Abstract
Nonpharmaceutical interventions (NPIs) intended to reduce infectious contacts between persons form an integral part of plans to mitigate the impact of the next influenza pandemic. Although the potential benefits of NPIs are supported by mathematical models, the historical evidence for the impact of such interventions in past pandemics has not been systematically examined. We obtained data on the timing of 19 classes of NPI in 17 U.S. cities during the 1918 pandemic and tested the hypothesis that early implementation of multiple interventions was associated with reduced disease transmission. Consistent with this hypothesis, cities in which multiple interventions were implemented at an early phase of the epidemic had peak death rates ≈50% lower than those that did not and had less-steep epidemic curves. Cities in which multiple interventions were implemented at an early phase of the epidemic also showed a trend toward lower cumulative excess mortality, but the difference was smaller (≈20%) and less statistically significant than that for peak death rates. This finding was not unexpected, given that few cities maintained NPIs longer than 6 weeks in 1918. Early implementation of certain interventions, including closure of schools, churches, and theaters, was associated with lower peak death rates, but no single intervention showed an association with improved aggregate outcomes for the 1918 phase of the pandemic. These findings support the hypothesis that rapid implementation of multiple NPIs can significantly reduce influenza transmission, but that viral spread will be renewed upon relaxation of such measures.
Keywords: mitigation, nonpharmaceutical interventions, closures
Influenza pandemics have occurred periodically in human populations, with three pandemics in the 20th century. The 1918 influenza pandemic resulted in unprecedented mortality, with an estimated 500,000–675,000 deaths in the U.S. and 50–100 million deaths worldwide (1–3). The spread of H5N1 avian influenza has provoked public concern (4) and accelerated efforts to plan for the next pandemic. Because antiviral medications and effective vaccines may not be widely available at the beginning of a pandemic, many authorities have suggested using nonpharmaceutical interventions (NPIs; i.e., voluntary quarantine of infected households, closure of schools, bans on public gatherings, and other measures) to decrease disease transmission. This approach is supported by mathematical models, which suggest that multiple simultaneous NPIs applied early in an epidemic may significantly reduce disease transmission (5). A recent review, however, concluded that the evidence base for recommending such interventions is limited, consisting primarily of historical and contemporary observations, rather than controlled studies (6).
The intensity of the 1918 pandemic, whether assessed as total excess deaths, the rate of increase in the epidemic curve, or peak death rates, varied widely among U.S. cities. Cities also varied widely in their choice and timing of implementation of NPIs designed to reduce disease spread. Many cities closed schools, churches, theaters, dance halls, or other public accommodations; made influenza a notifiable disease; banned funerals or other public gatherings; or introduced isolation of sick persons. In some cases, these NPIs were put in place in the first days of epidemic spread in a city, whereas in other cases, they were introduced late or not at all (Table 1).
Table 1.
Summary of interventions and their timing across 17 cities
| Intervention | Number of 17 cities implementing | Median (interquartile range) epidemic stage (CEPID) at time of implementation* |
|---|---|---|
| Making influenza a notifiable disease | 15 | 5.6 (3.1, 25.9) |
| Emergency declarations | 4 | — |
| Isolation policies | 14 | 15.7 (7.6, 30.8) |
| Quarantine of households where infection identified | 5 | — |
| School closures | 14 | 30.8 (15.1, 96.3) |
| Church closures | 15 | 29.9 (12.4, 130.6) |
| Theater closures | 15 | 29.9 (10.3, 66.9) |
| Dance hall closures | 11 | 44.7 (12.4, –) |
| Other closures | 13 | 84.7 (29.9, 322.0) |
| Staggered business hours to reduce congestion in stores and on transit systems | 8 | — |
| Mask ordinances | 2 | — |
| Rules forbidding crowding on streetcars | 6 | — |
| Private funerals | 11 | 92.1 (30.8, –) |
| Bans on door-to-door sales | 1 | — |
| Interventions designed to reduce transmission in the workplace | 0 | — |
| Protective sequestration of children | 3 | — |
| Bans on public gatherings | 15 | 30.8 (12.4, 118.1) |
| No-crowding rules in locations other than transit systems | 3 | — |
| Community-wide business closures | 1 | — |
*Shown only for interventions implemented in at least nine cities (>50%); 75th percentile not shown for interventions implemented in <13 cities.
