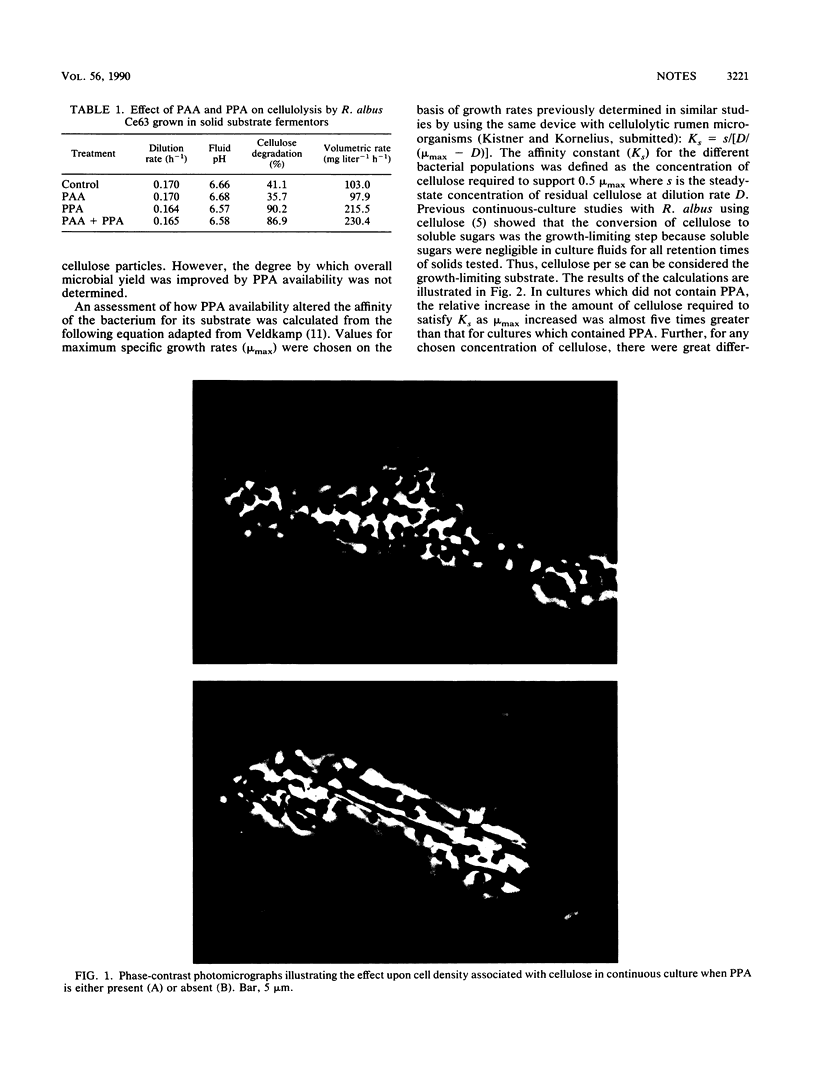
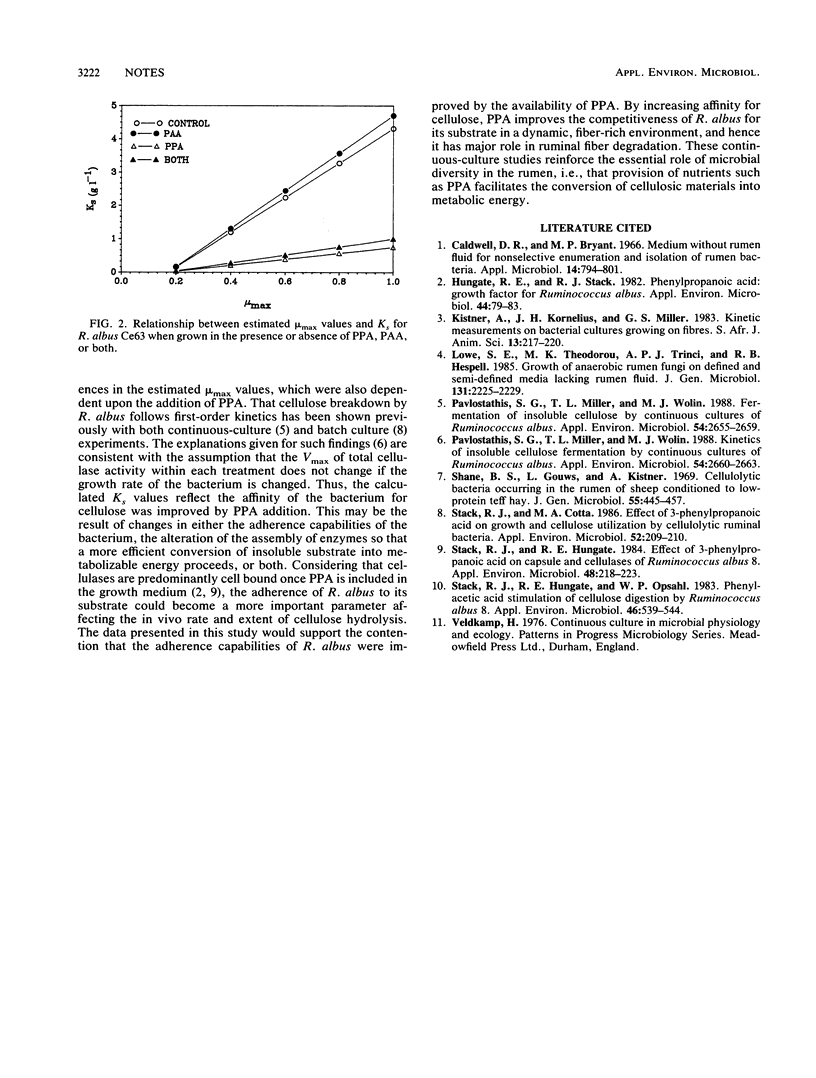
Abstract
A continuous-culture device, adapted for use with solid substrates, was used to evaluate the effects of 3-phenylpropanoic acid (PPA) upon the ability of the South African strain Ruminococcus albus Ce63 to ferment cellulose. Steady states of fermentation were established with a dilution rate of 0.17 h−1, and the extent and volumetric rates of cellulose fermentation were determined over four consecutive days. When the growth medium contained no additions (control), 25 μM phenylacetate alone, 25 μM PPA alone, or 25 μM each of phenylacetate and PPA, the extent of cellulose hydrolysis was determined to be 41.1, 35.7, 90.2, and 86.9%, respectively, and the volumetric rate of cellulose hydrolysis was 103.0, 97.9, 215.5, and 230.4 mg liter−1 h−1, respectively. To evaluate the effect of PPA availability on affinity for cellulose, the values for dilution rate and extent of cellulose hydrolysis were used in combination with values for maximum specific growth rate determined from previous studies of growth rates and kinetics of cellulose hydrolysis. The findings support the contention that PPA maintains a competitive advantage for R. albus when grown in a dynamic, fiber-rich environment.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Caldwell D. R., Bryant M. P. Medium without rumen fluid for nonselective enumeration and isolation of rumen bacteria. Appl Microbiol. 1966 Sep;14(5):794–801. doi: 10.1128/am.14.5.794-801.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hungate R. E., Stack R. J. Phenylpropanoic Acid: Growth Factor for Ruminococcus albus. Appl Environ Microbiol. 