterone secretion (50–53). Lesions of the VMH, but not the suprachiasmatic nucleus, abolish this change in circadian corticosterone levels in food restricted rats (50, 51). Additionally, lesions or inhibition of the VMH alter diurnal corticosterone rhythms (54–56). These findings suggest that the VMH may be critical in linking nutritional status into circadian neuroendocrine responses. Interestingly, an inverse relationship of plasma leptin and corticosteroid levels exist in mice and humans (7, 57). Food restriction reverses the phase of both the corticosterone and leptin circadian rhythms such that they maintain their inverse relationship (R.S.A. and J.S.F., unpublished observations). Finally, the dorsomedial VMH contains leptin receptors (15, 16) and is activated by i.v. leptin administration (14). Our findings demonstrate a population of leptin-activated neurons in the VMH that innervate the subparaventricular zone, providing an anatomic substrate by which leptin may regulate the secretion of hormones such as corticosterone across the circadian cycle. Moreover, the subparaventricular zone projects densely to the DMH and not to the PVH (49), so the effects of leptin-activated neurons in the VMH that innervate the subparaventricular zone ultimately may be mediated by projections of the DMH to the PVH (see Fig. 2).
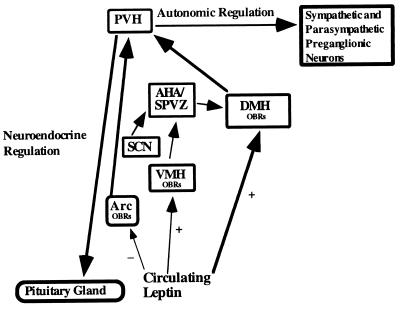
Figure 2.
A schematic drawing of the rat brain in sagittal section demonstrating a neuroanatomical model of leptin action. Circulating leptin acts on cell groups containing leptin receptors (OBRs) within the arcuate (Arc), dorsomedial (DMH), and ventromedial (VMH) hypothalamic nuclei. Ultimately, activation of the autonomic and neuroendocrine components of the paraventricular hypothalamic nucleus (PVH) is responsible for the physiological effects of leptin. We hypothesize that intravenous leptin inhibits Arc neuropeptide Y (NPY) neurons that innervate the PVH. Simultaneously, circulating leptin activates neurons in the DMH whose efferent projections converge on PVH neurons that innervate sympathetic and parasympathetic preganglionic neurons in the medulla and spinal cord. Additionally, circulating leptin activates neurons in the VMH whose efferent projections converge on the anterior hypothalamic area/subparaventricular zone (AHA/SPVZ). The AHA/SPVZ also receives dense innervation from the suprachiasmatic nucleus (SCN). Engagement of these parallel pathways is responsible for the manifestation of the physiological effects of circulating leptin.
Multiple Leptin-Sensitive Hypothalamic Pathways.
Recent studies have focused on NPY neurons in the arcuate nucleus as targets of leptin. Expression of NPY mRNA in the arcuate nucleus is increased after fasting and in ob/ob mice, and leptin repletion normalizes this overexpression (2, 15). In addition, leptin receptors colocalize with NPY mRNA in arcuate neurons (58). Recently, Palmiter and colleagues (59) found that ob/ob mice that also lacked the NPY gene (double knockouts) had an attenuated phenotype when compared with the ob/ob mutation alone. However, NPY neurons are not the only target of circulating leptin. For example, although lack of NPY did attenuate the ob/ob phenotype, these mice were still markedly overweight and had neuroendocrine abnormalities. Additionally, NPY single knockout mice have normal feeding behavior, body weight, neuroendocrine status, responses to exogenous leptin, and responses to starvation (60, 61).