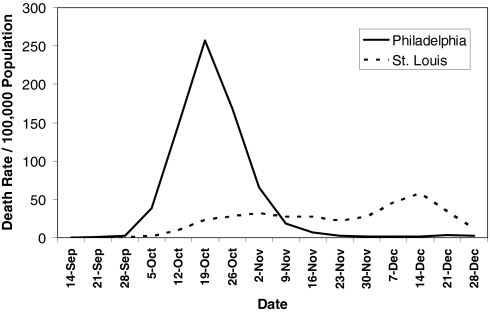
We noted that, in some cases, outcomes appear to have correlated with the quality and timing of the public health response. The contrast of mortality outcomes between Philadelphia and St. Louis is particularly striking (Fig. 1). The first cases of disease among civilians in Philadelphia were reported on September 17, 1918, but authorities downplayed their significance and allowed large public gatherings, notably a city-wide parade on September 28, 1918, to continue. School closures, bans on public gatherings, and other social distancing interventions were not implemented until October 3, when disease spread had already begun to overwhelm local medical and public health resources. In contrast, the first cases of disease among civilians in St. Louis were reported on October 5, and authorities moved rapidly to introduce a broad series of measures designed to promote social distancing, implementing these on October 7. The difference in response times between the two cities (≈14 days, when measured from the first reported cases) represents approximately three to five doubling times for an influenza epidemic. The costs of this delay appear to have been significant; by the time Philadelphia responded, it faced an epidemic considerably larger than the epidemic St. Louis faced. Philadelphia ultimately experienced a peak weekly excess pneumonia and influenza (P&I) death rate of 257/100,000 and a cumulative excess P&I death rate (CEPID) during the period September 8–December 28, 1918 (the study period) of 719/100,000. St. Louis, on the other hand, experienced a peak P&I death rate, while NPIs were in place, of 31/100,000 and had a CEPID during the study period of 347/100,000. Consistent with the predictions of modeling, the effect of the NPIs in St. Louis appear to have had a less-pronounced effect on CEPID than on peak death rates, and death rates were observed to climb after the NPIs were lifted in mid-November (7–9).
Fig. 1.
Excess P&I mortality over 1913–1917 baseline in Philadelphia and St. Louis, September 8–December 28, 1918. Data are derived from ref. 10.
To investigate whether early implementation of individual interventions or of multiple measures reduces disease transmission during influenza pandemics, we analyzed the NPIs used in a collection of U.S. cities during the fall wave of the 1918 pandemic, identifying the NPIs used in each city as well as the timing of their implementation [details of individual city outcomes and interventions are included in supporting information (SI) Appendix]. We then related this information to the observed outcomes of the peak weekly death rate and CEPID during the period September–December, 1918. Excess death rates were used as a proxy for case incidence because of the more accurate reporting of deaths than cases. We hypothesized that early implementation of multiple NPIs in an immunologically naïve population would slow the progression of the epidemic, resulting in a flatter epidemic curve, but that over time aggregate outcomes would approach those observed in cities not implementing such measures, until roughly comparable levels of herd immunity were achieved.
Results
Effect of Early Interventions on Epidemic Spread.
We assessed the relationship between the timing of NPIs and three measures of epidemic outcome: (i) the peak weekly rate of excess P&I deaths per 100,000 population (peak death rate) during the study period; (ii) the “normalized” peak weekly excess P&I death rate (peak weekly death rate during the study period divided by the median weekly rate during the period); and (iii) the CEPID per 100,000 population during the study period. The stage of the epidemic at the time of each intervention was defined as the CEPID from the start of the study period until the date on which the intervention was announced. Thus, early interventions in a given city were those that were implemented when relatively few individuals had died, whereas later ones were those implemented after more excess P&I deaths had occurred.