1982 Jul;44(1):79–83. doi: 10.1128/aem.44.1.79-83.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlostathis S. G., Miller T. L., Wolin M. J. Fermentation of Insoluble Cellulose by Continuous Cultures of Ruminococcus albus. Appl Environ Microbiol. 1988 Nov;54(11):2655–2659. doi: 10.1128/aem.54.11.2655-2659.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlostathis S. G., Miller T. L., Wolin M. J. Kinetics of Insoluble Cellulose Fermentation by Continuous Cultures of Ruminococcus albus. Appl Environ Microbiol. 1988 Nov;54(11):2660–2663. doi: 10.1128/aem.54.11.2660-2663.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shane B. S., Gouws L., Kistner A. Cellulolytic bacteria occurring in the rumen of sheep conditioned to low-protein teff hay. J Gen Microbiol. 1969 Mar;55(3):445–457. doi: 10.1099/00221287-55-3-445. [DOI] [PubMed] [Google Scholar]
- Stack R. J., Cotta M. A. Effect of 3-phenylpropanoic Acid on growth of and cellulose utilization by cellulolytic ruminal bacteria. Appl Environ Microbiol. 1986 Jul;52(1):209–210. doi: 10.1128/aem.52.1.209-210.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stack R. J., Hungate R. E. Effect of 3-Phenylpropanoic Acid on Capsule and Cellulases of Ruminococcus albus 8. Appl Environ Microbiol. 1984 Jul;48(1):218–223. doi: 10.1128/aem.48.1.218-223.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stack R. J., Hungate R. E., Opsahl W. P. Phenylacetic acid stimulation of cellulose digestion by Ruminococcus albus 8. Appl Environ Microbiol. 1983 Sep;46(3):539–544. doi: 10.1128/aem.46.3.539-544.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]