Abstract
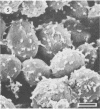
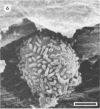
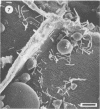
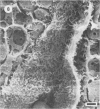
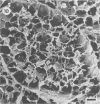
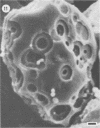
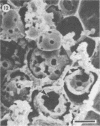
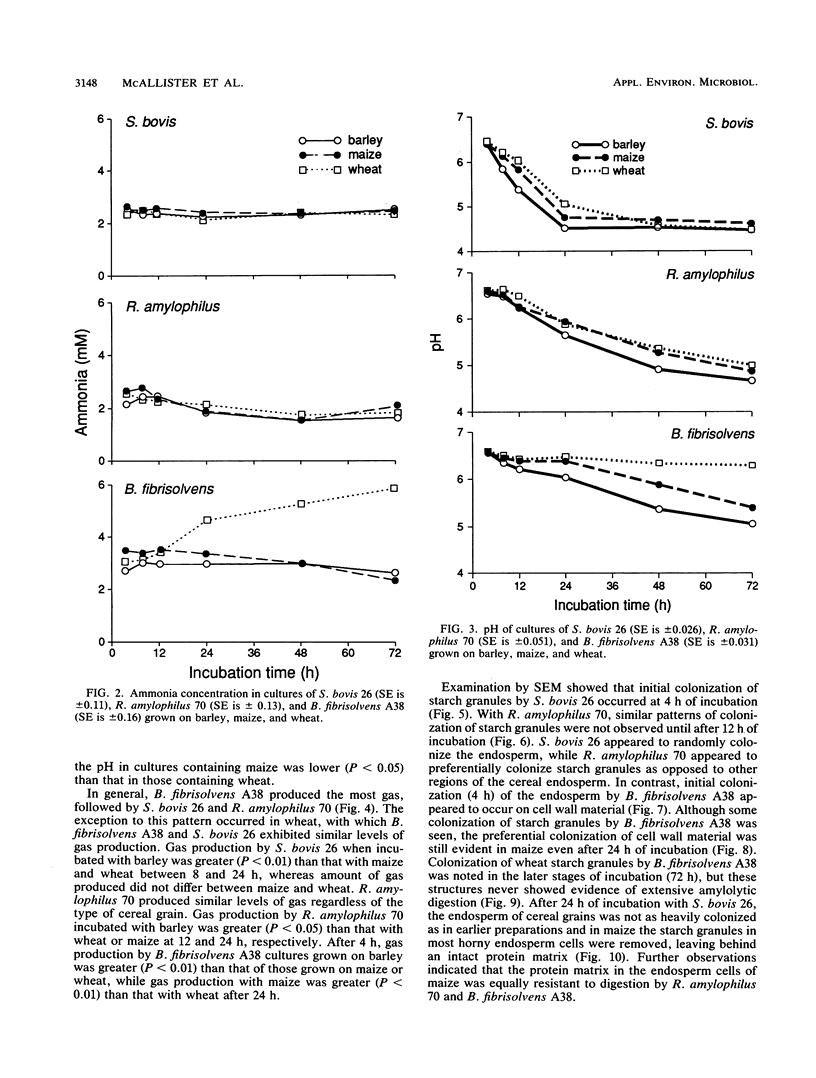
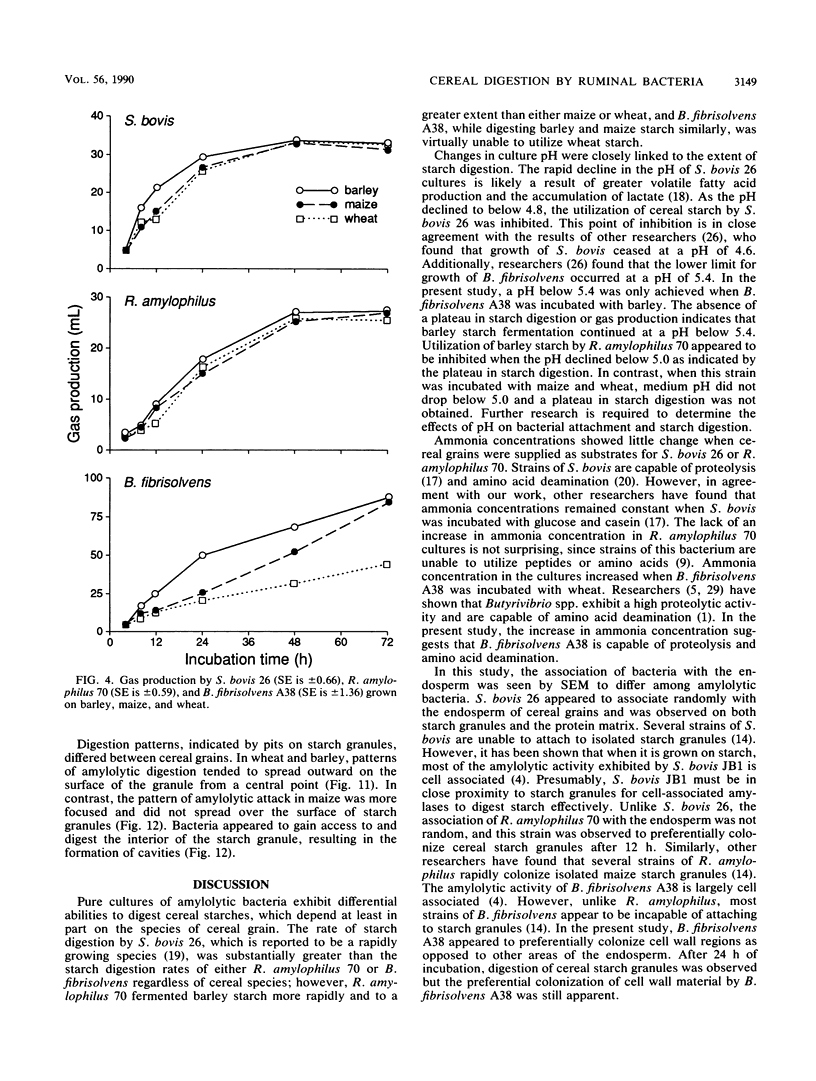
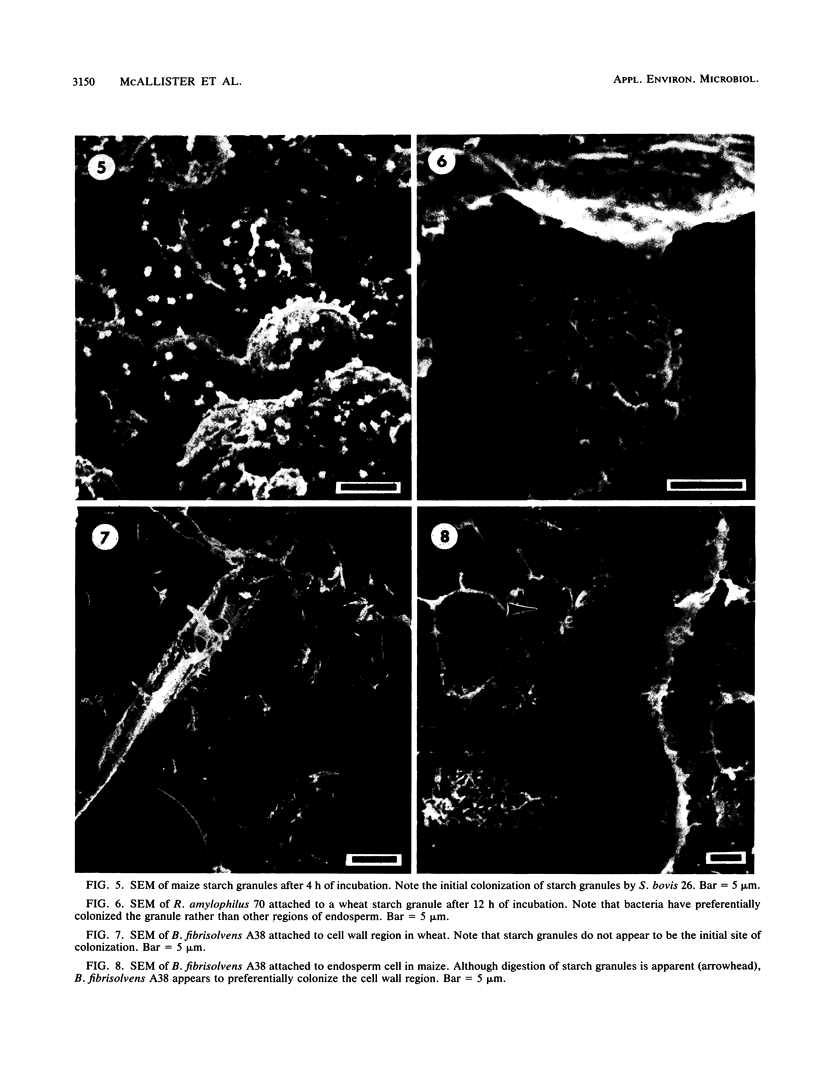
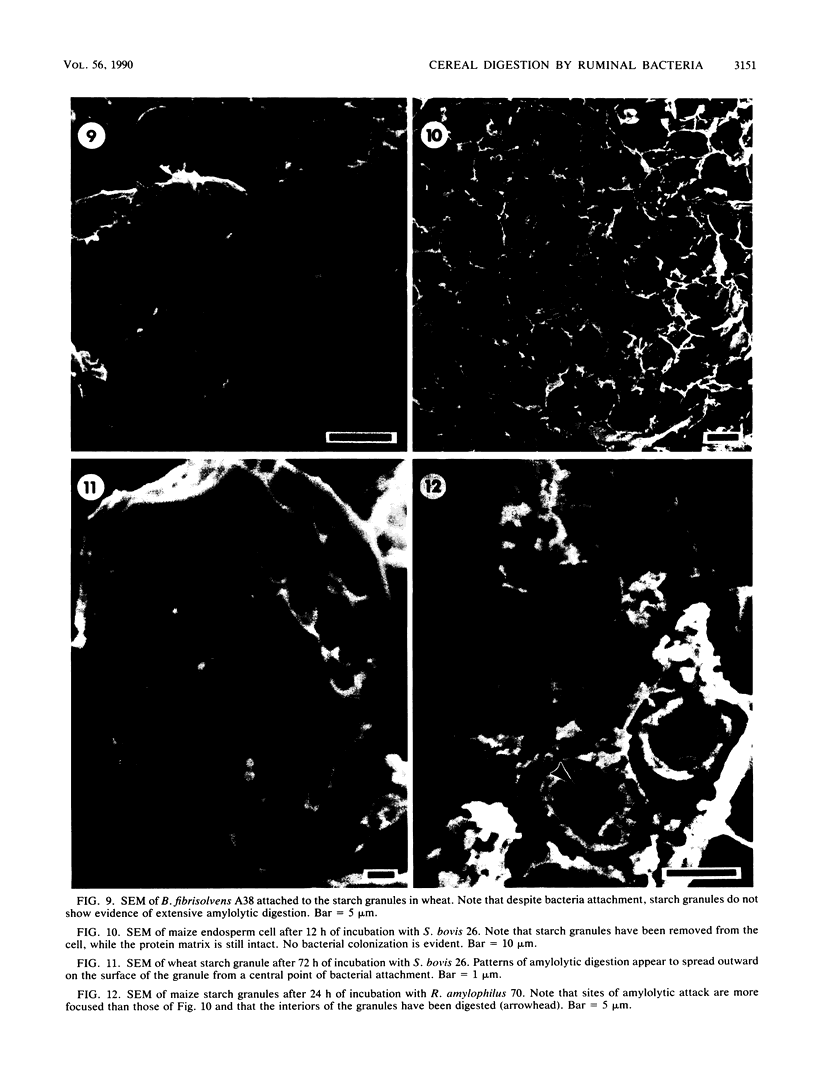
Differences in the digestion of barley, maize, and wheat by three major ruminal starch-digesting bacterial species, Streptococcus bovis 26, Ruminobacter amylophilus 50, and Butyrivibrio fibrisolvens A38, were characterized. The rate of starch digestion in all cereal species was greater for S. bovis 26 than for R. amylophilus 50 or B. fibrisolvens A38. Starch digestion by S. bovis 26 was greater in wheat than in barley or maize, whereas starch digestion by R. amylophilus 50 was greater in barley than in maize or wheat. B. fibrisolvens A38 digested the starch in barley and maize to a similar extent but was virtually unable to digest the starch in wheat. The higher ammonia concentration in cultures of B. fibrisolvens A38 when grown on wheat than when grown on barley or maize suggests that B. fibrisolvens A38 utilized wheat protein rather than starch. Scanning electron microscopy