Abstract
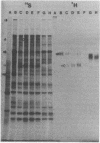
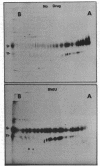
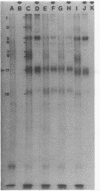
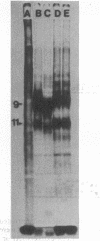
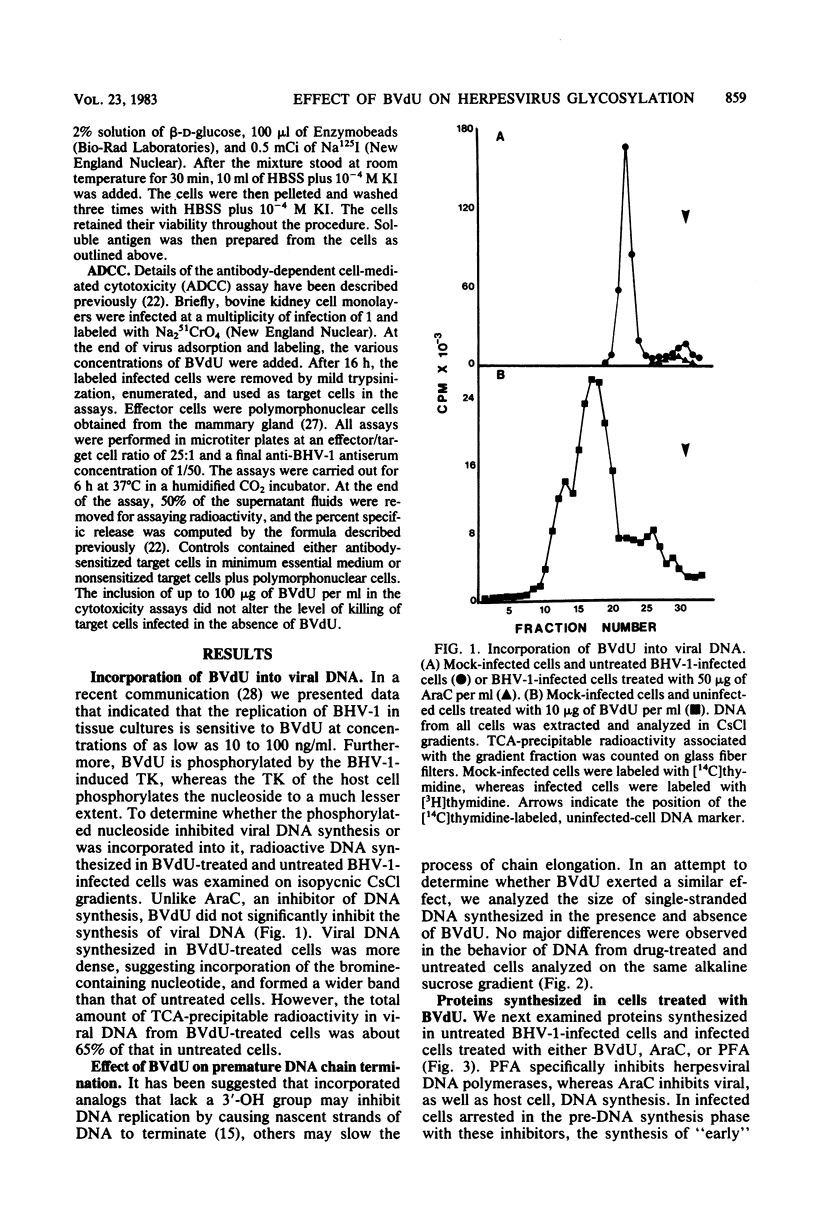
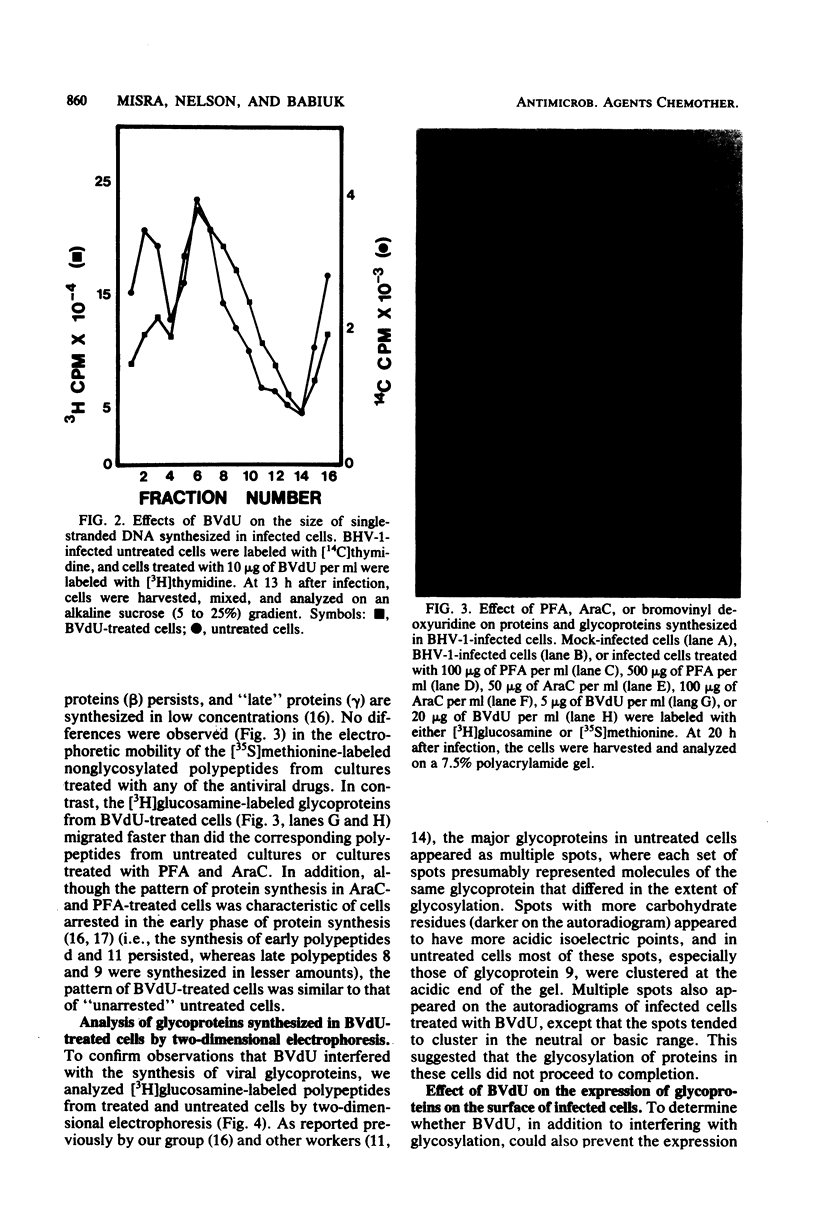
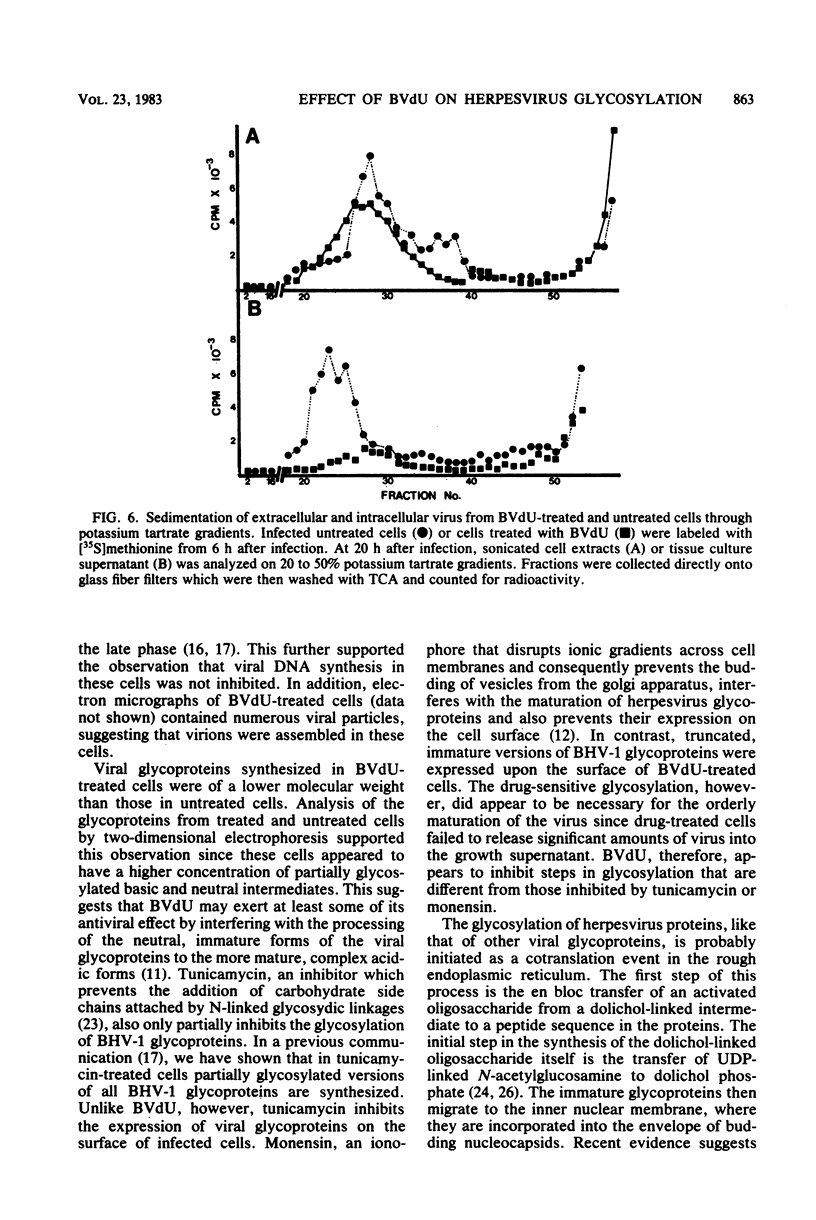
(E)-5-(2-Bromovinyl)-2'-deoxyuridine (BVdU) was phosphorylated by the bovine herpesvirus 1 (BHV-1)-induced thymidine kinase and subsequently incorporated into viral DNA, resulting in DNA that was more dense than DNA from untreated cells. Incorporation of the drug did not result in the termination of replicating BHV-1 DNA molecules since radioactively labeled DNA synthesized in drug-treated and untreated cells sedimented at similar rates in alkaline sucrose gradients. No differences were observed in the electrophoretic mobility of [35S]methionine-labeled viral polypeptides synthesized in treated and untreated cells, although [3H]glucosamine-labeled viral glycoproteins synthesized in treated cells were of a lower molecular weight than those in untreated cells. In BVdU-treated cells, unlike untreated cells, immature neutral and basic precursors of the mature viral glycoproteins accumulated. Although BVdU-treated and untreated cells contained similar amounts of virus, very little virus was released into the culture supernatant from BVdU-treated cells. Our results suggest that BVdU partially inhibits the glycosylation of BHV-1 glycoproteins. BVdU-sensitive glycosylation, however, is not necessary for expression of these glycoproteins on the surface of infected cells since the glycoproteins could be labeled on intact cells with 125I and because BVdU-treated cells remained sensitive to antibody-dependent, cell-mediated cytotoxity mediated by anti-BHV-1 serum. The phosphorylation of BVdU was a prerequisite for its effect on glycosylation since the glycoproteins of a thymidine kinase-deficient mutant of BHV-1 were not affected.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allaudeen H. S., Chen M. S., Lee J. J., De Clercq E., Prusoff W. H. Incorporation of E-5-(2-halovinyl)-2'-deoxyuridines into deoxyribonucleic acids of herpes simplex virus type 1-infected cells. J Biol Chem. 1982 Jan 25;257(2):603–606. [PubMed] [Google Scholar]
