Abstract
During the past 5 decades, the recognition and management of thoracic outlet syndrome (TOS) have evolved. This article elucidates these changes and improvements in the diagnosis and management of TOS at Baylor University Medical Center. The most remarkable change over the past 50 years is the use of nerve conduction velocity to diagnose and monitor patients with nerve compression. Recognition that procedures such as breast implantation and median sternotomy may produce TOS has been revealing. Prompt thrombolysis followed by surgical venous decompression for Paget-Schroetter syndrome has markedly improved results compared with the conservative anticoagulation approach; thrombolysis and prompt first rib resection is the optimal treatment for most patients with Paget-Schroetter syndrome. Complete first rib extirpation at the initial procedure markedly reduces the incidence of recurrent neurologic symptoms or the need for a second procedure. Chest pain or pseudoangina can be caused by TOS. Dorsal sympathectomy is helpful for patients with sympathetic maintained pain syndrome or causalgia and patients with recurrent TOS symptoms who need a second procedure.
Thoracic outlet syndrome (TOS) refers to compression of one or more of the neurovascular structures traversing the superior aperture of the chest. Previously, the name was designated according to the etiologies of compression, such as scalenus anticus, costoclavicular, hyperabduction, cervical rib, or first rib syndromes. Peet (1) coined the term “thoracic outlet syndrome” in 1956 to designate compression at the neurovascular bundle at the thoracic outlet.
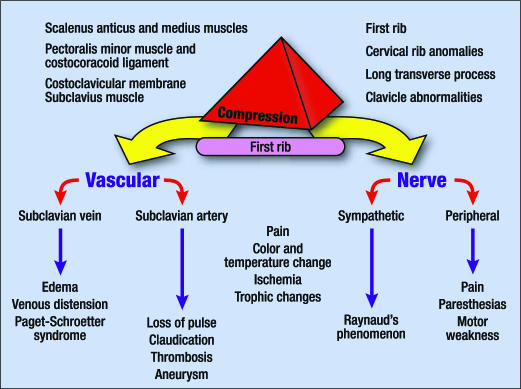
Most compressive factors operate against the first rib and produce a variety of symptoms, depending on which neurovascular structures are compressed. The functional anatomy and pathophysiology of compression in the thoracic outlet and the symptomatology of each of the specific structures compressed are summarized in Figure 1.
Figure 1.
Compression factors in the thoracic outlet with the signs and symptoms produced.
This article discusses the changes in the diagnosis and management of TOS at Baylor University Medical Center. From 1947 through 2005, in our group practice of six surgeons and three physiatrists, more than 20,000 patients were evaluated for TOS. Of these, 5102 underwent primary neurovascular decompression procedures and 2305 (most of whom were from other centers) had second procedures for recurrent symptoms. Evaluation of these patients provides the basis for this report.
A PERSONAL INTEREST
One of the authors, Dr. Urschel, developed a personal interest in the diagnosis and treatment of TOS while he played college football at Princeton from 1947 to 1951. Recruited from Ohio, where he was all-state, to play blocking back in Charles Caldwell's single-wing formation, he noticed that when his neck was knocked to the right, his arm would be paralyzed for 2 days. He was sent to Johns Hopkins Hospital to see Dr. George Bennett, who had just operated on Joe DiMaggio's knee. After the examination, Dr. Bennett said, “Urschel, you have an extra (cervical) rib for which I can either operate or build you a brace.” Realizing early that “surgery was for others,” he elected the brace. They forged a piece of steel to his shoulder pad and covered it with leather. It was excellent and highly successful in alleviating his symptoms. However, because players didn't wear face masks, occasionally the steel brace would take a nose off. It was prohibited at the end of the year, and Urschel was given the first doughnut pad to wear around his neck, like many players wear today. That Princeton football team was outstanding in the “golden era” of Ivy League football: they were undefeated 3 out of 4 years; were ranked second in the nation and first in offense and rushing; and never played with <70,000 people in the stands. Caldwell was named college coach of the year, and Dick Kazmaier, triple-threat tailback, won the Heisman trophy.
When Dr. Urschel attended Harvard Medical School and was chief surgical resident at the Massachusetts General Hospital, many teachers, particularly neurologists, dismissed the diagnosis of TOS and said it didn't exist. Dr. Urschel knew better, and it has been one of his avocations ever since.
HISTORICAL NOTE
Until 1927 the cervical rib was commonly thought to be the cause of symptoms of TOS. Galen and Vesalius first described the presence of a cervical rib (2). Hunauld, who published an article in 1742, is credited by Keen (3) as being the first to describe the importance of the cervical rib in causing symptoms. In 1818, Cooper treated symptoms of cervical rib with some success (4), and in 1861, Coote (5) performed the first cervical rib removal. Sir James Paget (6), in 1875 in London, and von Schroetter (7), in 1884 in Vienna, described the syndrome of thrombosis of the axillary-subclavian vein (Paget-Schroetter syndrome [PSS]). Halsted (8) stimulated interest in dilatation of the subclavian artery distal to cervical ribs, and Law (9) reported the role of adventitious ligaments in the cervical rib syndrome. Naffziger and Grant (10) and Ochsner and associates (11) popularized section of the scalenus anticus muscle. Falconer and Weddell (12) and Brintnall and associates (13) incriminated the costoclavicular membrane in the production of neurovascular compression. Wright (14) described the hyperabduction syndrome with compression in the costoclavicular area by the tendon of the pectoralis minor.
Rosati and Lord (15) added claviculectomy to anterior exploration, scalenotomy, resection of the cervical rib (when one was present), and section of the pectoralis minor and subclavian muscles as well as of the costoclavicular membrane. The role of the first rib in causing symptoms of neurovascular compression was recognized by Bramwell (16) in 1903. Murphy (17) is credited with the first resection of the first rib and in 1916 provided a collective review of 112 articles related to compression from the cervical ribs. Brinckner and Milch (18), Brinckner (19), Telford and Stopford (20), and Telford and Mottershead (21) suggested that the first rib was the culprit. Clagett (22) emphasized the first rib and its resection through the posterior thoracoplasty approach to relieve neurovascular compression. Falconer and Li (23) reported the anterior approach for first rib resection, whereas Roos (24) introduced the transaxillary route for first rib resection and extirpation. Krusen (25) and Caldwell and coworkers (26) introduced the measurement of motor conduction velocities across the thoracic outlet in diagnosing TOS. Urschel and associates (27) popularized reoperation for recurrent TOS and thrombolysis with prompt transaxillary rib resection for PSS (28). Wilbourn (29) emphasized the controversial nature of neurogenic TOS without intrinsic muscle wasting. Mackinnon (30–32) stressed the merits of appropriate physical therapy and recognition of associated distal entrapment neuropathies in the management of the patient with TOS.
SURGICAL ANATOMY
At the superior aperture of the thorax, the subclavian vessels and the brachial plexus traverse the cervicoaxillary canal to reach the upper extremity (Figure 2) (33). The cervicoaxillary canal is divided by the first rib into two sections: the proximal one, composed of the scalene triangle and the costoclavicular space (the space bounded by the clavicle and the first rib), and the distal one, composed of the axilla. The proximal division is the more critical for neurovascular compression. It is bounded superiorly by the clavicle, inferiorly by the first rib, anteromedially by the costoclavicular ligament, and posterolaterally by the scalenus medius muscle and the long thoracic nerve. The scalenus anticus muscle, which inserts on the scalene tubercle of the first rib, divides the costoclavicular space into two compartments: the anteromedial compartment, which contains the subclavian vein, and the scalene triangle, which is bounded by the scalenus anticus anteriorly, the scalenus medius posteriorly, and the first rib inferiorly and contains the subclavian artery and brachial plexus. This region of neurovascular compression is in anatomical reality the thoracic inlet and not the thoracic outlet (34). Cervical ribs narrow the space more (Figure 3).