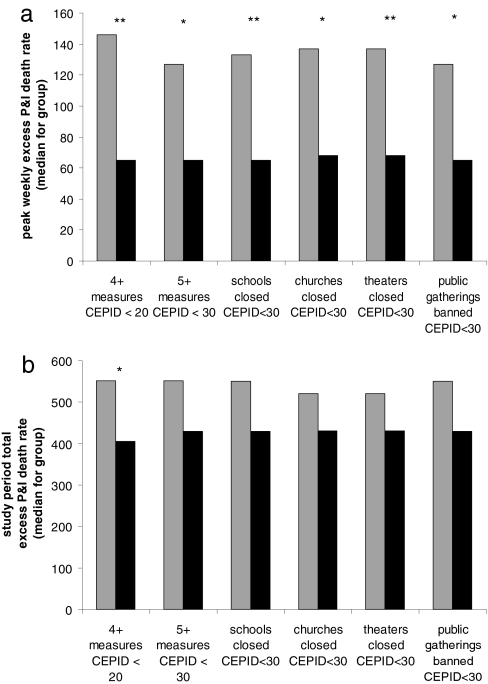
In comparisons across cities (Fig. 2a, Table 2), we found that aggressive early intervention was significantly associated with a lower peak of excess mortality (Spearman ρ = −0.49 to −0.68, P = 0.002–0.047; see Table 2, Number of interventions before, for the number of NPIs before a given CEPID cutoff vs. peak mortality). Cities that implemented three or fewer NPIs before 20/100,000 CEPID had a median peak weekly death rate of 146/100,000, compared with 65/100,000 in those implementing four or more NPIs by that time (Fig. 2a, P = 0.005). The relationship was similar for normalized peak death rates and for a range of possible cutoffs (see Table 2, CEPID at time of intervention), although the relationship became weaker as later interventions were included. Cities with more early NPIs also had fewer total excess deaths during the study period (Fig. 2b, Table 2, 1918 total), but this association was weaker: cities with three or fewer NPIs before CEPID = 20/100,000 experienced a median total excess death rate of 551/100,000, compared with a median rate of 405/100,000 in cities with four or more NPIs (P = 0.03).
Fig. 2.
Relationship of (a) peak weekly excess P&I death rate and (b) total excess P&I death rate during the study period to the timing of various NPIs. Cities were divided evenly into those intervening early (black bars) vs. late or not at all (gray bars), and the median outcome for the early and late groups was plotted. The first two groups of bars assess overall timing of intervention, comparing those cities that announced four or more NPIs before experiencing 20/100,000 CEPID with those with three or fewer and those that announced five or more NPIs before experiencing 30/100,000 CEPID with those with four or fewer. The remaining groups compare those cities that announced particular measures before experiencing 30/100,000 CEPID with those that did not. Significance by Mann–Whitney U test: ∗, P < 0.05; ∗∗, P < 0.01.
Table 2.
Correlation between influenza epidemic outcomes and timing of interventions in 17 U.S. cities in 1918
| Measure of interventions | Outcome: Excess weekly P&I deaths |
||
|---|---|---|---|
| Peak | Normalized peak | 1918 total | |
| Number of interventions before: | |||
| 10/100,000 CEPID | −0.53, P = 0.03 | −0.53, P = 0.03 | −0.31, P = 0.22 |
| 20/100,000 CEPID | −0.68, P = 0.002 | −0.64, P = 0.005 | −0.52, P = 0.03 |
| 30/100,000 CEPID | −0.51, P = 0.04 | −0.55, P = 0.02 | −0.29, P = 0.27 |
| 40/100,000 CEPID | −0.32, P = 0.21 | −0.40, P = 0.11 | −0.07, P = 0.80 |
| CEPID at time of intervention: | |||
| First | 0.08, P = 0.76 | 0.004, P = 0.87 | 0.07, P = 0.79 |
| Second | 0.54, P = 0.02 | 0.47, P = 0.06 | 0.39, P = 0.12 |
| Third | 0.54, P = 0.02 | 0.52, P = 0.03 | 0.31, P = 0.22 |
| Fourth | 0.66, P = 0.004 | 0.70, P = 0.002 | 0.38, P = 0.13 |
| Fifth | 0.55, P = 0.02 | 0.67, P = 0.003 | 0.27, P = 0.30 |
| Sixth | 0.26, P = 0.31 | 0.44, P = 0.08 | 0.05, P = 0.84 |
| CEPID at time of: | |||
| Closing schools | 0.54, P = 0.02 | 0.63, P = 0.007 | 0.25, P = 0.34 |
| Closing theaters | 0.56, P = 0.02 | 0.72, P = 0.001 | 0.17, P = 0.52 |
| Closing churches | 0.56, P = 0.02 | 0.70, P = 0.002 | 0.17, P = 0.53 |
| Closing dance halls | 0.03, P = 0.90 | 0.04, P = 0.87 | 0.15, P = 0.57 |
| Other closures | 0.33, P = 0.19 | 0.34, P = 0.18 | 0.24, P = 0.35 |
| Making influenza notifiable | 0.01, P = 0.97 | −0.07, P = 0.79 | 0.11, P = 0.67 |
| Bans on public gatherings | 0.46, P = 0.06 | 0.56, P = 0.02 | 0.27, P = 0.30 |
| Imposing case isolation | 0.16, P = 0.53 | 0.14, P = 0.59 | 0.13, P = 0.62 |
| Bans on public funerals | −0.09, P = 0.75 | 0.09, P = 0.72 | −0.41, P = 0.10 |
Three measures of epidemic intensity. Peak weekly excess P&I death rate, normalized peak weekly excess P&I death rate (peak divided by median weekly rate during the study period), and 1918 study period total excess P&I death rate are related to number of interventions before reaching a specified CEPID, CEPID at time when specified numbers of interventions had been imposed, and CEPID at time when specific interventions had been imposed. Spearman rank correlations and associated P values are shown, with bold type for P < 0.05.
