Abstract
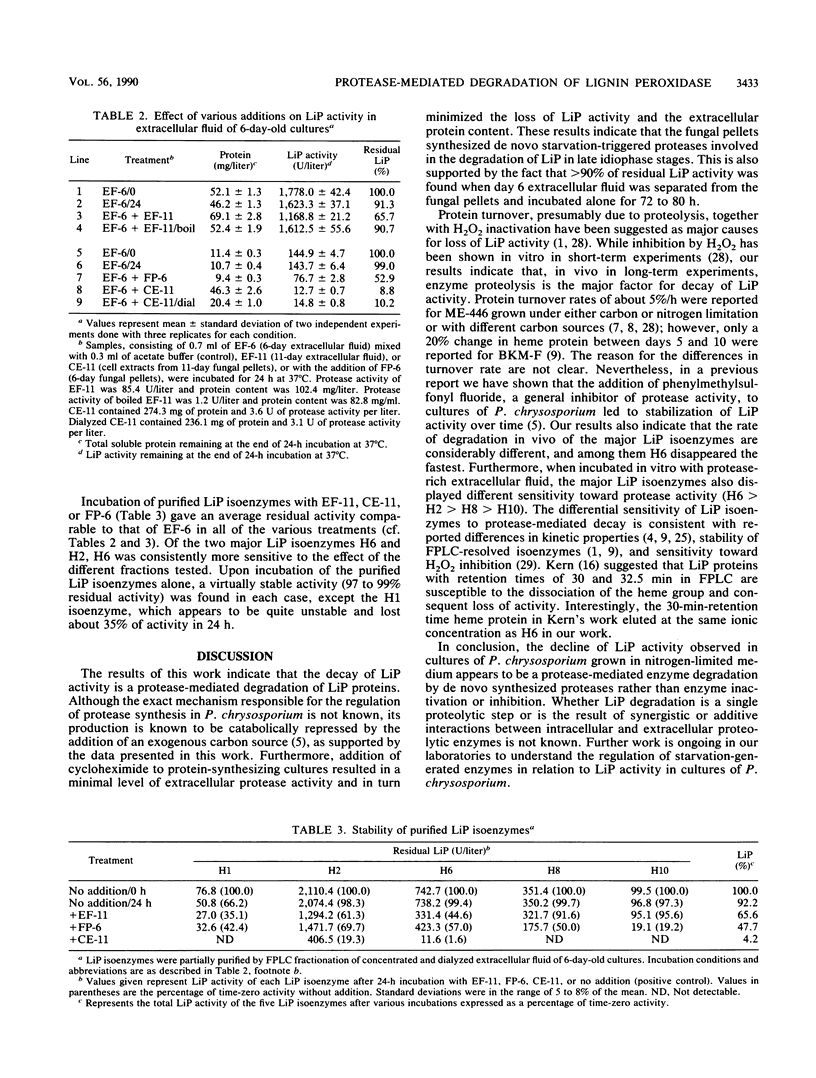
The decline of lignin peroxidase (LiP) activity observed after day 6 in cultures of Phanerochaete chrysosporium was found to be correlated with the appearance of idiophasic extracellular protease activity. Daily addition of glucose started on day 6 resulted in low protease levels and in turn in stable LiP levels. Addition of cycloheximide to day 6 cultures resulted in virtually no change of LiP activity and extracellular protein and negligible levels of protease activity, indicating that this protease is synthesized de novo. LiP activity was found to be stable upon removal of the fungal pellets on day 6 and incubation of the extracellular fluid alone. An almost complete disappearance of LiP activity and LiP proteins and high levels of protease activity were observed upon incubation of 6-day extracellular fluid in the presence of fungal pellets. Moreover, incubation of crude or purified LiP isoenzymes with protease-rich extracellular fluid of day 11 or 11-day cell extracts resulted in a marked loss of activity. In contrast, incubation of crude LiP with boiled and clarified extracellular fluid of day 11 cultures resulted in virtually no loss of activity. These results indicate that protease-mediated degradation of LiP proteins is a major cause for the decay of LiP activity during late secondary metabolism in cultures of P. chrysosporium.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonnarme P., Jeffries T. W. Mn(II) Regulation of Lignin Peroxidases and Manganese-Dependent Peroxidases from Lignin-Degrading White Rot Fungi. Appl Environ Microbiol. 1990 Jan;56(1):210–217. doi: 10.1128/aem.56.1.210-217.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dass S. B., Reddy C. A. Characterization of extracellular peroxidases produced by acetate-buffered cultures of the lignin-degrading basidiomycete Phanerochaete chrysosporium. FEMS Microbiol Lett. 1990 Jun 1;57(3):221–224. doi: 10.1111/j.1574-6968.1990.tb04233.x. [DOI] [PubMed] [Google Scholar]
- Dosoretz C. G., Chen H. C., Grethlein H. E. Effect of Environmental Conditions on Extracellular Protease Activity in Lignolytic Cultures of Phanerochaete chrysosporium. Appl Environ Microbiol. 1990 Feb;56(2):395–400. doi: 10.1128/aem.56.2.395-400.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faison B. D., Kirk T. K., Farrell R. L. Role of Veratryl Alcohol in Regulating Ligninase Activity in Phanerochaete chrysosporium. Appl Environ Microbiol. 1986 Aug;52(2):251–254. doi: 10.1128/aem.52.2.251-254.