Figure 2.
Neurovascular structures traversing the thoracic outlet. Netter illustration used with permission of Elsevier Inc. All rights reserved.
Figure 3.
Cervical ribs and related anomalies. Netter illustration used with permission of Elsevier Inc. All rights reserved.
Compression may occur at several different sites: the cervical disc, the thoracic outlet, the cubital tunnel, median nerve compression in the forearm, the carpal tunnel, and Guyon's canal. There may be multiple points of compression of the peripheral nerves between the cervical spine and hand, in addition to the thoracic outlet. When there are multiple compression sites, less pressure is required at each site to produce symptoms. Thus, a patient may have concomitant TOS, ulnar nerve compression at the elbow, and carpal tunnel syndrome. This phenomenon has been called the “multiple crush” syndrome.
TOS is commonly caused by repetitive motion, particularly in the shoulder and hand. Numerous baseball pitchers, for example, have had TOS, including “Whitey” Ford of the Yankees, who had dorsal sympathectomy; J. R. Richards of the Astors, who had a stroke; “Oil Can” Boyd of the Red Sox, who had PSS; and David Cone of the Yankees, who had an aneurysm resection. Clavicular trauma is another cause of TOS. Contributing factors include poor posture, polio, and pregnancy. Another contributing factor is hypertrophy or surgery of the breast, including both implants and radical mastectomy. Extreme opening of the median sternotomy retractor may produce TOS as well.
NERVE COMPRESSION
In the late 1950s and early 1960s, the surgical procedure of choice in our practice was the supraclavicular scalenectomy: partial scalenectomy with neurolysis of the brachial plexus when indicated combined with resection of a cervical rib if present. Of our 336 patients treated with this approach, 310 did well in the short term. However, the long-term outcomes were not as satisfactory. At 5 years, 150 of 336 patients had improvement of their symptoms, but at 20 years only 31 did (with 20 patients lost to follow-up). Because of these results and the 1962 presentation by Clagett (22), the posterior approach was used for resection of the first rib, the common denominator for thoracic outlet compression forces. Subsequently, the initial procedure was usually performed through the transaxillary approach because no muscle division was required and morbidity rates were lower. The supraclavicular or infraclavicular approach or the combined approach was used for arterial lesions. The posterior approach is now reserved for reoperative procedures in patients with recurrent TOS symptoms for removal of rib remnants and regenerated fibrocartilage with neurolysis of C7, C8, and T1 nerve roots and the brachial plexus.
The symptoms of nerve compression most frequently observed are pain and paresthesias (present in approximately 95% of patients) and motor weakness (<10%). Pain and paresthesias are segmental in 75% of cases, 90% involving the ulnar nerve distribution (35). TOS can occur in older patients (the oldest reported has been 87 years). However, when nerve compression symptoms occur in patients older than 60, causes such as degenerative or traumatic cervical spine, cardiac, or pulmonary pathologies should be suspected and ruled out.
DIAGNOSIS
A careful history and physical examination are critical for accurate diagnosis. Several tests are used in the diagnosis, including nerve conduction velocity (NCV), electromyography, and radiographic studies of the chest and cervical spine (radiographs, computed tomography scans, and magnetic resonance images). Consultations with specialists in neurology, physical medicine, cardiology, and angiography may also be appropriate.
The multiple physical signs of thoracic outlet compression and the classic diagnostic tests have been thoroughly reviewed (36). Other causes of TOS-like symptoms, such as cardiac or pulmonary disease, must be ruled out. The electromyogram should be normal, eliminating other neuromuscular disorders.
The primary objective test for thoracic outlet peripheral nerve compression in our clinic is the NCV (Figure 4). This test was improved and popularized at Baylor University Medical Center by Krusen, Caldwell, and Crane. Reduction in NCV to <85 m/s of either the ulnar or median nerves across the thoracic outlet corroborates the clinical diagnosis. More than 8000 NCV studies were performed annually at our medical center for many years, with approximately 3200 patients per year demonstrating TOS (38%) (37).
Figure 4.
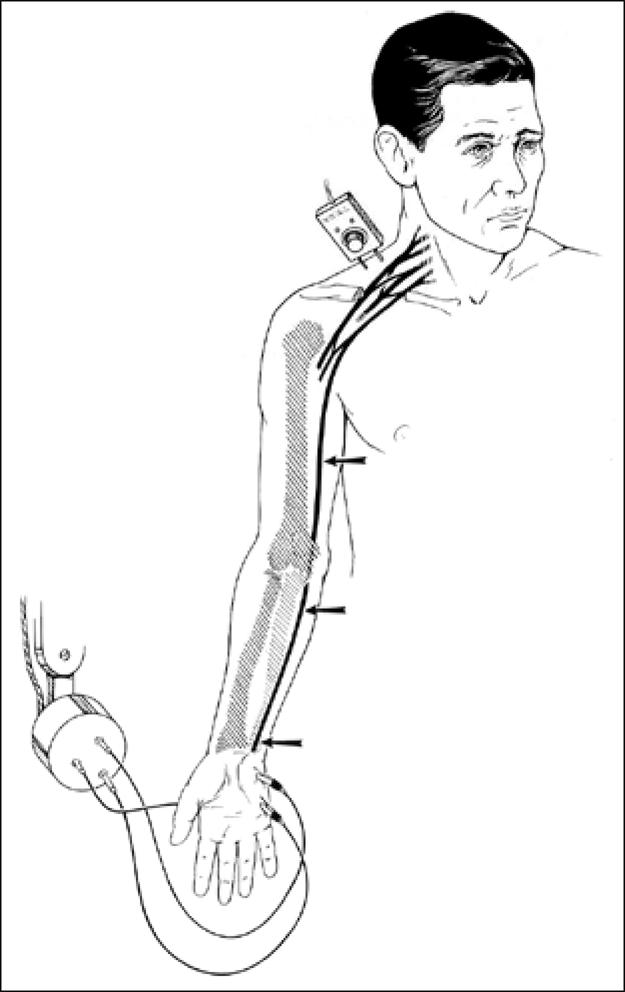
Nerve conduction velocity measurement technique. There is a “blip” on the oscilloscope when the electric current hits the electrode.
Initially, most patients, except those with vascular and motor nerve problems, were treated conservatively with physical therapy. Patients with an NCV exceeding 60 m/s were especially likely to benefit from this approach. Excellent principles of conservative management have been outlined by Mackinnon and Novak (30) and Caldwell, Crane, and Krusen (26). The primary goals of physical therapy are to open up the space between the clavicle and first rib, improve posture, strengthen the shoulder girdle, and loosen the neck muscles. These goals are accomplished by exercises that stretch the pectoralis muscles, exercises that strengthen the muscles between the shoulder blades, and neck exercises, including chin tuck, flexion, rotation, lateral bending, and circumduction. In addition, patients' work habits and sleep positions occasionally need to be modified, and some patients can benefit from weight loss.