Leptin receptors also are found in several other hypothalamic nuclear groups, including the DMH, VMH and ventral premamillary nuclei (15, 16), all of which contain Fos-IR after i.v. leptin administration (14). The ventral premammillary nucleus is thought to play a role in regulating reproductive physiology and behavior (62, 63). Recent studies have suggested a role for leptin in regulating the reproductive axis (64, 65) and the onset of puberty (66–68). We found very few double-labeled cells in the ventral premammillary nucleus after CTb injections into either the PVH or subparaventricular zone. These leptin-activated cells, therefore, project to other hypothalamic sites and represent yet another leptin sensitive pathway that may be involved in regulating the effects of leptin on the reproductive axis. We hypothesize that multiple pathways are simultaneously engaged by circulating leptin, and these pathways acting in concert underlie the multiple central nervous system responses to circulating leptin. Our results suggest that the VMH and DMH projections to the subparaventricular zone and the PVH, respectively, may be components of this circuitry.
Acknowledgments
We thank Quan Ha, Minh Ha, and Joseph Kelly for expert technical assistance and Eli Lilly for providing the recombinant leptin. This work was supported by U.S. Public Health Service Grants NS33987 and DKR3728082, American Heart Association Grant-In Aid 9413110 and Scientist Development Grant 9630014, and a National Research Service Award to J.K.E. (MH10832) and research support from Eli Lilly (J.K.E. and J.S.F.)
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: PVH, paraventricular hypothalamic nucleus; VMH, ventromedial hypothalamic nucleus; DMH, dorsomedial hypothalamic nucleus; NPY, neuropeptide Y; CTb, cholera toxin-b; Fos-IR, Fos-like immunoreactivity; CTb-IR, CTb-like immunoreactivity.
References
- 1.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman J M. Nature (London) 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 2.Spiegelman B M, Flier J S. Cell. 1996;87:377–389. doi: 10.1016/s0092-8674(00)81359-8. [DOI] [PubMed] [Google Scholar]
- 3.Caro J F, Sinha M K, Kolaczynski J W, Zhang P L, Considine R V. Diabetes. 1996;45:1455–1462. doi: 10.2337/diab.45.11.1455. [DOI] [PubMed] [Google Scholar]
- 4.Campfield L A, Smith F J, Guisez Y, Devos R, Burn P. Science. 1995;269:546–549. doi: 10.1126/science.7624778. [DOI] [PubMed] [Google Scholar]
- 5.Halaas J L, Gajiwala K S, Maffei M, Cohen S L, Chait B T, Rabinowitz D, Lallone R L, Burley S K, Friedman J M. Science. 1995;269:543–546. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- 6.Pelleymounter M A, Cullen M J, Baker M B, Hecht R, Winters D, Boone T, Collins F. Science. 1995;269:540–543. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- 7.Ahima R S, Prabakaran D, Mantzoros C, Qu D, Lowell B, Maratos-Flier E, Flier J S. Nature (London) 1996;382:250–252. doi: 10.1038/382250a0. [DOI] [PubMed] [Google Scholar]