The association of early intervention and lower peak death rates was also observed when cities were ranked according to the CEPID in each city at the time of the second, third, fourth, or fifth intervention (Table 2, CEPID at time of intervention). Similar relationships were again detected for the normalized peak death rate [Table 2, CEPID at time of intervention/Normalized peak]. Again, the relationship with total death rate was weaker and in this case not statistically significant.
Effects of Individual Interventions.
To assess whether particular NPIs were associated with better outcomes, we calculated a Spearman rank correlation coefficient between outcome measures and the stage at which individual NPIs were implemented in each city (cities that never implemented a given intervention were ranked last in each analysis). Results are shown in Table 2, CEPID at time of. Early school, church, or theater closure was associated with lower peak excess death rates (Spearman ρ = 0.54–0.56, P = 0.02). Cities that made each of these interventions before they reached 30/100,000 CEPID had a median peak death rate of 65–68/100,000, compared with median peaks of 127–146/100,000 for cities that made these interventions later or not at all (Fig. 2a, P = 0.005–0.01). Announcements of school, church, and theater closures were linked in most cities, occurring within a span of ≤6 days in the majority, and this near simultaneity of implementation precludes multivariate analysis or strong inference about the relative importance of the individual NPIs. Early bans on public gatherings were also associated with lower peak excess death rates, but the statistical significance of this result depended on the test used [Table 2, CEPID at time of, and Fig. 2a]. Of the other NPIs considered (closure of dance halls, other closures, isolation of cases, bans on public funerals, and making influenza notifiable), none showed a statistically significant association between the stage of implementation and the peak or cumulative excess death rates (Table 2, CEPID at time of, and Fig. 2).
Other Predictors of Epidemic Severity.
We assessed the correlation between peak mortality rate and each of the following variables: latitude, longitude, 1910 population density, 1920 population density, 1918 population size, and epidemic start week, defined as the first week in which excess P&I mortality exceeded 10/100,000. Of these variables, only longitude (Spearman ρ = −0.61, P = 0.009) and epidemic start week (Spearman ρ = −0.55, P = 0.02) were significantly associated with the peak weekly excess P&I mortality rate, and these two variables were strongly associated with one another (Spearman ρ = 0.66, P = 0.004), indicating that eastern U.S. cities were hit earlier in our data set. In addition, cities whose epidemics began later tended to intervene at an earlier stage of their epidemics (Spearman ρ = 0.77, P = 0.0003), presumably because local officials in these cities observed the effects of the epidemic along the Eastern seaboard and resolved to act quickly.
In linear regressions of peak death rates vs. stage of the epidemic at the time of interventions (number of NPIs before CEPID = 20/100,000) and timing of epidemic onset, the association of peak with intervention stage was statistically significant and stronger than that with epidemic onset in univariate models (SI Table 3). If both predictors are used in a bivariate regression, the point estimate for interventions remains unchanged while the p value increases to 0.13; no independent effect of week of onset is seen in this bivariate model. Similar results are found for longitude (data not shown). Subject to the caveats of performing a linear regression on only 17 cities with such highly correlated explanatory variables, this finding suggests that the relationship between early intervention and lower peak death rates is explained by factors of geography or timing of epidemic onset only to the extent that these factors influenced the quality of the public health response.
Sensitivity Analyses.
Similar results were obtained when the intervention date was defined as the date public health orders were promulgated (Table 2) or the last date a particular type of gathering was permitted (e.g., Sunday church service; SI Table 4). Results were identical or improved when 7- and 10-day lags in assessing CEPID were introduced to account for the lag between infection and death (SI Tables 5 and 6).
Relationship Between Interventions and Subsequent Waves.
Although it was not the primary intent of this paper to analyze pandemic wave dynamics, it is possible to formulate descriptive observations from the data at hand (SI Table 7). In offering these observations, it is important to underscore that in some cities, information about the dates of relaxation of the interventions used was incomplete.
