Abstract
Minimally invasive techniques for ablation of incompetent saphenous veins using either radiofrequency or laser energy sources have gained acceptance and are being widely applied today. Tumescent local anesthesia provides protection to tissues surrounding the treated veins and allows the procedures to be done on conscious patients. Results thus far compare favorably with surgical stripping procedures. Efficacy rates and complication rates support these techniques, and patient satisfaction appears high. Microphlebectomy, also minimally invasive, has advantages over direct incisional excision of varicosities. Foam sclerosant injection therapy has been used extensively in Europe and is proving useful in treating refluxing pelvic veins and recurrent varicosities.
Over the past 30 years, much has been learned about the pathophysiology of venous insufficiency, and in the last few years new and exciting treatment modalities have been developed. These new methods of resolving venous hypertension in the superficial veins of the lower extremities are safe and offer better patient outcomes with significantly less patient inconvenience than prior surgical remedies.
It is well established that superficial venous insufficiency (reflux) and its clinical presentation of varicose veins and related symptoms are the result of venous hypertension, which in turn is the result of failure of the venous valves. The complications of venous hypertension in addition to venous varicosities are chronic edema, hyperpigmentation, skin changes (lipodermatosclerosis), chronic ulceration, and variceal hemorrhage. It has been estimated that more than 25 million people in the USA have superficial venous reflux in their legs, and perhaps 7 million exhibit serious complications such as chronic edema, skin changes, and ulceration (1, 2). Perhaps in excess of 500,000 Americans suffer from debilitating chronic venous ulcers. Such ulcers are notoriously difficult to heal using usual wound management measures. It is generally accepted that many patients with varicose veins will go on to develop one or more of these complications if the venous hypertension is left untreated.
Friedrich von Trendelenburg (3) introduced modern surgical treatment of varicose veins in 1860 with an operation through a transverse incision in the upper thigh through which he ligated and divided the great saphenous vein. Charles Mayo (4), soon after graduating from medical school, began excising the great saphenous vein through a single incision from the groin to just below the knee. In 1906 (5), he reported a series of 185 patients treated in this fashion. Mayo (5) and Keller (6) published separate descriptions of procedures using “stripping” devices each had devised early in the 20th century. Later, Thomas T. Myers (7) of the Mayo Clinic published results using a flexible intraluminal stripper for removal of the great saphenous vein and the direct excision of the varicosities. This popular vein-stripping operation was the treatment of choice from 1950 until recently; it has been widely applied and is still performed by many surgeons. The operation has major drawbacks, however: it requires general or spinal anesthesia; limits patients' activity and causes them to miss work; and is associated with a significant infection rate, a high incidence of postoperative paresthesias and pain, and a high incidence of recurrent varicosities, which has been reported to be as high as 70% at 10 years (8).
Fortunately today varicose veins can be treated with new and promising treatment modalities. These include venous ablation procedures using radiofrequency, laser, and foam sclerotherapy. While these procedures have been available only in the last few years, a growing body of data supports their effectiveness.
SAPHENOUS VEIN ABLATION: RADIOFREQUENCY AND LASER
The modern surgical treatment of venous insufficiency of the lower extremities is possible because of endovenous technology, the application of color duplex sonography, and the use of tumescent anesthesia. With ultrasound scanning by a skilled sonographer knowledgeable about venous disease, it is now possible to identify the point of origin of the venous hypertension, i.e., the site of failure of the most proximal venous valve, and to map completely the extent of the venous reflux. Endovenous methods and the Seldinger technique permit a minimally invasive approach. Tumescent anesthesia (9, 10), the injection of a dilute concentration of local anesthesia in relatively large volumes into the surrounding tissues, allows the patient to remain conscious and avoid general or spinal anesthesia. It also serves to insulate the treated vein from other structures and thus avoid thermal injury to adjacent tissues and overlying skin. Because the vein is compressed down around the radiofrequency catheter or the laser fiber by the tumescence, better contact against the vein wall is achieved.
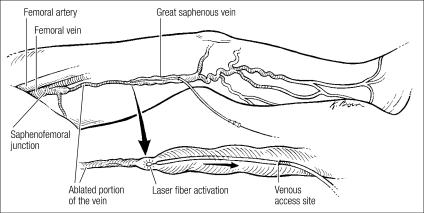
There are two new options to achieve saphenous vein ablation that have good outcomes and few complications. The first minimally invasive endovenous ablation device to gain approval was the radiofrequency catheter, which was followed a few years later by the laser fiber (Figure 1). Both have proved effective at causing thermal injury to the vein wall and subsequent thrombosis.