The usual indications for surgery are failure of appropriate conservative therapy in a patient with a significantly reduced NCV (<60 m/s; normal is 85 m/s) and the elimination of other possible etiologies for the symptoms (Table 1).
Table 1.
Indications for surgery in thoracic outlet syndrome
| Area of compression | Symptoms indicating the need for surgery |
| Nerve | Sensory: Persistent symptoms in spite of physical therapy Motor: Weakness or atrophy |
| Artery | Aneurysm or symptomatic insufficiency |
| Vein | Occlusion (Paget-Schroetter syndrome) |
| Multiple | Therapeutic trial |
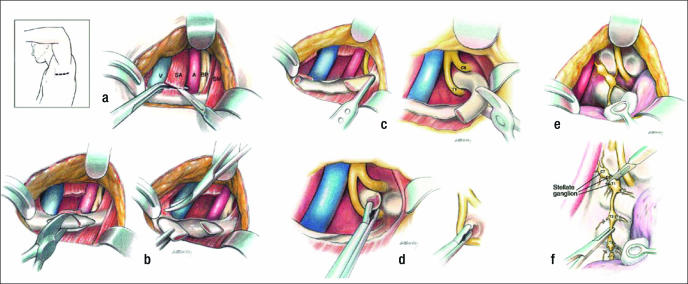
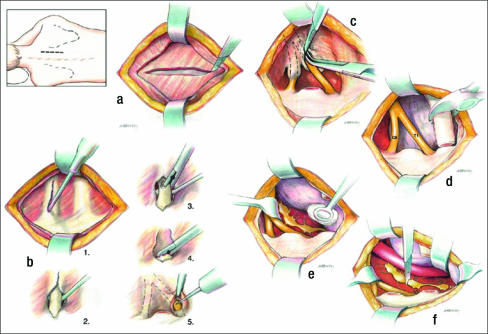
Initial surgical therapy involves complete first rib resection, anterior scalenectomy, resection of the costoclavicular ligament, and neurolysis of C7, C8, and T1 nerve roots and the brachial plexus through a transaxillary approach (38) (Figure 5). Various instruments have been developed to expedite the procedure (Figure 6). The first rib with the compressive elements may also be removed using the supraclavicular approach (39). This approach, however, requires working through and retracting the brachial plexus and produces a visible scar in women (the preponderant gender with TOS). The posterior thoracoplasty approach for first rib resection may be used for initial therapy, but in our clinic it is reserved for a second procedure and neurolysis of the brachial plexus (40, 41). Cervical ribs may be removed using any of the approaches described. Dorsal sympathectomy may also be performed with neurovascular decompression through any of the above incisions for sympathetic maintained pain syndrome (SMPS), reflex sympathetic dystrophy, causalgia, and Raynaud's phenomenon and disease (42). Table 2 summarizes the surgical approaches that are employed at BUMC.
Figure 5.
Transaxillary first rib resection. (a) Division of the scalenus anticus muscle. (b) Division of the first rib and anterior resection. (c) Posterior resection of the first rib. (d) Resection of head and neck of rib. (e) Identification of the dorsal sympathetic chain. (f) Division through the lower stellate ganglion above T1 and below T3 ganglia. V indicates subclavian vein; SA, scalenus anticus muscle; A, subclavian artery; BP, brachial plexus; SM, sternocleido-mastoid muscle.
Figure 6.
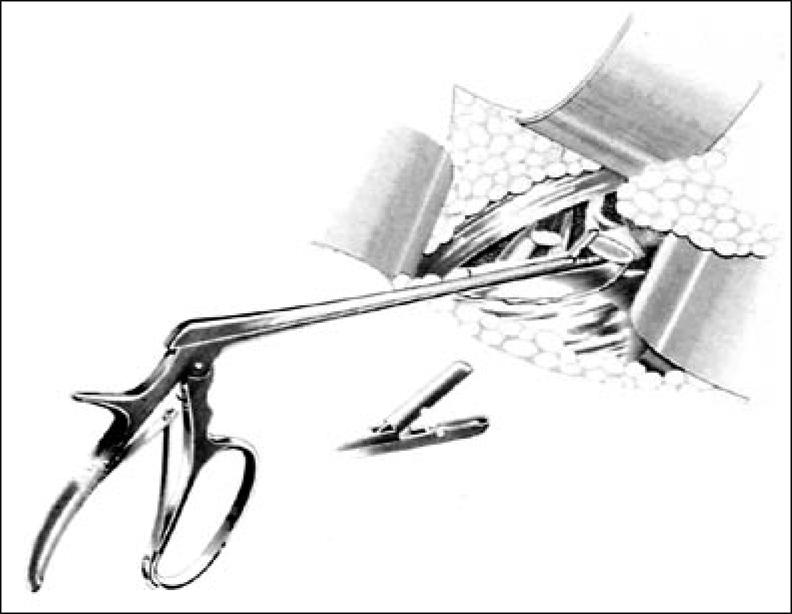
Urschel first rib rongeur.
Table 2.
Surgical approaches for thoracic outlet syndrome used at Baylor University Medical Center
| Type of problem | Surgical approach |
| Nerve compression | Transaxillary |
| Arterial compression | Supra- and infraclavicular |
| Venous compression | Transaxillary |
| Recurrent TOS | Posterior high thoracoplasty |
TOS indicates thoracic outlet syndrome.
UPPER PLEXUS VS LOWER PLEXUS
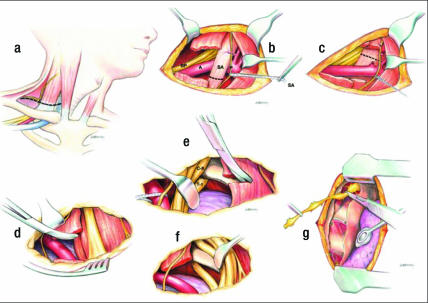
Most cases of neurologic TOS requiring surgery have been successfully managed with transaxillary resection of the first rib. However, for upper plexus (median nerve) compression, Stoney et al (43) and Urschel and Razzuk (44) wrote that transaxillary rib resection alone was not enough and that it should be combined with the supraclavicular approach (Figure 7) to achieve best results.
Figure 7.
Supraclavicular approach. (a) Supraclavicular incision. (b) Division of the scalenus anticus muscle. (c) Supraclavicular retraction of the neurovascular structures. (d–f) Resection of first rib. (g) Supraclavicular dorsal sympathectomy. BP indicates brachial plexus; A, subclavian artery; SA, scalenus anticus muscle; P, phrenic nerve.
Upper plexus compression was initially described by Swank and Simeone (45) with symptoms secondary to C5, C6, and C7 nerve root compression. Sensory changes occur primarily in the first three fingers, and muscle weakness or pain occurs in the anterior chest, triceps, deltoids, and parascapular muscle areas, as well as down the outer arm to the extensor muscles of the forearm. In contrast, lower plexus irritation involves C8 and T1 nerve root compression and includes sensory changes primarily in the fourth and fifth fingers, with muscle weakness or pain from the rhomboid and scapular muscles to the posterior axilla, down the ulnar distribution to the forearm, involving the elbow, flexors of the wrist, and intrinsic muscles of the hand. Urschel and Wood expanded the upper plexus symptoms to involve pain in the neck, face, mandible, and ear with occipital headaches. Wood and Ellison also noted dizziness, vertigo, and blurred vision in some patients with upper plexus lesions. In addition to these clinical symptoms and signs, median nerve conduction slowing indicates upper plexus compression, whereas ulnar nerve conduction slowing suggests lower plexus compression.