- 8.Heiman M L, Ahima R S, Craft L S, Schoner B, Stephens T W, Flier J S. Endocrinology. 1997;138:3859–3863. doi: 10.1210/endo.138.9.5366. [DOI] [PubMed] [Google Scholar]
- 9.Legradi G, Emerson C H, Ahima R S, Flier J S, Lechan R M. Endocrinology. 1997;138:2569–2576. doi: 10.1210/endo.138.6.5209. [DOI] [PubMed] [Google Scholar]
- 10.Swanson L W, Sawchenko P E. Annu Rev Neurosci. 1983;6:269–324. doi: 10.1146/annurev.ne.06.030183.001413. [DOI] [PubMed] [Google Scholar]
- 11.Saper C B. In: Central Autonomic System. Paxinos G, editor. San Diego: Academic; 1995. pp. 107–135. [Google Scholar]
- 12.Gold R M. Science. 1973;182:488–490. doi: 10.1126/science.182.4111.488. [DOI] [PubMed] [Google Scholar]
- 13.Leibowitz S F. Brain Res Bull. 1991;27:333–337. doi: 10.1016/0361-9230(91)90121-y. [DOI] [PubMed] [Google Scholar]
- 14.Elmquist J K, Ahima R S, Maratos-Flier E, Flier J S, Saper C B. Endocrinology. 1997;138:839–842. doi: 10.1210/endo.138.2.5033. [DOI] [PubMed] [Google Scholar]
- 15.Schwartz M W, Seeley R J, Campfield L A, Burn P, Baskin D G. J Clin Invest. 1996;98:1101–1106. doi: 10.1172/JCI118891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mercer J G, Hoggard N, Williams L M, Lawrence C B, Hannah L T, Trayhurn P. FEBS Lett. 1996;387:113–116. doi: 10.1016/0014-5793(96)00473-5. [DOI] [PubMed] [Google Scholar]
- 17.Fei H, Okano H J, Li C, Lee G H, Zhao C, Darnell R, Friedman J M. Proc Natl Acad Sci USA. 1997;94:7001–7005. doi: 10.1073/pnas.94.13.7001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoffman G E, Smith M S, Verbalis J G. Front Neuroendocrinol. 1993;14:173–213. doi: 10.1006/frne.1993.1006. [DOI] [PubMed] [Google Scholar]
- 19.Sagar S M, Sharp F R, Curran T. Science. 1988;240:1328–1331. doi: 10.1126/science.3131879. [DOI] [PubMed] [Google Scholar]
- 20.Elmquist J K, Scammell T E, Jacobson C D, Saper C B. J Comp Neurol. 1996;371:85–103. doi: 10.1002/(SICI)1096-9861(19960715)371:1<85::AID-CNE5>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 21.Elmquist J K, Saper C B. J Comp Neurol. 1996;374:315–331. doi: 10.1002/(SICI)1096-9861(19961021)374:3<315::AID-CNE1>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 22.Sherin J E, Shiromani P J, McCarley R W, Saper C B. Science. 1996;271:216–219. doi: 10.1126/science.271.5246.216. [DOI] [PubMed] [Google Scholar]
- 23.Abercrombie M. Anat Rec. 1946;94:239–247. doi: 10.1002/ar.1090940210. [DOI] [PubMed] [Google Scholar]
- 24.Coggeshall R E, Lekan H A. J Comp Neurol. 1996;364:6–15. doi: 10.1002/(SICI)1096-9861(19960101)364:1<6::AID-CNE2>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 25.Moga M M, Saper C B. J Comp Neurol. 1994;346:137–150. doi: 10.1002/cne.903460110. [DOI] [PubMed] [Google Scholar]
- 26.Sawchenko P E, Swanson L W. J Comp Neurol. 1983;218:121–144. doi: 10.1002/cne.902180202. [DOI] [PubMed] [Google Scholar]
- 27.McKellar S, Loewy A D. Brain Res. 1981;217:351–357. doi: 10.1016/0006-8993(81)90010-x. [DOI] [PubMed] [Google Scholar]
- 28.Watts A G, Swanson L W, Sanchez-Watts G. J Comp Neurol. 1987;258:204–229. doi: 10.1002/cne.902580204. [DOI] [PubMed] [Google Scholar]