Figure 1.
Endovenous laser ablation of the great saphenous vein.
Merchant et al (11) reported the results of radiofrequency ablation of truncal veins in 1222 limbs. Vein occlusion as evaluated by ultrasound duplex scanning was 96.8% at 1 week and 87% at 1 and 5 years. Absence of reflux in the limb was 96.6% at 1 week and decreased to 88% and 83.8% at 1 and 5 years, respectively. The late reappearance of venous reflux was attributed to missed refluxing veins at earlier examinations or the development of reflux in veins that appeared normal at the time of treatment. Complications included deep vein thrombosis, 0.9%; skin burns, 1.2%; clinical phlebitis, 2.9%; infection, 0.2%; and paresthesias, 12.3%. In a recent review of published clinical series, the incidence of thrombus extension into the common femoral vein or deep vein thrombosis was 2.1% after radiofrequency ablation and 0.3% after laser ablation (12).
When evaluating the results of endovenous ablation of truncal veins, it is necessary to consider the wavelength of the laser. Min and Khilnani (13) reported the results in 499 limbs treated with the 810-nm diode laser. Vein occlusion was documented in 98% of limbs at 1 week and in 93% of limbs at 2 years. There were no instances of deep vein thrombosis, skin burns, or paresthesias. Increased bruising was noted in 24% of limbs. In a series of 452 limbs treated with the 1320-nm laser, vein closure occurred in 98.6% at both 6 months and 1 year (J. R. Kingsley, personal communication, 2006).
It is necessary to compare results of the minimally invasive endovenous ablation procedures using either radiofrequency or laser against the results of the vein-stripping surgical procedure. Multiple series of vein-stripping patients have demonstrated significant recurrence rates of 15% to 70% over the course of 5 to 10 years (8, 14–17). Patient satisfaction with the vein-stripping procedure has been disappointing, with only 85% of patients rating the results excellent or good. This comparison does not take into account the increased pain and morbidity, the requirements for general or spinal anesthesia and hospitalization and the resulting expense, and the physical limitations and absence from work following the stripping operation. It is important to note that long-term follow-up of patients undergoing radiofrequency or laser venous ablation is limited.
FOAM CHEMICAL ABLATION
Foam sclerotherapy was developed in Europe and has gained popularity over the past few years. Using Tessari's method (18), the sclerosant solution is mixed forcefully with air or carbon dioxide using a technique employing two syringes and a three-way connector to create a foam, which is injected into veins being treated. The sclerosant, which coats the gas bubbles, tends to linger against the endothelial surface of the veins rather than be washed out. This allows more contact time for the sclerosant to cause endothelial and vein wall damage by interfering with the function of endothelial and subendothelial cell surface proteins that are necessary for cell survival. The end result is thrombosis and fibrous obliteration of the vein.
Cabrera (19) has used foam sclerotherapy as a single therapy to ablate truncal veins such as the great saphenous vein. Others have used foam in conjunction with radiofrequency to achieve results superior to either alone. Morrison (20) reported 1445 great saphenous veins treated with radiofrequency. At a mean follow-up of 27 months, success, as measured by sonographic absence of the great saphenous vein, occurred in <50% of the limbs. Of the veins with persistent flow, 80% were successfully closed with ultrasound-guided foam sclerotherapy. However, with the combination of radiofrequency and foam sclerotherapy, he achieved 94% closure (N. Morrison, personal communication, 2006). Adverse events are rare, but reported symptoms following foam injection include visual disturbance, cough, and migraine, all of which are transient and usually clear within minutes.
Clearly, foam chemical ablation has been a useful tool in treating recurrent veins after treatment with endovenous ablation methods. It has also been successful in treating varicosities emanating from pelvic venous reflux, which produces vulvar, high thigh, and posterior thigh painful varicosities. It is the best method of ablating incompetent communicating veins and perforators. These veins, which perforate the fascia generalis overlying the muscles of the leg, carry venous flow and transmit high pressure from the deep veins of the muscles into the superficial veins of the leg.