The rationale for why upper plexus symptoms are relieved by transaxillary first rib resection alone is based on several factors. Anatomic observations show that the median nerve, usually implicated in upper plexus compression of C5, C6, and C7 nerve roots, also receives significant fibers from C8 and T1 nerve roots. In addition, most muscles and ligaments that compress the upper plexus attach to the first rib. Thus, removing the first rib, with release of all the muscles and ligaments involved in compression, theoretically should relieve upper plexus compression.
To assess the optimal management of upper plexus TOS, we reviewed 2210 primary procedures for TOS in 1988 patients, 222 undergoing bilateral transaxillary resections, over a period of 30 years (44). Of the procedures, 250 were performed for symptoms, sings, and NCVs showing median nerve or upper plexus compression only; 452 were performed for compression of both the median and ulnar nerves or the combination of the upper plexus and lower plexus by symptoms, signs, and NCVs; and 1058 were performed for symptoms, signs, and NCVs demonstrating predominantly ulnar nerve or lower plexus compression. This study showed that transaxillary first rib resection with anterior scalenectomy relieved symptoms of upper plexus (96%) and combined upper and lower plexus (95%) compression as well as it did for lower plexus compression (95%). Patients were followed at 3 weeks and 3 months, and yearly contacts were made by phone. Wood, Ellison, and Sanders (46) independently corroborated these findings.
ARTERIAL COMPRESSION
The diagnosis of arterial compression is suspected by the results of the history, physical examination, and Doppler studies and is confirmed with arteriography (47). Therapy for arterial compression depends on its degree of involvement (Figure 8).
Figure 8.
Examples of arterial compression resulting from thoracic outlet compression. (a) Poststenotic dilatation of the axillary-subclavian artery. (b) Sacular aneurysm of the axillary-subclavian artery. (c) Total occlusion of the axillary subclavian artery.
An asymptomatic patient with cervical or first rib arterial compression producing poststenotic dilatation of the axillary subclavian artery should undergo rib resection, preferably using the transaxillary approach, removing the ribs, both first and cervical, without resecting the artery. The dilatation usually returns toward normal after removal of compression.
Patients with compression from the first or cervical rib producing aneurysm with or without thrombus should undergo rib resection and aneurysm excision with graft using the supraclavicular and infraclavicular combined approach.
Thrombosis of the axillary subclavian artery or distal emboli secondary to TOS compression should be treated with first rib resection, thrombectomy, embolectomy, arterial repair or replacement, and dorsal sympathectomy.
We successfully treated 196 patients with axillary subclavian artery aneurysm and 98 patients with occlusion. Dorsal sympathectomy was performed when indicated. In the occlusion group were 62 bypass grafts: 58 were successful; 3 occluded, requiring a second surgical procedure; and one could not prevent amputation because of the delay before the patient presented for therapy. No cerebrovascular accidents occurred.
SYMPATHETIC NERVE COMPRESSION
Compression of the sympathetic nerves in the thoracic outlet may occur alone or in combination with peripheral nerve and blood vessels. The sympathetics are intimately attached to the artery as well as adjacent to the bone. They may be compressed or irritated in primary or recurrent TOS. Atypical chest pain (pseudoangina) simulates cardiac pain (48).
Many arterial compressions result in more severe symptoms because of the additive or synergistic sympathetic stimulation. Trauma frequently is associated with SMPS or reflex sympathetic dystrophy.
For uncomplicated, nontraumatic TOS symptoms, usually first rib resection alone with neurovascular decompression relieves the sympathetic symptoms; dorsal sympathectomy is not required. However, if trauma is significant in the etiology, causalgia or SMPS is often present and concomitant dorsal sympathectomy is routinely required to ameliorate the symptoms. Also, if surgery is required for recurrent TOS symptoms, the relief of accompanying causalgia usually requires dorsal sympathectomy. Initially, dorsal sympathectomies were performed at an interval after procedures for traumatic or recurrent TOS, if necessary. However, because they were necessary in so many cases and because of the inconvenience of a second procedure, dorsal sympathectomy is now routinely combined with the initial TOS procedure for either trauma or recurrence cases (40, 41).
Major indications for dorsal sympathectomy include hyperhidrosis, Raynaud's phenomenon or disease, causalgia, SMPS, reflex sympathetic dystrophy, and vascular insufficiency of the upper extremity. Except for hyperhidrosis, most indications for sympathectomy require the usual diagnostic techniques, including cervical sympathetic block, to assess the relief of symptoms with temporary sympathetic blockade. When Raynaud's phenomenon of a minor to moderate degree is associated with TOS, the simple removal of the first rib with any cervical rib, in addition to stripping the axillary subclavian artery (neurectomy), generally relieves most symptoms after the initial procedure (49, 50). It is rarely necessary to perform a sympathectomy unless Raynaud's phenomenon is severe, in which case a dorsal sympathectomy is performed with first rib resection. The only contraindication to dorsal sympathectomy is venous obstruction (PSS; effort thrombosis of the axillary-subclavian vein).
SURGICAL APPROACHES FOR DORSAL SYMPATHECTOMY
Historically, the anterior cervical approach to the cervical sympathetic chain has been used for dorsal sympathectomy (51). The stellate ganglion lies on the transverse process of C6, and this approach is used primarily by neurosurgeons and vascular surgeons. For hypertension, Smithwick and Urschel popularized the posterior approach, with a longitudinal paraspinal incision with the patient in the prone position (52). Small pieces of the second ribs are removed, and the sympathetic chain is identified in the usual position. This approach has the advantage of allowing bilateral procedures to be performed at the same time without changing the patient's position. The most common current approach is the transaxillary, transthoracic approach, which is performed through the second interspace with a transverse subhairline incision (53). This is more painful than the other approaches, but with video-assisted thoracoscopy it can be performed with minimal discomfort (50).
The approach most frequently used for TOS and dorsal sympathectomy is the transaxillary approach, for first rib resection. This causes minimal pain and combines two procedures with a low morbidity rate. Video assistance is also used frequently with this approach.
Dorsal sympathectomy has been performed in 5147 extremities; 2602 of these were associated with neurologic TOS, causalgia, and SMPS, and 240 were associated with arterial complications of TOS. In the patients with neurologic TOS, 2305 dorsal sympathectomies were related to recurrent disease, and most were referred from elsewhere.
If symptoms recur, they happen, on average, in 3 years (range, 6 months to 25 years). In 46 patients, symptoms of sympathetic activity were apparent in <6 months. These symptoms are probably related to sprouting, or failure to strip the artery of its sympathetic nerves. This complication seems to occur less often if the bed of the sympathetic chain is cauterized after dorsal sympathectomy. It can also be explained by high circulating concentrations of catecholamines. The postsympathetic syndrome was observed in 39 extremities. This complication involves excessive postsurgical pain (as long as 6 months) in several nerve root distributions of the involved extremity and may be the result of injury to the somatic nerve. Unexpected Horner's syndrome was noted in 27 cases; 21 of these were transient and gradually resolved.
