Abstract
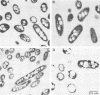
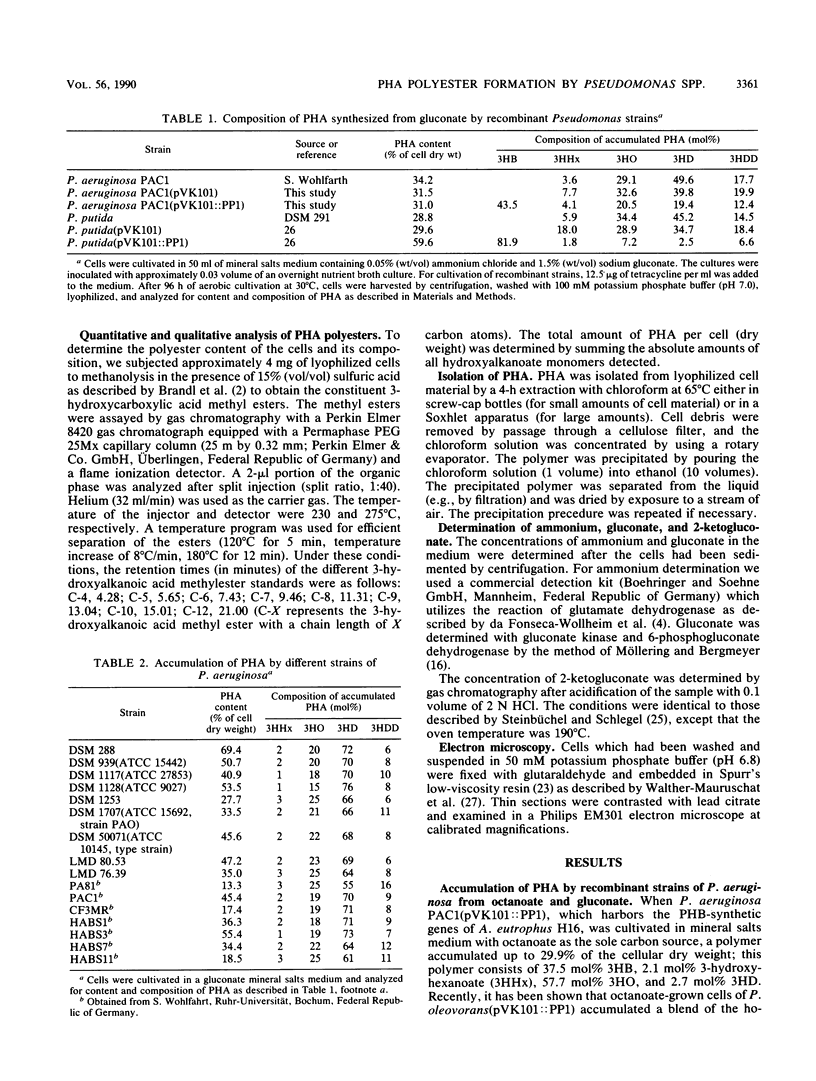
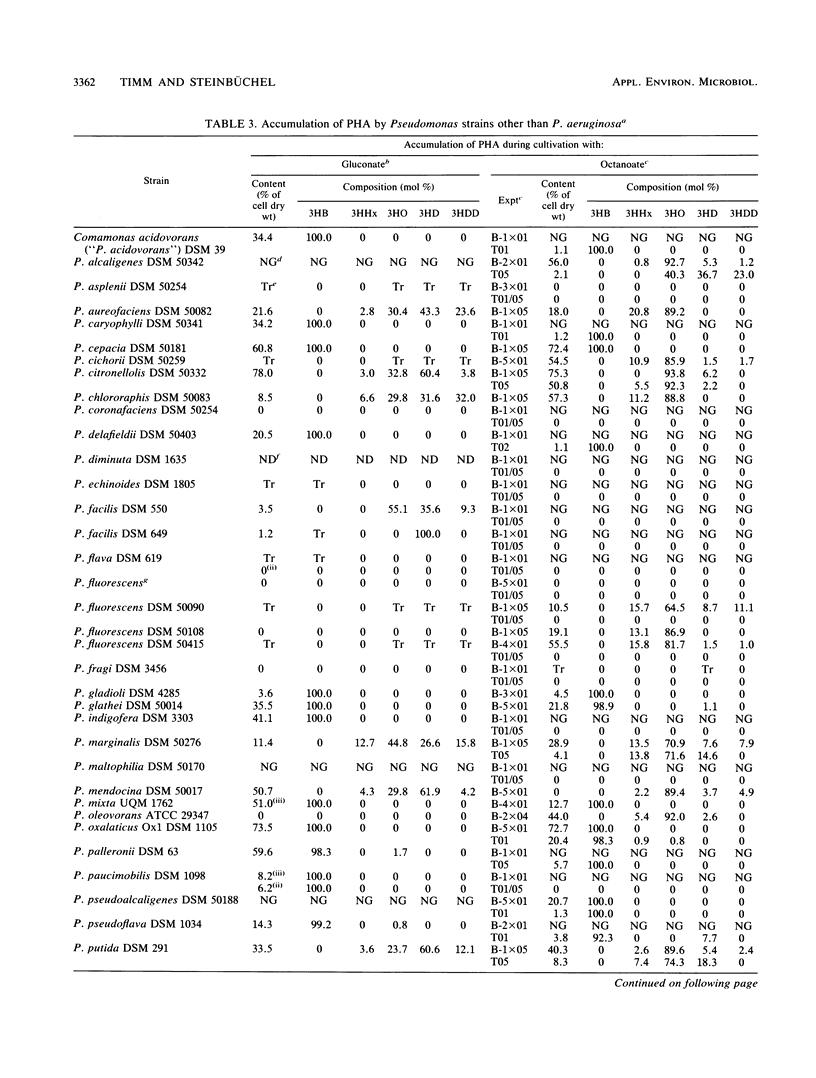
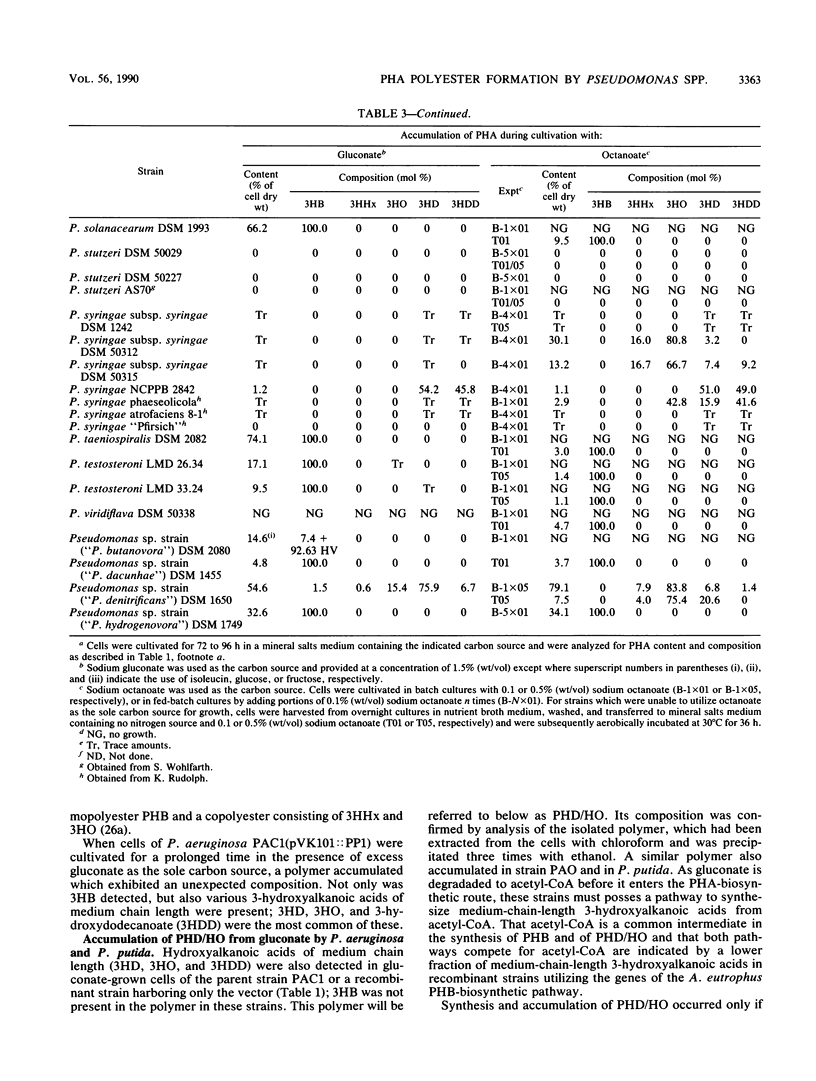
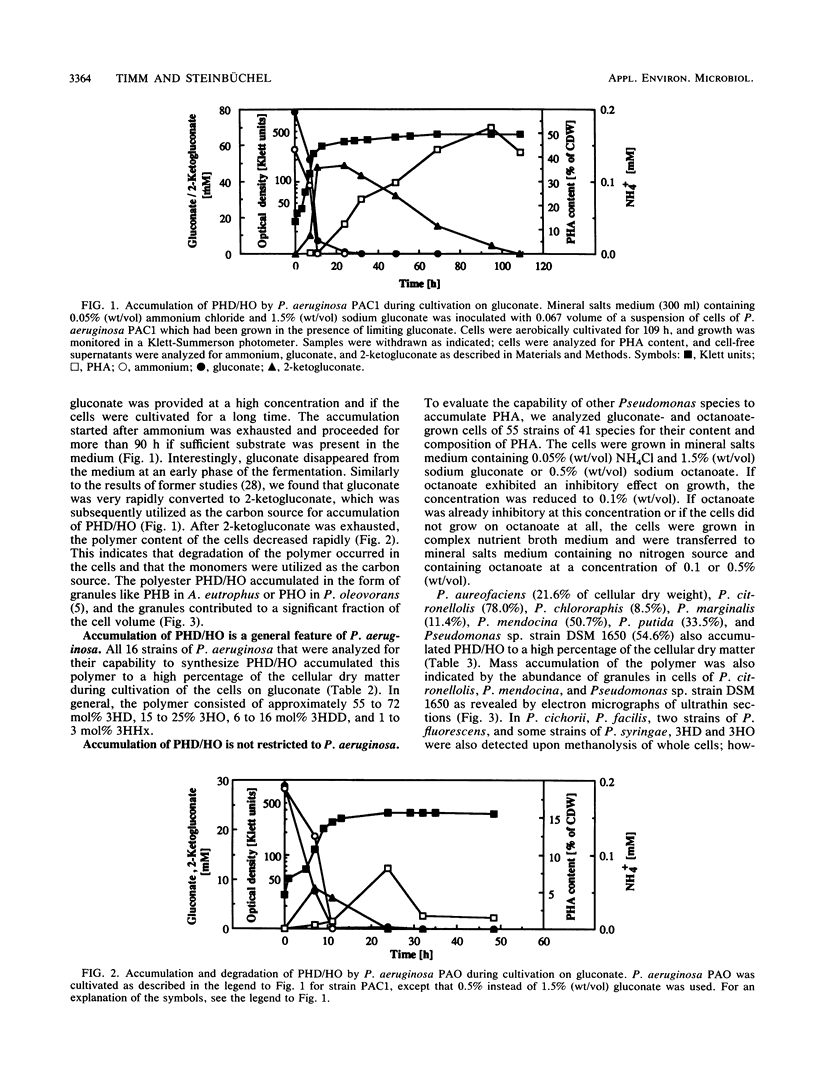
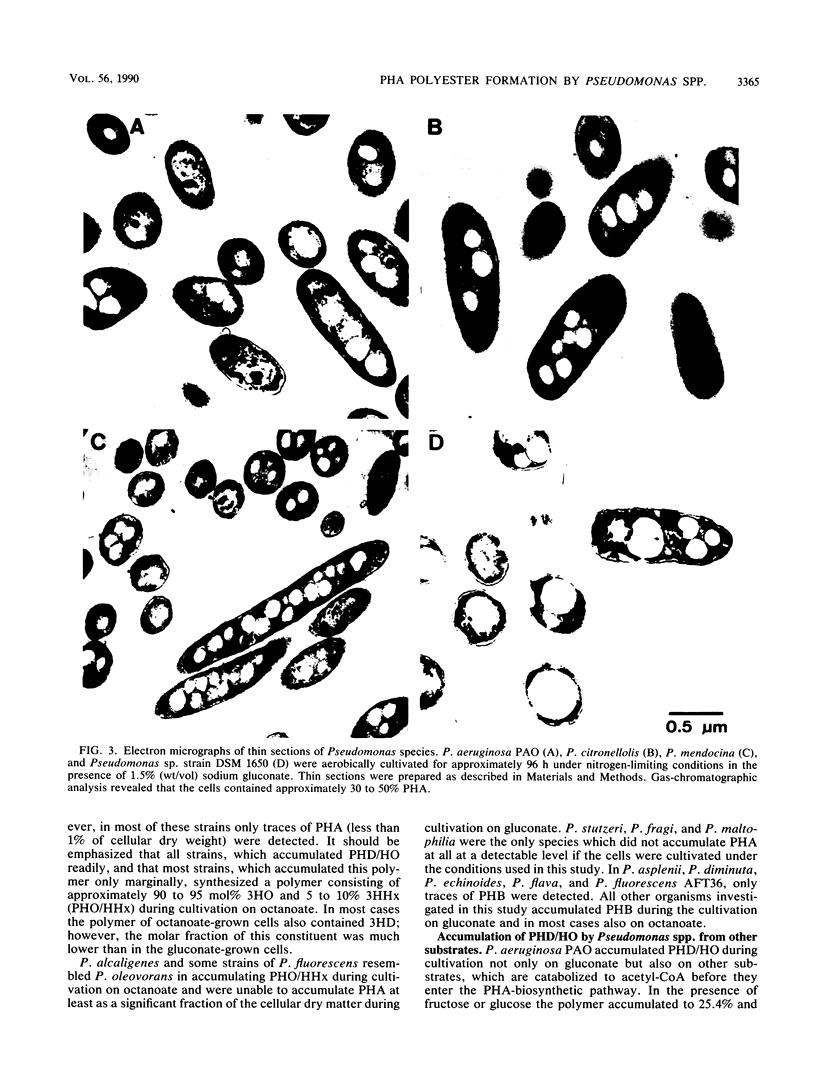
Pseudomonas aeruginosa PAO and 15 other strains of this species synthesized a polyester with 3-hydroxydecanoate as the main constituent (55 to 76 mol%) if the cells were cultivated in the presence of gluconate and if the nitrogen source was exhausted; 3-hydroxyhexanoate, 3-hydroxyoctanoate, and 3-hydroxydodecanoate were minor constituents of the polymer. The polymer was deposited in granules within the cell and amounted to 70% of the cell dry matter in some strains. Among 55 different strains of 41 Pseudomonas species tested, P. aureofaciens (21.6% of cellular dry matter), P. citronellolis (78.0%), P. chlororaphis (8.5%), P. marginalis (11.4%), P. mendocina (50.7%), P. putida (33.5%), and Pseudomonas sp. strain DSM 1650 (54.6%) accumulated this type of polymer at significant levels (greater than 5%) during cultivation on gluconate. In two strains of P. facilis and P. fluorescens, as well as in one strain of P. syringae, this polymer was detected as