All cities showed some fluctuation in mortality rates after the main wave of the 1918 pandemic subsided. The peak weekly mortality rates observed in “second waves” in the cities we studied ranged from 13.60 to 79.69/100,000, as compared with 31.29–256.96/100,000 during the first wave. There was a statistically significant inverse correlation of the height of the first and second peaks (Spearman ρ = −0.53, P = 0.03), so that cities that had low peaks during the first wave were at greater risk of a large second wave. Cities that had lower peak mortality rates during the first wave also tended to experience their second waves after a shorter interval of time, ≈6–8 weeks after the first peak vs. 10–14 weeks for cities with higher peak mortality rates (Spearman ρ = −0.84, P < 0.0001). These patterns were also observed in cities that implemented NPIs sooner [as assessed by ranking the cities according to their CEPID at school closure (Spearman ρ = 0.63, P = 0.006) or CEPID at time of the fourth intervention (Spearman ρ = 0.52, P = 0.03)]. Finally, and this is perhaps the most important observation, no city in our analysis experienced a second wave while its main battery of NPIs was in place. Second waves occurred only after the relaxation of interventions.
Discussion
Comparisons across 17 U.S. cities show that the first peak in excess P&I death rates during the fall wave of the 1918 influenza pandemic was ≈50% lower in cities that implemented multiple NPIs to control disease spread early in their epidemics than in cities that made such interventions late or not at all. This finding suggests that such interventions may be capable of significantly reducing the rate of disease transmission so long as they remain in effect.
If NPIs were maintained indefinitely once they were put in place, one would expect that early interventions would be associated with a reduction in both the peak incidence (and therefore peak death rate) and also in the cumulative incidence or cumulative excess death rate. However, NPIs used in 1918 did not last indefinitely; rather, most of the NPIs in the study cities appear to have been relaxed within 2–8 weeks, whereas opportunities for reintroduction and transmission of the pandemic virus extended for many months. If highly effective NPIs are put in place early in the epidemic, and these result in a smaller epidemic, then a large proportion of the population will remain susceptible to the renewed spread of the virus once interventions are relaxed. In the absence of an effective method of otherwise inducing immunity in the uninfected population (i.e., a well matched vaccine), such an epidemic is likely to have two phases, with the first phase mitigated by NPIs and the second commencing after NPIs are relaxed. In our review of 17 cities, we observed that cities that implemented NPIs sooner had lower peak mortality rates during the first wave and were at greater risk of a large second wave. These cities also tended to experience their second waves after a shorter interval of time. As described above, no city in our analysis experienced a second wave while its main battery of NPIs was in place, and second waves occurred only after the relaxation of NPIs.
A mitigated two-phase epidemic may result in a cumulative burden of morbidity and mortality less than that observed in a single unchecked epidemic because of reduced epidemic overshoot (7–9). However, the relationship between the timing of transiently maintained NPIs and final outcomes will be complicated and not necessarily monotonic (10). Because our goal was to assess the evidence for an effect of NPIs on transmission, rather than to assess whether the particular NPIs in 1918 were sustained long enough to prevent epidemic spread altogether, we defined peak death rates a priori as the main outcome measure. Consistent with these expectations, the relationship between intervention timing and peak death rates was stronger and statistically more convincing than that with total death rates in 1918.
The most important limitation of our study is that we used observed weekly excess fatality rates as a proxy for weekly community morbidity rates, which are not available for the study period. We believe that untransformed excess mortality rates are the most reliable (and least assumption-laden) record of the effects of the pandemic, but it is important to note that case fatality proportions (CFP) in 1918 appear to have varied between populations [being higher, for example, among the Inuit than in the general United States population (1)], likely as a result of differing levels of general public health, and it is possible that they varied between cities in the United States for similar reasons. Varying patterns of bacterial colonization or other, unidentified factors could also have contributed to variation in CFP. Differences in CFP between the cities could introduce a systematic error into our results (because they would lead to higher total deaths at a given stage of the epidemic, and higher peaks, in the same subset of cities). Our use of a normalized peak death rate was designed to avoid this error. If our results were artifacts of city-to-city variation in CFP, then the associations found should become weaker after this normalization; in fact, each of the strongest associations was at least comparably strong after the normalization (Table 2, Normalized peak), suggesting that variation in CFP did not create the associations we found.
More generally, a possible explanation for our findings is that inherently small epidemics (i.e., epidemics with flatter and smaller overall mortality curves, because of variation in CFP or in other factors not considered in our analysis) could appear to be associated with earlier interventions as an artifact of how we defined “early.” If this were the case, however, even ineffectual NPIs, considered individually, should correlate with lower peak mortality rates. In fact, NPIs that seem less likely to block transmission directly (e.g., making influenza a notifiable disease, closing dance halls, and bans on public funerals) had no such association. That several individual interventions were found not to be associated with lower peaks suggests this statistical artifact is not present.