- Allaudeen H. S., Kozarich J. W., Bertino J. R., De Clercq E. On the mechanism of selective inhibition of herpesvirus replication by (E)-5-(2-bromovinyl)-2'-deoxyuridine. Proc Natl Acad Sci U S A. 1981 May;78(5):2698–2702. doi: 10.1073/pnas.78.5.2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aswell J. F., Allen G. P., Jamieson A. T., Campbell D. E., Gentry G. A. Antiviral activity of arabinosylthymine in herpesviral replication: mechanism of action in vivo and in vitro. Antimicrob Agents Chemother. 1977 Aug;12(2):243–254. doi: 10.1128/aac.12.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayisi N. K., Gupta V. S., Meldrum J. B., Taneja A. K., Babiuk L. A. Combination chemotherapy: interaction of 5-methoxymethyldeoxyuridine with adenine arabinoside, 5-ethyldeoxyuridine, 5-iododeoxyuridine, and phosphonoacetic acid against herpes simplex virus types 1 and 2. Antimicrob Agents Chemother. 1980 Apr;17(4):558–566. doi: 10.1128/aac.17.4.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M. S., Ward D. C., Prusoff W. H. Specific herpes simplex virus-induced incorporation of 5-iodo-5'-amino-2',5'-dideoxyuridine into deoxyribonucleic acid. J Biol Chem. 1976 Aug 25;251(16):4833–4838. [PubMed] [Google Scholar]
- De Clercq E., Descamps J., De Somer P., Barr P. J., Jones A. S., Walker R. T. (E)-5-(2-Bromovinyl)-2'-deoxyuridine: a potent and selective anti-herpes agent. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2947–2951. doi: 10.1073/pnas.76.6.2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Clercq E., Descamps J., Verhelst G., Walker R. T., Jones A. S., Torrence P. F., Shugar D. Comparative efficacy of antiherpes drugs against different strains of herpes simplex virus. J Infect Dis. 1980 May;141(5):563–574. doi: 10.1093/infdis/141.5.563. [DOI] [PubMed] [Google Scholar]