a minor constituent (much less than 5%). All other strains accumulated either poly(3-hydroxybutyrate) or a polymer consisting mainly of 3-hydroxyoctanoate with octanoate but no polyester with gluconate as the carbon source. Only a few species (e.g., P. stutzeri) were unable to accumulate poly(hydroxyalkanoic acids) (PHA) at all. These results indicated that the formation of PHA depends on a pathway which is distinct from all other known PHA-biosynthetic pathways. The polyesters accumulated by gluconate- or octanoate-grown cells of recombinant strains of P. aeruginosa and P. putida, which harbored the Alcaligenes eutrophus poly(3-hydroxybutyrate)biosynthetic genes, contained 3-hydroxybutyrate as an additional constituent.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brandl H., Gross R. A., Lenz R. W., Fuller R. C. Pseudomonas oleovorans as a Source of Poly(beta-Hydroxyalkanoates) for Potential Applications as Biodegradable Polyesters. Appl Environ Microbiol. 1988 Aug;54(8):1977–1982. doi: 10.1128/aem.54.8.1977-1982.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandl H., Knee E. J., Jr, Fuller R. C., Gross R. A., Lenz R. W. Ability of the phototrophic bacterium Rhodospirillum rubrum to produce various poly (beta-hydroxyalkanoates): potential sources for biodegradable polyesters. Int J Biol Macromol. 