Previous authors have noted that epidemics that started later tended to be milder and have speculated that this might be due to attenuation of the causative virus (3). Although viral attenuation may explain changes in the CFP over the course of the pandemic period (which extended to approximately March 1920), this mechanism seems an unlikely explanation for the striking variability of outcomes during the 1918 fall wave, given the marked transmissibility of the lethal virus and the short intervals between the onset of epidemics in different cities. A potentially more plausible explanation is that public health and political authorities in cities that were struck later responded more quickly and aggressively because they had several weeks' notice of the severity of the pandemic. Subject to the caveats attendant on a linear regression in such a small data set, we found that the stage of the epidemic at the time of interventions predicted peak mortality better than timing of epidemic onset. This finding suggests that the association between early intervention and lower peak mortality may be explained in large part by the fact that later-hit cities responded more promptly. Similar results were obtained when longitude was included in the analysis along with or in place of time of epidemic onset. Although we do not know of any mechanistic hypothesis connecting longitude directly to epidemic severity, our analysis similarly suggests that longitude is not an important confounder of our results.
In a related vein, the analysis of second peaks adds credence to the inference that NPIs were responsible for the observed lower first peaks in cities that implemented NPIs promptly. If lower first peaks were attributable to some other mechanism (e.g., a less virulent virus, seasonal changes in transmission, etc.), it is difficult to explain why, upon relaxation of NPIs, these low-peak cities tended to have larger second peaks. On the other hand, if NPIs curtailed the first wave, leaving more susceptibles in the early-intervention cities, then one would expect a more severe second wave in these cities, as was observed. Altogether, we take these findings as evidence that NPIs were capable of reducing influenza transmission in 1918, but that their benefits (as one would expect) were limited to the time they remained in effect.
In sensitivity analyses, we found that associations between early intervention and better outcomes were strengthened when we timed interventions based on the cumulative excess deaths up to 7 or 10 days after the intervention, an effort to account for the delay expected from case incidence (which is affected by interventions) to mortality. In part, this strengthening likely is due to the fact that delayed death figures better reflect the true stage of the epidemic at time of intervention. However, use of a delay time in this fashion raises concerns about reverse causality. If a delay longer than the shortest time from infection to death is used (e.g., the median, rather than the minimum, time to death), then the number of deaths before intervention, the independent variable in our analysis, is affected by the intervention itself. To avoid such difficulties, we took as our primary analysis the simpler, more conservative approach of defining the stage of the epidemic based on the date of intervention, with no delay. This choice has the additional benefit that in future pandemics, the cumulative excess death rate at the time of an intervention is in principle knowable in nearly real time, whereas the delayed death rate cannot by definition be known at the time of an intervention.
The implications of our analysis should be interpreted with care. Our univariate analyses of the relationship between individual NPIs and outcomes are consistent with the hypothesis that social distancing through closure of particular institutions (schools, churches, and theaters) led to reduced transmission, but the similarities in timing of various NPIs within a given city make it very difficult to discriminate the relative contributions of individual interventions (Fig. 2). Similarly, it was not possible to evaluate the effects of NPIs that were undertaken only in a small number of cities, or that were generally implemented only late in the epidemic, if at all, such as mass transit interventions (rules forbidding crowding and introduction of staggered business hours to reduce crowding on mass transit) or mask ordinances. Whether these NPIs might have made a difference in particular cities where they were implemented early, such early implementation was not common enough to evaluate whether it was associated with better outcomes. A third consideration is that the historical record is not seamless, and it is possible that our source material did not capture the full range of interventions used or reflect the true timing of implementation of those it identifies. Finally, we note that causality may be complicated; the interventions used may themselves have produced the observed effects, or they could have worked by shaping perceptions about the epidemic and causing changes in unmeasured private behaviors. Despite these caveats about the details of interpretation, the relationships detected in our analyses strongly suggest that the aggressive implementation of NPIs resulted in flatter epidemic curves and a trend toward better overall outcomes in the fall of 1918.