SUBFASCIAL ENDOSCOPIC PERFORATOR VEIN SURGERY
With the popularity of minimally invasive endoscopic surgery, surgeons began to apply the technique to interrupt incom petent perforator veins in the subfascial space. The subfascial endoscopic perforator vein surgery (SEPS) approach avoided the larger and in many cases multiple incisions necessary to divide the communicating veins, as described by Linton (21). The SEPS operation has been shown to be effective in accelerating ulcer healing and in preventing recurrences (22, 23). It has the disadvantage of requiring general or spinal anesthesia, and patients experience some wound pain and disability for a while. It appears that foam chemical ablation using a sclerosant is less invasive and safely accomplishes perforator closure without incisions and division of the vessels.
MICROPHLEBECTOMY
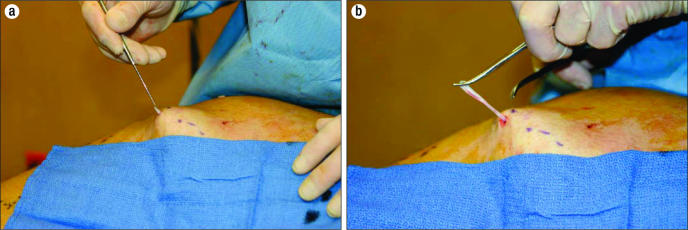
In the past, incisions have been used to excise bulging superficial varices, which typically course in the fat layer above the muscle fascia and below the skin. These unsightly and painful veins are removed for cosmetic reasons and to prevent superficial thrombophlebitis that often occurs in these varicosities once the venous hypertension has been alleviated by one of the techniques described above. By removing the varicosities, skin hyperpigmentation is also avoided should clotting of the varicosities occur. Large incisions have been effective but leave significant scars. Microphlebectomy, which was described by Muller (24) and is often referred to as ambulatory phlebectomy, allows the removal of the varicosities quite effectively without leaving objectionable scars. Tiny 2- to 3-mm incisions are made over the bulging varicosities, which have been marked with the patient in a standing position. Through these incisions, a small vein hook delivers the varicose vein, which is clamped, teased out, and excised (Figure 2). Microphlebectomy has proven to be a very esthetic method of removing the varicosities and is easily accomplished using tumescent local anesthesia.
Figure 2.
Microphlebectomy of superficial venous varicosity. The vein is (a) hooked through a tiny incision and (b) clamped, teased out, and excised.
CONCLUSIONS
Most patients require a combination of treatment methods depending on the point of highest venous reflux, the presence of bulging varicosities, the presence of incompetent perforator veins, and the depth of subcutaneous tissue overlying the muscle fascia. Radiofrequency or laser ablation in combination with microphlebectomy and foam sclerotherapy has been shown to achieve excellent clinical and cosmetic outcomes.
Thanks to developments in endovenous ablation, the treatment of venous hypertension in the lower extremities may be performed as a minimally invasive procedure under local anesthesia in an outpatient setting. Patients return to usual activities in 1 or 2 days. Recurrence rates are acceptably low, and if a recurrence should occur, early treatment with foam chemical ablation is easy and effective. Overall patient satisfaction with these modern techniques surpasses that with the vein-stripping operation of the past.
References
- 1.Brand FN, Dannenberg AL, Abbott RD, Kannel WB. The epidemiology of varicose veins: the Framingham Study. Am J Prev Med. 1988;4(2):96–101. [PubMed] [Google Scholar]
- 2.Madar G, Widmer LK, Zemp E, Maggs M. Varicose veins and chronic venous insufficiency disorder or disease? A critical epidemiological review. Vasa. 1986;15(2):126–134. [PubMed] [Google Scholar]
- 3.Trendelenburg F. Über die Unterbindung der Vena saphena magna bei Unterschenkelvaricen. Beiträge zur klinischen Chirurgie. 1891;7:195–210. [Google Scholar]
- 4.Mayo CH. Varicose veins of the lower extremity. St. Paul Med J. 1900;2:595. [Google Scholar]
- 5.Mayo CH. Treatment of varicose veins. Surg Gynecol Obstet. 1906;2:385–388. [Google Scholar]
- 6.Keller WL. A new method of extirpating the internal saphenous and similar veins in varicose conditions. A preliminary report. N Y Med J. 1905;82:385–386. [Google Scholar]