PSEUDOANGINA
The pain of pseudoangina is frequently insidious in onset, commonly involving the neck, shoulder, arm, and hand. In some patients, the pain is atypical, involving the anterior chest wall and parascapular area, and is termed pseudoangina because it simulates angina pectoris. Late symptoms of pseudoangina can include paresthesias of the arms and hands. Laboratory tests are used to differentiate pseudoangina from angina pectoris. In patients with pseudoangina, the results of electrocardiography, exercise stress testing, and coronary angiography are normal, whereas NCV is abnormal. A group of 320 patients with chest pain simulating angina pectoris, but with normal coronary angiograms, were evaluated in 1973. When either medical therapy (194 patients) or surgical therapy (98 patients) of TOS relieved the symptoms of pseudoangina, the diagnosis was considered confirmed (48).
To explain chest pain from TOS compression, it is important to remember there are at least two types of pain pathways in the arm: the commonly acknowledged (C5 to T1) somatic fibers, which transmit more superficial pain, and the afferent sympathetic nerve fibers, which transmit deeper painful stimuli. The cell bodies of the two types of neurons are situated in the dorsal root ganglia of the corresponding spinal segments. They synapse in the dorsal gray matter of the spinal cord, and the axons of the second-order neurons ascend in the spinal cord up to the brain. Compression of the superficial C8 to T1 cutaneous afferent fibers elicits stimuli that are transmitted to the brain and are recognized as integumentary pain or paresthesias in the ulnar nerve distribution. In contrast, compression of the predominantly deeper sensory fibers elicits impulses that are appreciated by the brain as deep pain originating in the arm or the chest wall, even if the source of the impulses is cardiac (referred pain).
VENOUS COMPRESSION
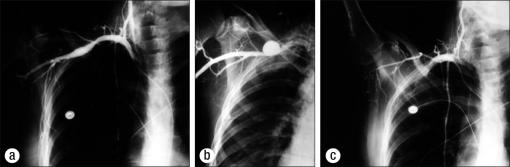
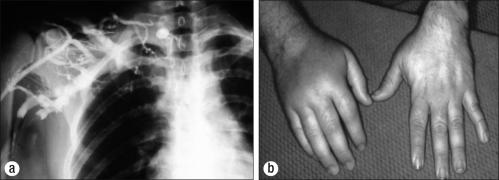
Effort thrombosis of the axillary-subclavian vein (PSS) is usually secondary to unusual, repetitive use of the arm, in addition to the presence of one or more compressive elements in the thoracic outlet. Sir James Paget (6) in 1875 in London and Von Schroetter (7) in 1884 in Vienna independently described this syndrome of thrombosis of the axillary-subclavian vein. The word “effort” was added to thrombosis because of the frequent association with exertion producing either direct or indirect compression of the vein. Phlegmasia cerulea dolens, or blue phlebitis, may occur in the arm. The thrombosis is caused by trauma or repetitive muscular activity, often occupational (e.g., professional athletes, linotype operators, painters, and cosmetologists). Cold and traumatic factors, such as carrying skis over the shoulder, tend to increase the proclivity for thrombosis. Elements of increased thrombogenicity also increase the incidence of this problem and exacerbate its symptoms on a long-term basis (54, 55). The diagnosis—suspected after a careful history, physical examination, and Doppler studies—is confirmed with venography (Figure 9).
Figure 9.
Paget-Schroetter syndrome. (a) Occlusion of the axillary-subclavian vein. (b) Swollen right hand.
The major pathophysiological cause of PSS is congenital aberration of the costoclavicular ligament, which inserts far lateral to its usual insertion on the first rib. If the scalenus anticus muscle hypertrophies, the vein is then compressed and occludes.
Intermittent or partial obstruction should be treated by first rib removal through the transaxillary approach, with resection of the costoclavicular ligament medially, the first rib inferiorly, and the scalenus anticus muscle laterally. The clavicle is left in place. The vein is decompressed, and all the bands and adhesions are removed.
For many years, complete thrombosis of the axillary-subcla-vian vein (PSS) was treated by elevation of the arm and the use of anticoagulants, with subsequent return to work. If symptoms recurred, a first rib resection, with or without thrombectomy, was considered, as well as resection of the scalenus anticus muscle and removal of any other compressive element in the thoracic outlet, such as the costoclavicular ligament, cervical rib, or abnormal bands (55). Of 36 patients treated by this approach, only 10 (28%) had a good to excellent result.
The availability of thrombolytic agents, combined with prompt surgical decompression of the neurovascular compressive elements in the thoracic outlet, has reduced the morbidity rate and the need for thrombectomy and has produced substantially improved clinical results, including the ability to return to work (28). Through an antecubital indwelling venous catheter, venography is performed and thrombolytic therapy is initiated. After lysis of the clot, prompt first rib resection with removal of compressive elements is performed. No postoperative anticoagulants are necessary.
Of the 625 patients treated for PSS, 581 underwent this newer technique. Thrombectomy was necessary in only four extremities. The long-term results indicate that 554 patients had good results (the patient returned to work without symptoms), 25 patients had fair results (intermittent swelling but able to work), and 2 patients had poor results (chronic swelling). Seven of the poor results occurred in the 35 patients initially seen more than 3 months after the thrombotic episode. No patient had phlegmasia cerulea dolens. There were no deaths. These results were in marked contrast to those of 35 patients treated. with only anticoagulants: 10 good results, 16 fair results, and 9 poor results. Delay in initiating thrombolytic therapy or the prolonged use of warfarin or heparin is contraindicated. There were no obvious complications of thrombolytic therapy. With a prolonged interval between venous occlusion and diagnosis (>3 months), the same management produced less favorable results. Attempts to open the occluded vein mechanically with the use of laser or bypass grafts have uniformly been unsatisfactory.
Claviculectomy is occasionally used for decompression, particularly if a fracture of the clavicle has occurred secondary to trauma (56, 57).
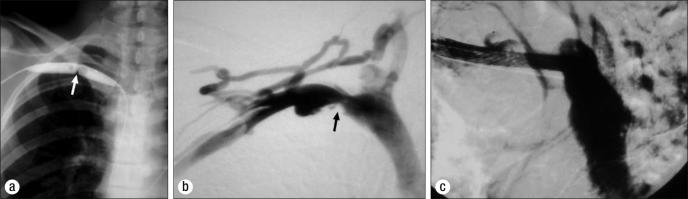
Many interventional radiologists or cardiologists respond to venous constriction as if it were arterial stenosis secondary to a plaque. They perform a percutaneous transluminal angiography and when the constriction appears to recoil after the balloon is deflated, they insert a stent “to hold it open.” This reaction shows poor understanding of the underlying pathophysiology, which is the external constriction by the congenitally abnormal lateral insertion of the costoclavicular ligament. The stent always occludes, making further treatment difficult (58) (Figure 10). The ideal management of PSS consists of an accurate diagnosis, expeditious thrombolytic therapy, and prompt first rib resection.
Figure 10.
Inappropriate treatment of Paget-Schroetter syndrome. (a) Pericutaneous balloon dilatation. (b) Venous recoil after balloon deflation. (c) Deleterious stent insertion.