1989 Feb;11(1):49–55. doi: 10.1016/0141-8130(89)90040-8. [DOI] [PubMed] [Google Scholar]
- Haywood G. W., Anderson A. J., Ewing D. F., Dawes E. A. Accumulation of a Polyhydroxyalkanoate Containing Primarily 3-Hydroxydecanoate from Simple Carbohydrate Substrates by Pseudomonas sp. Strain NCIMB 40135. Appl Environ Microbiol. 1990 Nov;56(11):3354–3359. doi: 10.1128/aem.56.11.3354-3359.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huisman G. W., de Leeuw O., Eggink G., Witholt B. Synthesis of poly-3-hydroxyalkanoates is a common feature of fluorescent pseudomonads. Appl Environ Microbiol. 1989 Aug;55(8):1949–1954. doi: 10.1128/aem.55.8.1949-1954.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lageveen R. G., Huisman G. W., Preusting H., Ketelaar P., Eggink G., Witholt B. Formation of Polyesters by Pseudomonas oleovorans: Effect of Substrates on Formation and Composition of Poly-(R)-3-Hydroxyalkanoates and Poly-(R)-3-Hydroxyalkenoates. Appl Environ Microbiol. 1988 Dec;54(12):2924–2932. doi: 10.1128/aem.54.12.2924-2932.