- 29.Watts A G. Front Neuroendocrinol. 1996;17:281–326. doi: 10.1006/frne.1996.0008. [DOI] [PubMed] [Google Scholar]
- 30.Swanson L W. Brain Maps: Structure of the Rat Brain. Amsterdam: Elsevier; 1992. [Google Scholar]
- 31.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic; 1997. , compact 3rd Ed. [DOI] [PubMed] [Google Scholar]
- 32.Herbert H, Moga M M, Saper C B. J Comp Neurol. 1990;293:540–580. doi: 10.1002/cne.902930404. [DOI] [PubMed] [Google Scholar]
- 33.van Dijk G, Thiele T E, Donahey J C, Campfield L A, Smith F J, Burn P, Bernstein I L, Woods S C, Seeley R J. Am J Physiol. 1996;271:R1096–1100. doi: 10.1152/ajpregu.1996.271.4.R1096. [DOI] [PubMed] [Google Scholar]
- 34.Chan R K, Brown E R, Ericsson A, Kovacs K J, Sawchenko P E. J Neurosci. 1993;13:5126–5138. doi: 10.1523/JNEUROSCI.13-12-05126.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kovacs K J, Sawchenko P E. Proc Natl Acad Sci USA. 1993;90:7681–7685. doi: 10.1073/pnas.90.16.7681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haynes W G, Morgan D A, Walsh S A, Mark A L, Sivitz W I. J Clin Invest. 1997;100:270–278. doi: 10.1172/JCI119532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Collins S, Kuhn C M, Petro A E, Swick A G, Chrunyk B A, Surwit R S. Nature (London) 1996;380:677. doi: 10.1038/380677a0. [DOI] [PubMed] [Google Scholar]
- 38.Yoshimatsu H, Niijima A, Oomura Y, Yamabe K, Katafuchi T. Brain Res. 1984;303:147–152. doi: 10.1016/0006-8993(84)90222-1. [DOI] [PubMed] [Google Scholar]
- 39.Frohman L A, Bernardis L L. Am J Physiol. 1971;221:1596–1603. doi: 10.1152/ajplegacy.1971.221.6.1596. [DOI] [PubMed] [Google Scholar]
- 40.Bernardis L L, Bellinger L L. Brain Res. 1987;434:321–381. doi: 10.1016/0165-0173(87)90004-x. [DOI] [PubMed] [Google Scholar]
- 41.DiMicco J A, Stotz-Potter E H, Monroe A J, Morin S M. Clin Exp Pharmacol Physiol. 1996;23:171–176. doi: 10.1111/j.1440-1681.1996.tb02592.x. [DOI] [PubMed] [Google Scholar]
- 42.Thompson R H, Canteras N S, Swanson L W. J Comp Neurol. 1996;376:143–173. doi: 10.1002/(SICI)1096-9861(19961202)376:1<143::AID-CNE9>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 43.ter Horst G J, Luiten P G. Brain Res Bull. 1986;16:231–248. doi: 10.1016/0361-9230(86)90038-9. [DOI] [PubMed] [Google Scholar]
- 44.Kesterson R A, Huszar D, Lynch C A, Simerly R B, Cone R D. Mol Endocrinol. 1997;11:639–637. doi: 10.1210/mend.11.5.9921. [DOI] [PubMed] [Google Scholar]
- 45.Dallman M F. Am J Physiol. 1984;246:R1–12. doi: 10.1152/ajpregu.1984.246.1.R1. [DOI] [PubMed] [Google Scholar]
- 46.Keesey R E, Powley T L. Annu Rev Psychol. 1986;37:109–133. doi: 10.1146/annurev.ps.37.020186.000545. [DOI] [PubMed] [Google Scholar]
- 47.Watts A G, Swanson L W. J Comp Neurol. 1987;258:230–252. doi: 10.1002/cne.902580205. [DOI] [PubMed] [Google Scholar]
- 48.Canteras N S, Simerly R B, Swanson L W. J Comp Neurol. 1994;348:41–79. doi: 10.1002/cne.903480103. [DOI] [PubMed] [Google Scholar]