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn J. K., Morgan M. A., Mayfield M. B., Kuwahara M., Gold M. H. An extracellular H2O2-requiring enzyme preparation involved in lignin biodegradation by the white rot basidiomycete Phanerochaete chrysosporium. Biochem Biophys Res Commun. 1983 Aug 12;114(3):1077–1083. doi: 10.1016/0006-291x(83)90672-1. [DOI] [PubMed] [Google Scholar]
- Holzbaur E. L., Andrawis A., Tien M. Structure and regulation of a lignin peroxidase gene from Phanerochaete chrysosporium. Biochem Biophys Res Commun. 1988 Sep 15;155(2):626–633. doi: 10.1016/s0006-291x(88)80541-2. [DOI] [PubMed] [Google Scholar]
- Jäger A., Croan S., Kirk T. K. Production of Ligninases and Degradation of Lignin in Agitated Submerged Cultures of Phanerochaete chrysosporium. Appl Environ Microbiol. 1985 Nov;50(5):1274–1278. doi: 10.1128/aem.50.5.1274-1278.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersten P. J., Kirk T. K. Involvement of a new enzyme, glyoxal oxidase, in extracellular H2O2 production by Phanerochaete chrysosporium. J Bacteriol. 1987 May;169(5):2195–2201. doi: 10.1128/jb.169.5.2195-2201.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk T. K., Farrell R. L. Enzymatic "combustion": the microbial degradation of lignin. Annu Rev Microbiol. 1987;41:465–505. doi: 10.1146/annurev.mi.41.100187.002341. [DOI] [PubMed] [Google Scholar]
- Leisola M. S., Kozulic B., Meussdoerffer F., Fiechter A. Homology among multiple extracellular peroxidases from Phanerochaete chrysosporium. J Biol Chem. 1987 Jan 5;262(1):419–424. [PubMed] [Google Scholar]
- Pease E. A., Andrawis A., Tien M. Manganese-dependent peroxidase from Phanerochaete chrysosporium. Primary structure deduced from cDNA sequence. J Biol Chem. 1989 Aug 15;264(23):13531–13535. [PubMed] [Google Scholar]
- Tien M., Kirk T. K. Lignin-Degrading Enzyme from the Hymenomycete Phanerochaete chrysosporium Burds. Science. 1983 Aug 12;221(4611):661–663. doi: 10.1126/science.221.4611.661. [DOI] [PubMed] [Google Scholar]
- Tien M. Properties of ligninase from Phanerochaete chrysosporium and their possible applications. Crit Rev Microbiol. 1987;15(2):141–168. doi: 10.3109/10408418709104456. [DOI] [PubMed] [Google Scholar]
- Tonon F., Odier E. Influence of Veratryl Alcohol and Hydrogen Peroxide on Ligninase Activity and Ligninase Production by Phanerochaete chrysosporium. Appl Environ Microbiol. 1988 Feb;54(2):466–472. doi: 10.1128/aem.54.2.466-472.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]