Abstract
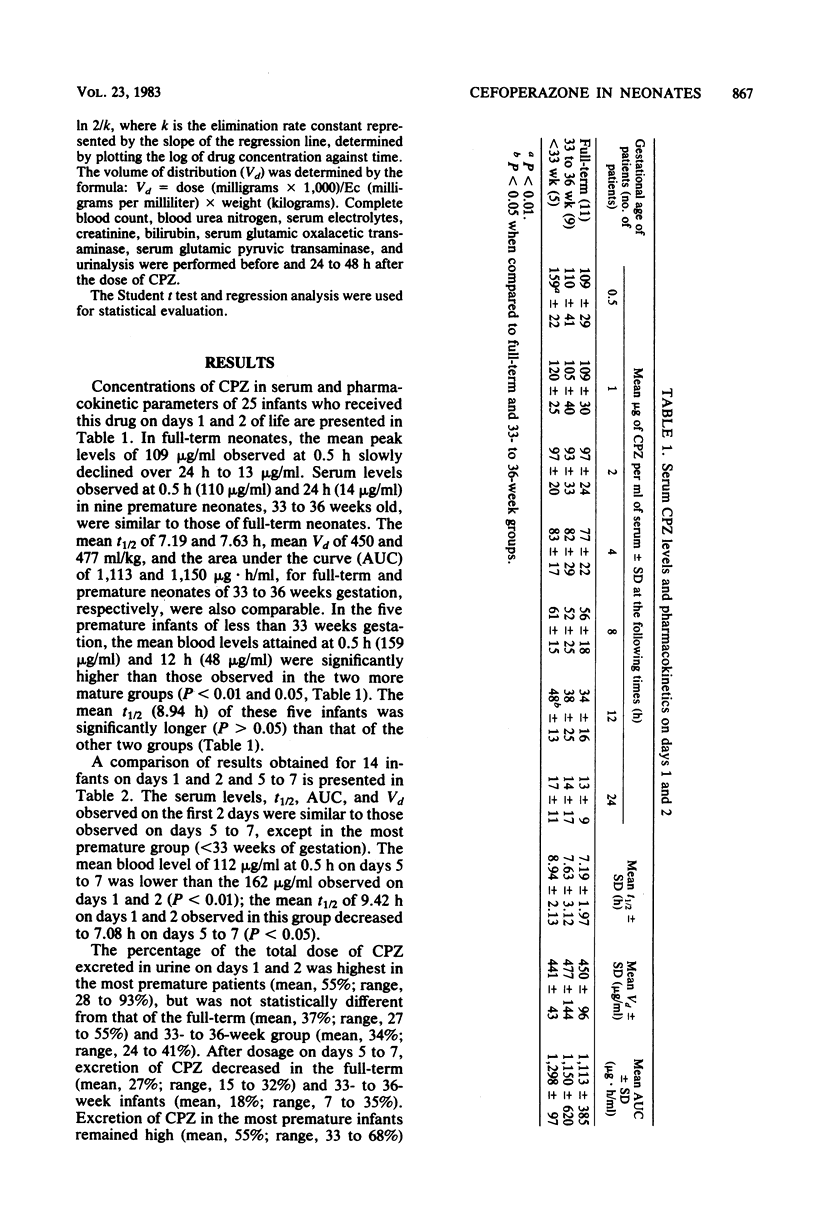
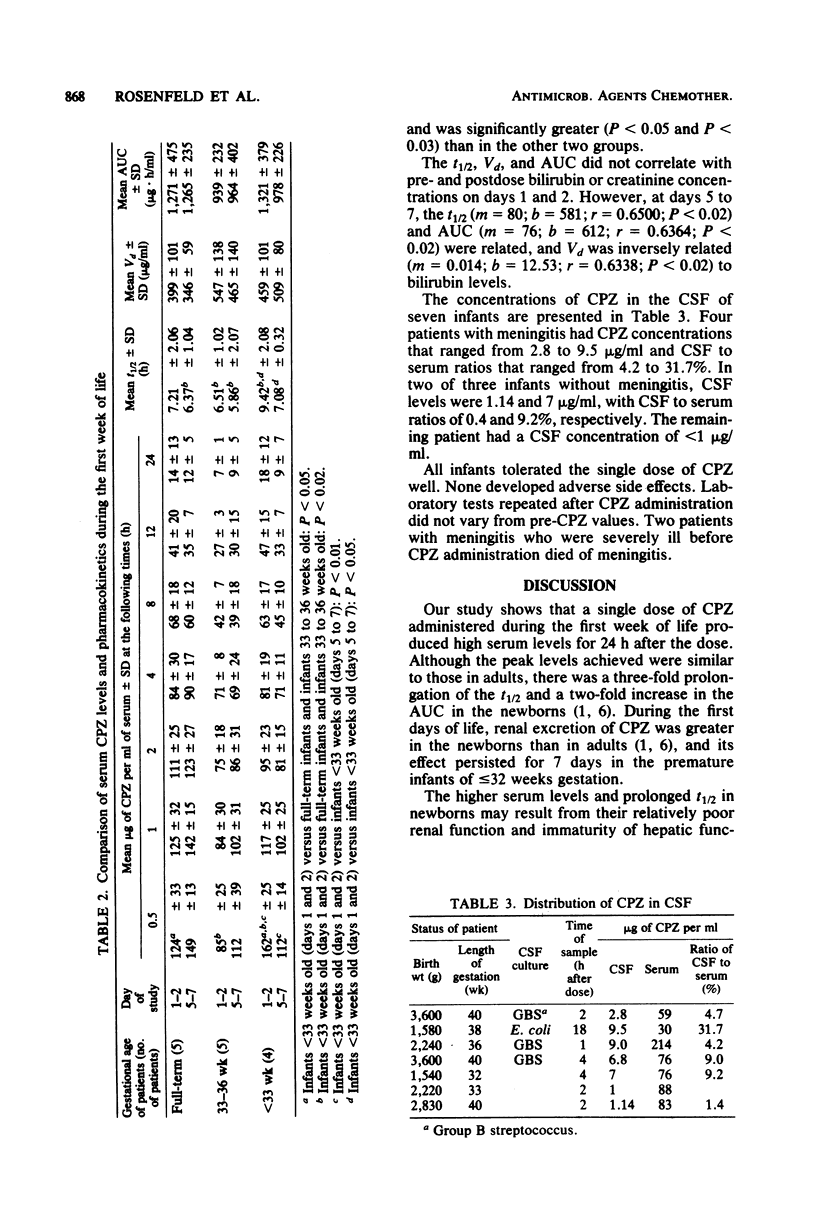
The pharmacokinetics of cefoperazone were evaluated in 28 newborn infants who were being treated for sepsis. A dose of 50 mg/kg was administered intravenously on days 0 to 2 in all, with a second dose administered on days 5 to 7 in 14 infants. Cerebrospinal fluid penetration was also studied in seven neonates. The mean peak concentration of cefoperazone in the serum of premature infants less than 33 weeks of gestational age, 159 (standard deviation, +/- 22) micrograms/ml, was higher than concentrations in premature infants 33 to 36 weeks of age and full-term infants (110 +/- 41 and 109 +/- 29 micrograms/ml, respectively). The mean concentrations 24 h after dosage were similar in all three groups, 13 to 17 micrograms/ml. The mean serum half-lives were similar in the three subgroups and ranged from 7 to 9 h. After the dose at 5 to 7 days, mean blood levels in the subgroups at 0.5 h were 149, 112, and 112 micrograms/ml; 24-h levels ranged from 9 to 12 micrograms/ml. The mean serum half-lives ranged from 5 to 7 h. Cerebrospinal fluid levels in patients with meningitis ranged from 2.8 to 9.5 micrograms/ml and in patients without meningitis from 1 to 7 micrograms/ml. Peak blood levels were 15 to 1,000 times higher than the 90% minimal inhibitory concentration of common pathogens found in newborns. These observations support the potential efficacy of cefoperazone in treatment of infections, including meningitis, in newborn infants.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brogden R. N., Carmine A., Heel R. C., Morley P. A., Speight T. M., Avery G. S. Cefoperazone: A review of its in vitro antimicrobial activity, pharmacological properties and therapeutic efficacy. Drugs. 1981 Dec;22(6):423–460. doi: 10.2165/00003495-198122060-00002. [DOI] [PubMed] [Google Scholar]