To the extent that these results provide evidence that multiple NPIs can reduce influenza transmission and mitigate the impact of a pandemic, they should inform current efforts related to pandemic preparedness. In particular, our results underscore the need for prompt action by public health authorities. The strongest relationship between peak death rates and timing of NPIs was observed for the number of interventions in place before the CEPID exceeded 20/100,000. If we assume a 2% CFP, this approximately corresponds to interventions undertaken before the deaths caused by infections in 1% of the population in a given city had occurred. Given the rate of growth of the pandemic and the lag between infection and death, perhaps 3–6% of the population would have been infected at this time. This finding emphasizes the need for very rapid interventions to stem the spread of the disease. Communities that prepare to implement layered NPIs aggressively are likely to achieve better outcomes than communities that introduce such interventions reactively, and they may be better positioned to manage the disruption caused by the more stringent interventions, such as school closure.
Finally, an important practical issue that requires further study is the question of when such interventions can be relaxed. The implication of patterns observed in the timing and severity of second waves in 1918 seems clear, however. In the absence of an effective vaccine, cities that use NPIs to mitigate the impact of a pandemic remain vulnerable. In practice, and until emergency vaccine production capacity increases, this means that in the event of a severe pandemic, cities will likely need to maintain NPIs for longer than the 2–8 weeks that was the norm in 1918.
Methods
Historical Data.
We defined our study period as September 8–December 28, 1918, encompassing the first 16 weeks for which excess P&I death rates were reported by ref. 11. Of the 45 cities reported in ref. 11, we eliminated those cities for which >4 weeks during the study period had missing or partial data (partial data included excess pneumonia deaths only or excess influenza deaths only). Of the remaining cities, we included in the final analysis those 17 cities for which we were able to obtain a complete account of public health responses during the study period from our research in period newspapers, public health reports, or municipal records; from consultations with current public health officials in the study cities; or from well documented secondary sources. We defined 19 categories of public health responses (NPIs, interventions, or measures) and scored the date on which a city implemented each of these interventions. Citations for the scoring of individual NPIs in each city are provided in SI Appendix.
Interventions.
Cities were scored as implementing an intervention if available evidence suggested that a measure was implemented on a community-wide basis through policy actions. Cities attempting to influence public behavior through exhortation alone (e.g., a recommendation to “avoid crowds” without an explicit ban on their formation) were not scored as implementing an intervention. Where possible, dates of implementation of NPIs were cross-checked against multiple sources.
Timing of NPIs was assessed relative to the epidemic in each city by defining the “stage” of an epidemic for a given intervention as the estimated CEPID from September 8, 1918, through the calendar date on which the intervention was announced. Linear interpolation was used for cumulative deaths when this date was between weekly reporting dates in ref. 11. In sensitivity analyses performed to account for the interval between infection (the true measure of transmission) and death (an outcome of infection), we also considered lags of 7 or 10 days in calculating the CEPID, that is, defining the stage of the epidemic at which an intervention was implemented as the CEPID 7 or 10 days after the date of intervention (the median time from infection to death in autopsy reports tabulated in ref. 12 was ≈10 days). In a separate sensitivity analysis, we defined the date of the intervention as the last day that a particular activity was possible, rather than the date on which it was banned. Thus, for example, if a ban on church services was announced on a day other than Sunday, the last activity date was defined as the preceding Sunday; likewise, if school closure was announced during a weekend, the last activity date was the preceding Friday.
The timing of a city's overall response was scored in two closely related ways. First, the number of NPIs (of a possible 19) announced by a city before the CEPID reached a particular threshold (e.g., 20/100,000) was quantified as “number of interventions before CEPID = 20/100,000.” This threshold was varied from 10 to 40/100,000 to encompass the range in which there was substantial intercity variation. Second, the CEPID at the time of the first intervention imposed in a city, the second intervention, and so on up to the sixth intervention was calculated.
Outcomes.
Epidemic outcomes were measured as (i) the first weekly peak excess death rate during the fall wave of the pandemic; (ii) normalized peak death rate: the ratio of i to the median weekly death rate for a given city during the study period; and (iii) cumulative excess deaths during the study period. Outcome ii was selected as a measure of the “peakedness” of the epidemic curve that would be insensitive to intercity differences in the CFP.
Data in SI Tables 8–11.
Outcomes and CEPID at the time of each intervention are provided in SI Table 8. Dates of intervention intent used in the primary analysis are provided in SI Table 9, whereas last activity dates used in sensitivity analyses are provided in SI Table 10. Weekly excess P&I death rate data transcribed from ref. 10 are provided in SI Table 11. SI Tables 7–11 are in Excel format.
Analysis.