RECURRENT THORACIC OUTLET SYNDROME
Recurrent symptoms, primarily neurogenic, should be documented by objective NCVs. When NCVs are depressed in a patient whose symptoms are unrelieved by prolonged conservative therapy, a posterior procedure should be considered. Removal of any rib remnants or regenerated fibrocartilage and neurolysis of C7, C8, and T1 nerve roots and the brachial plexus are performed (27). Dorsal sympathectomy is added to minimize the contribution of causalgia to symptoms. Methylprednisolone acetate and hyaluronic acid are employed to minimize recurrent scarring (40).
Two distinct groups of patients require a second procedure: those with pseudorecurrence and those with true recurrence. Pseudorecurrences were observed in 43 patients, all referred from other surgeons; these patients were never completely relieved of their symptoms after the initial procedure. They were separated into the following etiologies: mistaken resection of the second rib instead of the first (22 patients), resection of the first rib with a cervical rib left in place (11 patients), resection of a cervical rib with an abnormal first rib remaining (8 patients), and resection of a second rib with a rudimentary first rib left (2 patients). True recurrences occurred in 2305 extremities; some of these patients had relief of symptoms after the initial procedure, but the symptoms recurred 4 months to 18 years later.
The diagnosis and differential diagnosis for recurrence are similar to those for the original procedure. However, the indications for a second procedure are more stringent in that longer periods of conservative therapy are usually involved.
In the group needing a second procedure, a substantial posterior stump (>1 cm) of the first rib remained in 2106 patients (all referred from outside physicians). Complete resection of the first rib at the initial procedure was observed in 199 patients who had recurrent symptoms associated with excessive scar formation on the brachial plexus. We performed the original procedure on 98 of these patients, for a reoperation rate of 2.5% (3914 primary procedures). Even though some of our patients did not return to us for recurrent symptoms, this rate is much lower than in most series.
The preferred technique for reoperation is the posterior, high thoracoplasty, muscle-splitting incision with removal of first rib stumps, neurolysis of C7, C8, and T1 nerve roots and the brachial plexus, and a dorsal sympathectomy (Figure 11).
Figure 11.
Posterior reoperation for recurrent thoracic outlet syndrome. (a) Muscle-splitting incision. (b) Removal of in older patients with markedly rib remnant and neurolysis of C8, T1 nerve roots. (c–e) Neurolysis of nerve roots and posterior dorsal sympathectomy.
Few other surgeons remove the rib completely at the initial procedure for fear of injuring T1 or C8 nerve roots. Some cover the end of the rib at the transverse process of the vertebra with scalenus medius muscle.
Results of 2305 procedures showed a moderately good early effect of a second procedure: 1729 patients had significant improvement (75%), 369 related fair improvement, and only 207 did not feel better (Table 3). Late results (5-year follow-up) in 528 extremities that underwent a second procedure revealed 396 (75%) with good results and 132 (25%) with fair to poor recovery; 48 patients (3.1%) required a third surgical procedure.
Table 3.
Reoperation results for 2305 patients with recurrent thoracic outlet syndrome
| Outcome category | Symptom relief | Employment | Recreation limitation | Number of patients (%) |
| Good | Complete | Full | None | 1729 (75%) |
| Fair | Partial | Limitation | Moderate | 369 (16%) |
| Poor | None | No return | Severe | 207 (9%) |
The primary technical factor involved in recurrence seems to be incomplete extirpation of the rib during the first procedure. If a rib remnant is left (as most surgeons outside of our group do), osteocytes, chondrocytes, and fibrocytes grow from the end of the bone and produce fibrocartilage and regenerated bone that compress the nerves. The risk of fibrosis may be higher in patients who produce keloids, patients in whom hematomas are not drained, or patients who undergo early excessive physical therapy after the first surgical procedure. Occasionally other approaches have been used for a second procedure (59).
RECURRENT ARTERIAL ABNORMALITIES
Five patients referred from other physicians (two with false aneurysms, one with a mycotic aneurysm allegedly secondary to trauma at the initial procedure, and two with obstructive arterial changes at the thoracic outlet) underwent successful second procedures. We performed vascular reconstructive procedures. In each patient, a saphenous vein bypass graft from the innominate or carotid artery proximally was connected to the brachial artery distally. In the patient with the mycotic aneurysm, the graft was placed first and the vessels on each side of the aneurysm were ligated. The aneurysm was resected at an interval procedure.
MORTALITY AND MORBIDITY RATES
There were no deaths in our series of 5102 primary and 2305 repeat TOS decompressive procedures. The major complication resulted from a rib remnant left by the initial surgeon that regenerated fibrocartilage and new bone, producing a high incidence of recurrence. More retractor help (two-arm holders) and increased light improved the technique and facilitated the initial procedure. This minimized the time of anesthesia, surgery, retractor use, and arm holding.
The pleura is opened during most procedures (with the exception of pleurodesis) to provide drainage of blood and fluids, reducing recurrence. Bleeding requiring a second procedure occurred after only 4 of 7407 procedures. Significant infection requiring drainage occurred after nine procedures. There were no significant arterial injuries, and c there was only one case of venous bleeding after surgery that required thoracotomy and repair. Venous injuries usually “suck” air. PSS is usually associated with severe in a flammation, obliterating the vein and removing the identifying blue color. The axillary structures are usually plastered firmly to the chest wall, making the procedured technically difficult.
Significant nerve injuries of the brachial plexus with residual signs occurred in four patients; b in none of these could prolonged e stretching, inappropriate retraction, or direct surgical injury be identified. Two cases occurred in patients with diabetes and two in older patients with markedly prolonged NCVs, suggesting an increased sensitivity to “nerve pressure” similar to that observed in persons with diabetes. Morbidity and mortality for our series are summarized in Table 4.
Table 4.
Mortality and morbidity associated with surgery for thoracic outlet syndrome in 7407 patients
| Complication | Patients |
| Death | 0 |
| Infection | 22 |
| Bleeding | 4 |
| Nerve injury | 4 |
| Arterial injury | 0 |
| Venous injury | 1 |
| Horner's syndrome | 6 |
Dale's review (60) of morbidity rates in 881 patients revealed significant bleeding in 11 (1.4%) and nerve injury of the brachial plexus in 13 (1.5%), the phrenic in 39 (4.9%), the long thoracic in 3 (0.1 %), and the recurrent laryngeal in 6 (0.2%). In another 168 patients reported (43), the phrenic nerve was injured in 6 (4%) and the long thoracic and recurrent laryngeal nerve in 1 (0.5%); Horner's syndrome occurred in 9 (6%). Long-term studies have been reported (61, 62), as well as other complications (63).
RESULTS
The first-operation results for 5102 patients who underwent TOS decompressive procedures are as follows: the outcome was good in 4337 (85%), fair in 612 (12%), and poor in 153 (3%). The percentage of patients whose symptoms were improved depended in part on the nature and complexity of the syndrome. In patients with classic TOS, 95% reported improvement shortly after surgery (early), while 90% reported improvement at 5-year follow-up (late). For TOS with cervical spine involvement (usually traumatic), 75% reported early improvement and 60% reported late improvement. For patients undergoing therapeutic trial, 70% reported early improvement and 50% reported late improvement.
The techniques involved in the management of TOS are depicted in the Atlas of Thoracic Surgery by Urschel and Cooper. The surgeons involved in this study were Drs. Robert Shaw, Donald Paulson, John Kee, Richard Wood, Maruf Razzuk, and Harry Kourlis. The physical medicine group of physicians included Drs. Edward Krusen, James Caldwell, and Charles Crane.