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peoples O. P., Sinskey A. J. Poly-beta-hydroxybutyrate (PHB) biosynthesis in Alcaligenes eutrophus H16. Identification and characterization of the PHB polymerase gene (phbC). J Biol Chem. 1989 Sep 15;264(26):15298–15303. [PubMed] [Google Scholar]
- Peoples O. P., Sinskey A. J. Poly-beta-hydroxybutyrate biosynthesis in Alcaligenes eutrophus H16. Characterization of the genes encoding beta-ketothiolase and acetoacetyl-CoA reductase. J Biol Chem. 1989 Sep 15;264(26):15293–15297. [PubMed] [Google Scholar]
- SCHLEGEL H. G., KALTWASSER H., GOTTSCHALK G. [A submersion method for culture of hydrogen-oxidizing bacteria: growth physiological studies]. Arch Mikrobiol. 1961;38:209–222. [PubMed] [Google Scholar]
- Schubert P., Steinbüchel A., Schlegel H. G. Cloning of the Alcaligenes eutrophus genes for synthesis of poly-beta-hydroxybutyric acid (PHB) and synthesis of PHB in Escherichia coli. J Bacteriol. 1988 Dec;170(12):5837–5847. doi: 10.1128/jb.170.12.5837-5847.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater S. C., Voige W. H., Dennis D. E. Cloning and expression in Escherichia coli of the Alcaligenes eutrophus H16 poly-beta-hydroxybutyrate biosynthetic pathway. J Bacteriol. 1988 Oct;170(10):4431–4436. doi: 10.1128/jb.170.10.4431-4436.