To avoid issues of reverse causality and reduce some forms of confounding, the data were analyzed in a fashion similar to an “intention to treat” analysis: that is, NPIs were scored on the date they were announced, and the duration, effectiveness, or other features of the intervention were not considered in the analysis.
Associations between overall intervention timing and outcomes were assessed by Spearman rank correlation coefficients and associated P values calculated between the measures of overall response (number of interventions before CEPID = x or CEPID at the time of the xth intervention) and the three outcome measures. Univariate associations between the timing of particular NPIs and the outcomes were also assessed by Spearman rank correlation coefficients and associated P values. In these cases, multivariate analyses were not performed because of the small sample size and strong collinearity of many intervention timings.
Because of specific concerns that later-hit cities might have had milder epidemics for reasons other than interventions, we did perform linear regression of peak death rate on longitude and epidemic onset week, along with intervention timing (number of interventions before CEPID = 20/100,000) and eliminated model variables by backward selection.
For NPIs that showed significant or nearly significant overall correlations with outcomes, we divided cities as evenly as possible into early and late-intervening cities (eight in the early group and nine in the late or vice versa) and plotted the median outcome for each group. The round-numbered cutoff that created this division is shown in Fig. 2. Mann–Whitney U tests were used to assess statistical significance of differences in the distributions.
Supplementary Material
Acknowledgments
We thank Lisa Koonin for invaluable and indefatigable assistance; Katondra Lee for data retrieval and entry; and John Barry, Barry Bloom, Martin Cetron, Paul Glezen, Howard Markel, Christina Mills, and David Morens for constructive criticism. The analysis presented here would not have been possible without the contributions of a large number of public health and medical professionals, historians, librarians, journalists, and private citizens, especially Virginia Aita, Terry Allan, Jim Anderson, James Apa, Rex Archer, Steven Burg, Pat Cusick, Curt Dalton, Esther Day, Karen Evans, Evangeline Franklin, Jackie Frederick, Gary Gernhart, Anna Gillio, Rob Gillio, Gerald Hoff, Blythe Horman, Erika Janik, Lucy Killen, Chris Kippes, Judith Leavitt, Harry Levins, Meredith Li-Vollmer, Dorann Loehr, Mark McKinstry, Jackie Phillips, Shawn Richards, Kevin Stephens, and Dorothy Teeter. M.L. was supported by cooperative agreement 5U01GM076497 (Models of Infectious Disease Agent Study) from the National Institutes of Health.
Abbreviations
- P&I
pneumonia and influenza
- CEPID
cumulative excess P&I deaths
- NPI
nonpharmaceutical intervention
- CFP
case-fatality proportion.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 7313.
This article contains supporting information online at www.pnas.org/cgi/content/full/0610941104/DC1.
References
- 1.Crosby A. America's Forgotten Pandemic: The Influenza of 1918. 2nd Ed. Cambridge: Cambridge Univ Press; 2003. [Google Scholar]
- 2.Johnson NP, Mueller J. Bull Hist Med. 2002;76:105–115. doi: 10.1353/bhm.2002.0022. [DOI] [PubMed] [Google Scholar]
- 3.Patterson KD, Pyle GF. Bull Hist Med. 1991;65:4–21. [PubMed] [Google Scholar]
- 4.WHO. Wkly Epidemiol Rec. 2006;81:249–257. [Google Scholar]
- 5.Committee on Modeling Community Containment for Pandemic Influenza. Modeling Community Containment for Pandemic Influenza: A Letter Report. Washington, DC: Institute of Medicine of the National Academies; 2006. [Google Scholar]
- 6.World Health Organization Writing Group. Emerg Infect Dis. 2006;12:88–94. doi: 10.3201/eid1201.051371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bootsma MCJ, Ferguson NM. Proc Natl Acad Sci USA. 2007;104:7588–7593. doi: 10.1073/pnas.0611071104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Handel A, Longini IM, Antia R. Proc R Soc London Ser B. 2007;274:833–837. doi: 10.1098/rspb.2006.0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lipsitch M, Cohen T, Murray M, Levin BR. PLoS Med. 2007;4:e15. doi: 10.1371/journal.pmed.0040015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sattenspiel L, Herring DA. Bull Math Biol. 2003;65:1–26. doi: 10.1006/bulm.2002.0317. [DOI] [PubMed] [Google Scholar]
- 11.Collins SD, Frost WH, Gover M, Sydenstricker E. Public Health Rep. 1930;45:2277–2328. [Google Scholar]
- 12.Mills CE, Robins JM, Lipsitch M. Nature. 2004;432:904–906. doi: 10.1038/nature03063. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.