- Garrels J. I. Two dimensional gel electrophoresis and computer analysis of proteins synthesized by clonal cell lines. J Biol Chem. 1979 Aug 25;254(16):7961–7977. [PubMed] [Google Scholar]
- Glorioso J. C., Smith J. W. Immune interactions with cells infected with herpes simplex virus: antibodies to radioiodinated surface antigens. J Immunol. 1977 Jan;118(1):114–121. [PubMed] [Google Scholar]
- Haarr L., Marsden H. S. Two-dimensional gel analysis of HSV type 1-induced polypeptides and glycoprotein processing. J Gen Virol. 1981 Jan;52(Pt 1):77–92. doi: 10.1099/0022-1317-52-1-77. [DOI] [PubMed] [Google Scholar]
- Johnson D. C., Spear P. G. Monensin inhibits the processing of herpes simplex virus glycoproteins, their transport to the cell surface, and the egress of virions from infected cells. J Virol. 1982 Sep;43(3):1102–1112. doi: 10.1128/jvi.43.3.1102-1112.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Little S. P., Jofre J. T., Courtney R. J., Schaffer P. A. A virion-associated glycoprotein essential for infectivity of herpes simplex virus type 1. Virology. 1981 Nov;115(1):149–160. doi: 10.1016/0042-6822(81)90097-0. [DOI] [PubMed] [Google Scholar]
- McGuirt P. V., Furman P. A. Acyclovir inhibition of viral DNA chain elongation in herpes simplex virus-infected cells. Am J Med. 1982 Jul 20;73(1A):67–71. doi: 10.1016/0002-9343(82)90066-3. [DOI] [PubMed] [Google Scholar]
- Misra V., Blumenthal R. M., Babiuk L. A. Proteins Specified by bovine herpesvirus 1 (infectious bovine rhinotracheitis virus). J Virol. 1981 Nov;40(2):367–378. doi: 10.1128/jvi.40.2.367-378.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra V., Gilchrist J. E., Weinmaster G., Qualtiere L., Van den Hurk S., Babiuk L. A. Herpesvirus-induced "early" glycoprotein: characterization and possible role in immune cytolysis. J Virol. 