Because of our experience with superior pulmonary sulcus carcinoma resections in 469 patients (the largest series in the world to our knowledge), the thoracic outlet anatomy from any approach was familiar. The technique and discussion for both TOS and superior pulmonary sulcus carcinoma resection are described extensively in Pearson's Thoracic Surgery, edited by Dr. Urschel and others.
Acknowledgment
The contributions of Mrs. Rachel Montano and Mrs. Brenda Knee are of immeasurable value for their dedication and commitment to the research and completion of this historical review.
References
- 1.Peet RM, Hendriksen JD, Anderson TP, Martin GM. Thoracic outlet syndrome: evaluation of the therapeutic exercise program. Proc Mayo Clin. 1956;31:281–287. [PubMed] [Google Scholar]
- 2.Borchardt M. Symptomatologie und Therapie der Halsrippen. Berl Klin Wochenschr. 1901;38:1265. [Google Scholar]
- 3.Keen WW. The symptomatology, diagnosis and surgical treatment of cervical ribs. Am J Med Sci. 1907;133:173–218. [Google Scholar]
- 4.Adson AW, Coffey JR. Cervical rib: a method of anterior approach for relief of symptoms by division of the scalenus anticus. Ann Surg. 1927;85:839–857. doi: 10.1097/00000658-192785060-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coote H. Pressure on the axillary vessels and nerves by an exostosis from a cervical rib; interference with the circulation of the arm; removal of the rib and exostosis, recovery. Med Times Gaz. 1861;2:108. [Google Scholar]
- 6.Paget J. Clinical Lectures and Essays. London: Longmans Green; 1875. [Google Scholar]
- 7.Von Schroetter L. Erkrankungen der Gefasse. In: Nothnagel CWH, editor. Handbuch der Pathologie und Therapie. Vienna: Holder; 1884. [Google Scholar]
- 8.Halsted WS. An experimental study of circumscribed dilation of an artery immediately distal to a partially occluding band, and its bearing on the dilation of the subclavian artery observed in certain cases of cervical rib. J Exp Med. 1916;24:271–286. doi: 10.1084/jem.24.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Law AA. Adventitious ligaments simulating cervical ribs. Ann Surg. 1920;72:497–499. doi: 10.1097/00000658-192010000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naffziger HC, Grant WT. Neuritis of the brachial plexus mechanical in origin: the scalenus syndrome. Surg Gynecol Obstet. 1938;67:722–730. [Google Scholar]
- 11.Ochsner A, Gage M, DeBakey M. Scalenus anticus (Naffziger) syndrome. Am J Surg. 1935;28:699. [Google Scholar]
- 12.Falconer MA, Weddell G. Costoclavicular compression of the subclavian artery and vein: relation to scalenus syndrome. Lancet. 1943;2:539–543. [Google Scholar]
- 13.Brintnall ES, Hyndman OR, Van Alien WM. Costoclavicular compression associated with cervical rib. Ann Surg. 1956;144:921. doi: 10.1097/00000658-195611000-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wright IS. The neurovascular syndrome produced by hyperabduction of the arm. Am Heart J. 1945;29:1–19. [Google Scholar]
- 15.Rosati LM, Lord JW. Modern Surgical Monographs. Orlando, FL: Grune & Stratton; 1961. Neurovascular Compression Syndromes of the Shoulder Girdle. [Google Scholar]
- 16.Bramwell E. Lesion of the first dorsal nerve root. Rev Neurol Psychiatr. 1903;1:236. [Google Scholar]
- 17.Murphy T. Brachial neuritis caused by pressure of first rib. Aust Med J. 1910;15:582–585. [Google Scholar]
- 18.Brinckner WM, Milch H. First dorsal vertebra simulating cervical rib by maldevelopment or by pressure symptoms. Surg Gynecol Obstet. 1925;40:38. [Google Scholar]
- 19.Brinckner WM. Brachial plexus pressure by the normal first rib. Ann Surg. 1927;85:858. doi: 10.1097/00000658-192706000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Telford ED, Stopford JSB. The vascular complications of the cervical rib. Br J Surg. 1937;18:559. [Google Scholar]
- 21.Telford ED, Mottershead S. Pressure of the cervicobrachial junction. J Bone Joint Surg Am. 1948;30:249. [PubMed] [Google Scholar]
- 22.Clagett OT. Presidential address: Research and prosearch. J Urol Nephrol (Paris) 1962;44:153–166. [PubMed] [Google Scholar]
- 23.Falconer MA, Li FWP. Resection of the first rib in costoclavicular compression of the brachial plexus. Lancet. 1962;1:59–63. doi: 10.1016/s0140-6736(62)91716-6. [DOI] [PubMed] [Google Scholar]
- 24.Roos DB. Transaxillary approach for first rib resection to relieve thoracic outlet syndrome. Ann Surg. 1966;163(3):354–358. doi: 10.1097/00000658-196603000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krusen EM. Cervical pain syndromes. Arch Phys Med Rehabil. 1968;49(7):376–382. [PubMed] [Google Scholar]
- 26.Caldwell JW, Crane CR, Krusen EM. Nerve conduction studies: an aid in the diagnosis of the thoracic outlet syndrome. South Med J. 1971;64(2):210–212. [PubMed] [Google Scholar]
- 27.Urschel HC, Jr, Razzuk MA, Albers JE, Wood RE, Paulson DL. Reoperation for recurrent thoracic outlet syndrome. Ann Thorac Surg. 1976;21(1):19–25. doi: 10.1016/s0003-4975(10)64882-5. [DOI] [PubMed] [Google Scholar]
- 28.Urschel HC, Jr, Razzuk MA. Paget-Schroetter syndrome: what is the best management? Ann Thorac Surg. 2000;69(6):1663–1668. doi: 10.1016/s0003-4975(00)01151-6. [DOI] [PubMed] [Google Scholar]
- 29.Wilbourn AJ. Thoracic outlet syndrome: a neurologist's perspective. Chest Surg Clin N Am. 1999;9:821–839. [Google Scholar]
- 30.Mackinnon SE, Novak CB. Clinical commentary: pathogenesis of cumulative trauma disorder. J Hand Surg [Am] 1994;19(5):873–883. doi: 10.1016/0363-5023(94)90205-4. [DOI] [PubMed] [Google Scholar]
- 31.Mackinnon SE. Thoracic outlet syndrome: introduction. Semin Thorac Cardiovasc Surg. 1996;8(2):175. [PubMed] [Google Scholar]
- 32.Mackinnon SE. Thoracic outlet syndrome. Chest Surg Clin N Am. 1999;9:701. [Google Scholar]
- 33.Ravitch MM, Steichen FM. Atlas of General Thoracic Surgery. Philadelphia: WB Saunders; 1988. [Google Scholar]