- 49.Watts A G. In: The Suprachiasmatic Nucleus: The Mind’s Clock. Klein D, Moore R Y, Reppert S M, editors. London: Oxford Univ. Press; 1991. pp. 75–104. [Google Scholar]
- 50.Krieger D T. Endocrinology. 1980;106:649–654. doi: 10.1210/endo-106-3-649. [DOI] [PubMed] [Google Scholar]
- 51.Krieger D T, Hauser H, Krey L C. Science. 1977;197:398–399. doi: 10.1126/science.877566. [DOI] [PubMed] [Google Scholar]
- 52.Krieger D T. Endocrinology. 1974;95:1195–1201. doi: 10.1210/endo-95-5-1195. [DOI] [PubMed] [Google Scholar]
- 53.Moberg G P, Bellinger L L, Mendel V E. Neuroendocrinology. 1975;19:160–169. doi: 10.1159/000122436. [DOI] [PubMed] [Google Scholar]
- 54.Bellinger L L, Bernardis L L, Mendel V E. Neuroendocrinology. 1976;22:216–225. doi: 10.1159/000122628. [DOI] [PubMed] [Google Scholar]
- 55.Choi S, Horsley C, Aguila S, Dallman M F. J Neurosci. 1996;16:8170–8180. doi: 10.1523/JNEUROSCI.16-24-08170.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Suemaru S, Darlington D N, Akana S F, Cascio C S, Dallman M F. Neuroendocrinology. 1995;61:453–463. doi: 10.1159/000126868. [DOI] [PubMed] [Google Scholar]
- 57.Licinio J, Mantzoros C, Negrao A B, Cizza G, Wong M L, Bongiorno P B, Chrousos G P, Karp B, Allen C, Flier J S, Gold P W. Nat Med. 1997;3:575–579. doi: 10.1038/nm0597-575. [DOI] [PubMed] [Google Scholar]
- 58.Mercer J G, Hoggard N, Williams L M, Lawrence C B, Hannah L T, Morgan P J, Trayhurn P. J Neuroendocrinol. 1996;8:733–735. doi: 10.1046/j.1365-2826.1996.05161.x. [DOI] [PubMed] [Google Scholar]
- 59.Erickson J C, Hollopeter G, Palmiter R D. Science. 1996;274:1704–1707. doi: 10.1126/science.274.5293.1704. [DOI] [PubMed] [Google Scholar]
- 60.Erickson J C, Clegg K E, Palmiter R D. Nature (London) 1996;381:415–421. doi: 10.1038/381415a0. [DOI] [PubMed] [Google Scholar]
- 61.Erickson J C, Ahima R S, Hollopeter G, Flier J S, Palmiter R D. Regul Pept. 1997;70:199–102. doi: 10.1016/s0167-0115(97)01007-0. [DOI] [PubMed] [Google Scholar]
- 62.Canteras N S, Simerly R B, Swanson L W. J Comp Neurol. 1992;324:195–212. doi: 10.1002/cne.903240205. [DOI] [PubMed] [Google Scholar]
- 63.Simerly R B. In: Anatomical Substrates of Hypothalamic Integration. Paxinos G, editor. San Diego: Academic; 1995. pp. 353–376. [Google Scholar]
- 64.Yu W H, Kimura M, Walczewska A, Karanth S, McCann S M. Proc Natl Acad Sci USA. 1997;94:1023–1028. doi: 10.1073/pnas.94.3.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Barash I A, Cheung C C, Weigle D S, Ren H, Kabigting E B, Kuijper J L, Clifton D K, Steiner R A. Endocrinology. 1996;137:3144–3147. doi: 10.1210/endo.137.7.8770941. [DOI] [PubMed] [Google Scholar]
- 66.Ahima R S, Dushay J, Flier S N, Prabakaran D, Flier J S. J Clin Invest. 1997;99:391–395. doi: 10.1172/JCI119172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chehab F F, Mounzih K, Lu R, Lim M E. Science. 1997;275:88–90. doi: 10.1126/science.275.5296.88. [DOI] [PubMed] [Google Scholar]
- 68.Cheung C C, Thornton J E, Kuijper J L, Weigle D S, Clifton D K, Steiner R A. Endocrinology. 1997;138:855–858. doi: 10.1210/endo.138.2.5054. [DOI] [PubMed] [Google Scholar]