- 7.Myers TT. Results and technique of stripping operation for varicose veins. JAMA. 1957;163(2):87–92. doi: 10.1001/jama.1957.02970370001001. [DOI] [PubMed] [Google Scholar]
- 8.Winterborn RJ, Earnshaw JJ. Crossectomy and great saphenous vein stripping. J Cardiovasc Surg (Torino) 2006;47(1):19–33. [PubMed] [Google Scholar]
- 9.Klein JA. The tumescent techniques for liposuction surgery. Am J Cosmet Surg. 1987;4:263–267. [Google Scholar]
- 10.Klein J. The two standards of care for tumescent liposuction. Dermatol Surg. 1997;23(12):1194–1195. doi: 10.1111/j.1524-4725.1997.tb00473.x. [DOI] [PubMed] [Google Scholar]
- 11.Merchant RF, Pichot O, Closure Study Group Long-term outcomes of endovenous radiofrequency obliteration of saphenous reflux as a treatment for superficial venous insufficiency. J Vasc Surg. 2005;42(3):502–509. doi: 10.1016/j.jvs.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 12.Mozes G, Kalra M, Carmo M, Swenson L, Gloviczki P. Extension of saphenous thrombus into the femoral vein: a potential complication of new endovenous ablation techniques. J Vasc Surg. 2005;41(1):130–135. doi: 10.1016/j.jvs.2004.10.045. [DOI] [PubMed] [Google Scholar]
- 13.Min RJ, Khilnani NM. Reply: Re: endovenous laser treatment of saphenous vein reflux: long-term results. J Vasc Interv Radiol. 2004;15(2):203–204. doi: 10.1097/01.rvi.0000109404.52762.0a. [DOI] [PubMed] [Google Scholar]
- 14.Hartmann K, Klode J, Pfister R, Toussaint M, Weingart I, Waldermann F, Hartmann M. Recurrent varicose veins: sonography-based re-examination of 210 patients 14 years after ligation and saphenous vein stripping. Vasa. 2006;35(1):21–26. doi: 10.1024/0301-1526.35.1.21. [DOI] [PubMed] [Google Scholar]
- 15.Fischer R, Chandler JG, Stenger D, Puhan MA, De Maeseneer MG, Schimmelpfennig L. Patient characteristics and physician-determined variables affecting saphenofemoral reflux recurrence after ligation and stripping of the great saphenous vein. J Vasc Surg. 2006;43(1):81–87. doi: 10.1016/j.jvs.2005.09.027. [DOI] [PubMed] [Google Scholar]
- 16.Blomgren L, Johansson G, Dahlberg-Akerman A, Noren A, Brundin C, Nordstrom E, Bergqvist D. Recurrent varicose veins: incidence, risk factors and groin anatomy. Eur J Vasc Endovasc Surg. 2004;27(3):269–274. doi: 10.1016/j.ejvs.2003.12.022. [DOI] [PubMed] [Google Scholar]
- 17.MacKenzie RK, Paisley A, Allan PL, Lee AJ, Ruckley CV, Bradbury AW. The effect of long saphenous vein stripping on quality of life. J Vasc Surg. 2002;35(6):1197–1203. doi: 10.1067/mva.2002.121985. [DOI] [PubMed] [Google Scholar]
- 18.Tessari L. Nouvelle technique d'obtention de la sclero-mousse. Phlebologie. 2000;53:129–133. [Google Scholar]
- 19.Cabrera J, Cabrera J, Jr, Garcia-Olmedo MA. Treatment of varicose long saphenous veins with sclerosant in microfoam form: long-term outcomes. Phlebology. 2000;15:19–23. [Google Scholar]
- 20.Morrison N. Saphenous ablation: what are the choices, laser or RF energy. Semin Vasc Surg. 2005;18(1):15–18. doi: 10.1053/j.semvascsurg.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 21.Linton RR. The communicating veins of the lower leg and the operative technique for their ligation. Ann Surg. 1938;107:582–593. doi: 10.1097/00000658-193804000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bianchi C, Ballard JL, Abou-Zamzam AM, Teruya TH. Subfascial endoscopic perforator vein surgery combined with saphenous vein ablation: results and critical analysis. J Vasc Surg. 2003;38(1):67–71. doi: 10.1016/s0741-5214(03)00472-5. [DOI] [PubMed] [Google Scholar]
- 23.Gloviczki P, Bergan JJ, Rhodes JM, Canton LG, Harmsen S, Ilstrup DM, North American Study Group Mid-term results of endoscopic perforator vein interruption for chronic venous insufficiency: lessons learned from the North American subfascial endoscopic perforator surgery registry. J Vasc Surg. 1999;29(3):489–502. doi: 10.1016/s0741-5214(99)70278-8. [DOI] [PubMed] [Google Scholar]
- 24.Muller R. Ambulatory phlebectomy [article in German] Ther Umsch. 1992;49(7):447–450. [PubMed] [Google Scholar]