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Steinbüchel A., Schlegel H. G. A multifunctional fermentative alcohol dehydrogenase from the strict aerobe Alcaligenes eutrophus: purification and properties. Eur J Biochem. 1984 Jun 15;141(3):555–564. doi: 10.1111/j.1432-1033.1984.tb08229.x. [DOI] [PubMed] [Google Scholar]
- Walther-Mauruschat A., Aragno M., Mayer F., Schlegel H. G. Micromorphology of Gram-negative hydrogen bacteria. II. Cell envelope, membranes, and cytoplasmic inclusions. Arch Microbiol. 1977 Aug 26;114(2):101–110. doi: 10.1007/BF00410770. [DOI] [PubMed] [Google Scholar]
- Whiting P. H., Midgley M., Dawes E. A. The role of glucose limitation in the regulation of the transport of glucose, gluconate and 2-oxogluconate, and of glucose metabolism in Pseudomonas aeruginosa. J Gen Microbiol. 1976 Feb;92(2):304–310. doi: 10.1099/00221287-92-2-304. [DOI] [PubMed] [Google Scholar]
- de Smet M. J., Eggink G., Witholt B., Kingma J., Wynberg H. Characterization of intracellular inclusions formed by Pseudomonas oleovorans during growth on octane. J Bacteriol. 1983 May;154(2):870–878. doi: 10.1128/jb.154.2.870-878.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]