1982 Sep;43(3):1046–1054. doi: 10.1128/jvi.43.3.1046-1054.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Olofsson S., Blomberg J., Lycke E. O-glycosidic carbohydrate-peptide linkages of Herpes simplex virus glycoproteins. Arch Virol. 1981;70(4):321–329. doi: 10.1007/BF01320247. [DOI] [PubMed] [Google Scholar]
- Pogolotti A. L., Jr, Nolan P. A., Santi D. V. Methods for the complete analysis of 5-fluorouracil metabolites in cell extracts. Anal Biochem. 1981 Oct;117(1):178–186. doi: 10.1016/0003-2697(81)90708-9. [DOI] [PubMed] [Google Scholar]
- Prusoff W. H., Chen M. S., Fischer P. H., Lin T. S., Shiau G. T., Schinazi R. F. Role of nucleosides in virus and cancer chemotherapy. Adv Ophthalmol. 1979;38:3–16. [PubMed] [Google Scholar]
- Rouse B. T., Wardley R. C., Babiuk L. A. Antibody-dependent cell-mediated cytotoxicity in cows: comparison of effector cell activity against heterologous erthrocyte and herpesvirus-infected bovine target cells. Infect Immun. 1976 May;13(5):1433–1441. doi: 10.1128/iai.13.5.1433-1441.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipman C., Jr, Smith S. H., Carlson R. H., Drach J. C. Antiviral activity of arabinosyladenine and arabinosylhypoxanthine in herpes simplex virus-infected KB cells: selective inhibition of viral deoxyribonucleic acid synthesis in synchronized suspension cultures. Antimicrob Agents Chemother. 1976 Jan;9(1):120–127. doi: 10.1128/aac.9.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardley R. C., Rouse B. T., Babiuk L. A. Antibody dependent cytotoxicity mediated by neutrophils: a possible mechanism of antiviral defense. J Reticuloendothel Soc. 1976 May;19(5):323–332. [PubMed] [Google Scholar]
- Weinmaster G. A., Misra V., McGuire R., Babiuk L. A., DeClercq E. Bovid herpesvirus type-1 (infectious bovine rhinotracheitis virus)-induced thymidine kinase. Virology. 1982 Apr 15;118(1):191–201. doi: 10.1016/0042-6822(82)90332-4. [DOI] [PubMed] [Google Scholar]