revealed that B. fibrisolvens A38 initially colonized cell wall material, while S. bovis 26 randomly colonized the endosperm and R. amylophilus 50 preferentially colonized starch granules. There was subsequent colonization but only superficial digestion of wheat starch granules by B. fibrisolvens A38. Variation in the association between starch and protein within the endosperm of cereal grains contributes to the differential effectiveness with which amylolytic species can utilize cereal starch.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bladen H. A., Bryant M. P., Doetsch R. N. A Study of Bacterial Species from the Rumen Which Produce Ammonia from Protein Hydrolyzate. Appl Microbiol. 1961 Mar;9(2):175–180. doi: 10.1128/am.9.2.175-180.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotta M. A. Amylolytic activity of selected species of ruminal bacteria. Appl Environ Microbiol. 1988 Mar;54(3):772–776. doi: 10.1128/aem.54.3.772-776.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNGATE R. E. The anaerobic mesophilic cellulolytic bacteria. Bacteriol Rev. 1950 Mar;14(1):1–49. doi: 10.1128/br.14.1.1-49.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobson P. N., McDougall E. I., Summers R. The nitrogen sources of Bacteroides amylophilus. J Gen Microbiol. 1968 Mar;50(3 Suppl):i–i. [PubMed] [Google Scholar]
- McWethy S. J., Hartman P. A. Purification and some properties of an extracellular alpha-amylase from Bacteroides amylophilus. J Bacteriol. 1977 Mar;129(3):1537–1544. doi: 10.1128/jb.129.3.1537-1544.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney L. W., Pflugfelder R. L. Factors affecting starch digestibility with special emphasis on sorghum and corn. J Anim Sci. 1986 Nov;63(5):1607–1623. doi: 10.2527/jas1986.6351607x. [DOI] [PubMed] [Google Scholar]
- Russell J. B., Bottje W. G., Cotta M. A. Degradation of protein by mixed cultures of rumen bacteria: identification of Streptococcus bovis as an actively proteolytic rumen bacterium. J Anim Sci. 1981 Jul;53(1):242–252. doi: 10.2527/jas1981.531242x. [DOI] [PubMed] [Google Scholar]
- Russell J. B., Dombrowski D. B. Effect of pH on the efficiency of growth by pure cultures of rumen bacteria in continuous culture. Appl Environ Microbiol. 1980 Mar;39(3):604–610. doi: 10.1128/aem.39.3.604-610.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J. B., Robinson P. H. Compositions and characteristics of strains of Streptococcus bovis. J Dairy Sci. 1984 Jul;67(7):1525–1531. doi: 10.3168/jds.S0022-0302(84)81471-X. [DOI] [PubMed] [Google Scholar]