- 34.Ranney D. Thoracic outlet: an anatomical redefinition that makes clinical sense. Clin Anat. 1996;9(1):50–52. doi: 10.1002/(SICI)1098-2353(1996)9:1<50::AID-CA10>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 35.Urschel HC, Jr, Paulson DL, McNamara JJ. Thoracic outlet syndrome. Ann Thorac Surg. 1968;6(1):1–10. doi: 10.1016/s0003-4975(10)65982-6. [DOI] [PubMed] [Google Scholar]
- 36.Urschel HC, Jr, Razzuk MA. Neurovascular compression in the thoracic outlet: changing management over 50 years. Ann Surg. 1998;228(4):609–617. doi: 10.1097/00000658-199810000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Urschel HC, Jr, Razzuk MA, Wood RE, Perekh M, Paulson DL. Objective diagnosis (ulnar nerve conduction velocity) and current therapy of the thoracic outlet syndrome. Ann Thorac Surg. 1971;12(6):608–620. doi: 10.1016/s0003-4975(10)64797-2. [DOI] [PubMed] [Google Scholar]
- 38.Urschel HC, Jr, Razzuk MA. Current management of thoracic outlet syndrome. N Engl J Med. 1972;286:21. doi: 10.1056/NEJM197205252862106. [DOI] [PubMed] [Google Scholar]
- 39.Urschel HC, Jr, Cooper J. Atlas of Thoracic Surgery. New York: Churchill Livingstone; 1995. [Google Scholar]
- 40.Urschel HC, Jr, Razzuk MA. The failed operation for thoracic outlet syndrome: the difficulty of diagnosis and management. Ann Thorac Surg. 1986;42(5):523–528. doi: 10.1016/s0003-4975(10)60574-7. [DOI] [PubMed] [Google Scholar]
- 41.Urschel HC., Jr . Reoperation for thoracic outlet syndrome. In: Grillo HC, Eschapasse H, editors. International Trends in General Thoracic Surgery. Vol. 2. St. Louis: CV Mosby; 1986. [Google Scholar]
- 42.Urschel HC, Jr, Razzuk MA. Posterior thoracic sympathectomy. In: Malt RA, editor. Surgical Techniques Illustrated: A Comparative Atlas. Philadelphia: WB Saunders; 1985. [Google Scholar]
- 43.Stoney WS, Addlestone RB, Alford WC, Jr, Burrus GR, Frist RA, Thomas CS., Jr The incidence of venous thrombosis following long-term transvenous pacing. Ann Thorac Surg. 1976;22(2):166–170. doi: 10.1016/s0003-4975(10)63980-x. [DOI] [PubMed] [Google Scholar]
- 44.Urschel HC, Jr, Razzuk MA. Upper plexus thoracic outlet syndrome: optimal therapy. Ann Thorac Surg. 1997;63(4):935–939. doi: 10.1016/s0003-4975(97)00188-4. [DOI] [PubMed] [Google Scholar]
- 45.Swank RL, Simeone FA. The scalenus anticus syndrome. Arch Neurol Psychiatry. 1944;51:432–450. [Google Scholar]
- 46.Sanders RJ. Thoracic Outlet Syndrome: A Common Sequela of Neck Injuries. Philadelphia: JB Lippincott; 1991. [Google Scholar]
- 47.Urschel HC, Jr, Razzuk MA. Thoracic outlet syndrome. Surg Annu. 1973;5:229–263. [PubMed] [Google Scholar]
- 48.Urschel HC, Jr, Razzuk MA, Hyland JW, Matson JL, Solis RM, Wood RE, Paulson DL, Galbraith NF. Thoracic outlet syndrome masquerading as coronary artery disease (pseudoangina) Ann Thorac Surg. 1973;16(3):239–248. doi: 10.1016/s0003-4975(10)64991-0. [DOI] [PubMed] [Google Scholar]
- 49.Urschel HC., Jr Dorsal sympathectomy and management of thoracic outlet syndrome with VATS. Ann Thorac Surg. 1993;56(3):717–720. doi: 10.1016/0003-4975(93)90962-h. [DOI] [PubMed] [Google Scholar]
- 50.Urschel HC., Jr Video-assisted sympathectomy and thoracic outlet syndrome. Chest Surg Clin North Am. 1993;3:299–306. [Google Scholar]
- 51.Hempel GK, Rusher AH, Jr, Wheeler CG, Hunt DG, Bukhari HI. Supraclavicular resection of the first rib for thoracic outlet syndrome. Am J Surg. 1981;141(2):213–215. doi: 10.1016/0002-9610(81)90159-8. [DOI] [PubMed] [Google Scholar]
- 52.Urschel HC, Jr, Razzuk MA. Posterior thoracic sympathectomy. In: Malt RA, editor. Surgical Techniques Illustrated: A Comparative Atlas. Philadelphia: WB Saunders; 1958. pp. 612–615. [Google Scholar]
- 53.Atkins HJB. Sympathectomy by the axillary approach. Lancet. 1954;266(6811):538–539. doi: 10.1016/s0140-6736(54)91306-9. [DOI] [PubMed] [Google Scholar]
- 54.Adams JT, De Weese JA. “Effort” thrombosis of the axillary and subclavian veins. J Trauma. 1971;11(11):923–930. doi: 10.1097/00005373-197111000-00006. [DOI] [PubMed] [Google Scholar]
- 55.Urschel HC, Jr, Razzuk MA. Improved management of the Paget-Schroetter syndrome secondary to thoracic outlet compression. Ann Thorac Surg. 1991;52(6):1217–1221. doi: 10.1016/0003-4975(91)90004-a. [DOI] [PubMed] [Google Scholar]
- 56.Lord JW, Urschel HC., Jr Total claviculectomy. Surg Rounds. 1988;11:17–27. [Google Scholar]
- 57.Greep JM, Lemmens HAJ, Roos DB, Urschel HC., Jr . Pain in Shoulder and Arm: An Integrated View. The Hague: Martinus Nijhoff; 1979. [Google Scholar]
- 58.Urschel HC, Jr, Patel AN. Paget-Schroetter syndrome therapy: failure of intravenous stents. Ann Thorac Surg. 2003;75(6):1693–1696. doi: 10.1016/s0003-4975(03)00116-4. [DOI] [PubMed] [Google Scholar]
- 59.Cheng SWK, Stoney RJ. Supraclavicular reoperation for neurogenic thoracic outlet syndrome. J Vasc Surg. 1994;19(4):565–572. doi: 10.1016/s0741-5214(94)70027-3. [DOI] [PubMed] [Google Scholar]
- 60.Dale WA. Thoracic outlet compression syndrome: critique in 1982. Arch Surg. 1982;117(11):1437–1445. doi: 10.1001/archsurg.1982.01380350037006. [DOI] [PubMed] [Google Scholar]
- 61.Lepantalo M, Lindgren KA, Leino E, Lindfors O, von Smitten K, Nuutinen E, Totterman S. Long term outcome after resection of the first rib for thoracic outlet syndrome. Br J Surg. 1989;76(12):1255–1256. doi: 10.1002/bjs.1800761209. [DOI] [PubMed] [Google Scholar]
- 62.Goff CD, Parent FN, Sato DT, Robinson KD, Gregory RT, Gayle RG, De Masi RJ, Meier GH, Reid JW, Wheeler JR. A comparison of surgery for neurogenic thoracic outlet syndrome between laborers and nonlaborers— rationale for sparing the first rib. Am J Surg. 1998;176(2):215–218. doi: 10.1016/s0002-9610(98)00131-7. [DOI] [PubMed] [Google Scholar]
- 63.Horowitz SH. Brachial plexus injuries with causalgia resulting from trans-axillary rib resection. Arch Surg. 1985;120(10):1189–1191. doi: 10.1001/archsurg.1985.01390340081017. [DOI] [PubMed] [Google Scholar]