- Jones R. N., Fuchs P. C., Barry A. L., Gavan T. L., Sommers H. M., Gerlach E. H. Cefoperazone (T-1551), a new semisynthetic cephalosporin: comparison with cephalothin and gentamicin. Antimicrob Agents Chemother. 1980 Apr;17(4):743–749. doi: 10.1128/aac.17.4.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muytjens H. L., van der Ros-van de Repe J. Comparative activities of 13 beta-lactam antibiotics. Antimicrob Agents Chemother. 1982 Jun;21(6):925–934. doi: 10.1128/aac.21.6.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C., Fu K. P., Aswapokee N., Aswapokee P., Kung K. Comparative activity and beta-lactamase stability of cefoperazone, a piperazine cephalosporin. Antimicrob Agents Chemother. 1979 Aug;16(2):150–157. doi: 10.1128/aac.16.2.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaad U. B., McCracken G. H., Jr, Threlkeld N., Thomas M. L. Clinical evaluation of a new broad-spectrum oxa-beta-lactam antibiotic, moxalactam, in neonates and infants. J Pediatr. 1981 Jan;98(1):129–136. doi: 10.1016/s0022-3476(81)80559-8. [DOI] [PubMed] [Google Scholar]
- Shimizu K. Cefoperazone: absorption, excretion, distribution, and metabolism. Clin Ther. 1980;3(Spec Issue):60–79. [PubMed] [Google Scholar]
- Thirumoorthi M. C., Buckley J. A., Aravind M. K., Kauffman R. E., Dajani A. S. Diffusion of moxalactam into the cerebrospinal fluid in children with bacterial meningitis. J Pediatr. 1981 Dec;99(6):975–979. doi: 10.1016/s0022-3476(81)80036-4. [DOI] [PubMed] [Google Scholar]