- SCOTT H. W., DEHORITY B. A. VITAMIN REQUIREMENTS OF SEVERAL CELLULOLYTIC RUMEN BACTERIA. J Bacteriol. 1965 May;89:1169–1175. doi: 10.1128/jb.89.5.1169-1175.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheifinger C., Russell N., Chalupa W. Degradation of amino acids by pure cultures of rumen bacteria. J Anim Sci. 1976 Oct;43(4):821–827. doi: 10.2527/jas1976.434821x. [DOI] [PubMed] [Google Scholar]
- Slyter L. L. Influence of acidosis on rumen function. J Anim Sci. 1976 Oct;43(4):910–929. doi: 10.2527/jas1976.434910x. [DOI] [PubMed] [Google Scholar]
- Therion J. J., Kistner A., Kornelius J. H. Effect of pH on growth rates of rumen amylolytic and lactilytic bacteria. Appl Environ Microbiol. 1982 Aug;44(2):428–434. doi: 10.1128/aem.44.2.428-434.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALKER G. J. THE CELL-BOUND ALPHA-AMYLASES OF STREPTOCOCCUS BOVIS. Biochem J. 1965 Feb;94:289–298. doi: 10.1042/bj0940289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker G. J., Hope P. M. Degradation of starch granules by some amylolytic bacteria from the rumen of sheep. Biochem J. 1964 Feb;90(2):398–408. doi: 10.1042/bj0900398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace R. J., Broderick G. A., Brammall M. L. Protein degradation by ruminal microorganisms from sheep fed dietary supplements of urea, casein, or albumin. Appl Environ Microbiol. 1987 Apr;53(4):751–753. doi: 10.1128/aem.53.4.751-753.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]