Abstract
Kikuchi-Fujimoto disease (KFD), or histiocytic necrotizing lymphadenitis, is a benign and self-limited disease that mainly affects young women. Patients present with localized lymphadenopathy, fever, and leukopenia in up to half of the cases. KFD can occur in association with systemic lupus erythematosus. We present the case of a patient with KFD and systemic lupus erythematosus, as well as relapsing polychondritis. This patient had persistently low C4 complement levels, so she was evaluated for a genetic defect in complement production and was found to have two “null” C4 alleles. We believe that this may have contributed to the development of her diseases.
A 56-year-old white woman with no significant past medical history was initially seen at Baylor University Medical Center in April 1998. She presented with acute onset of bilateral tender cervical lymphadenopathy with associated malaise, fever to 103°F, and anorexia. She also had severe arthralgias in her hands and knees and had difficulty walking. Through a lymph node biopsy, the diagnosis of histiocytic necrotizing lymphadenitis, or Kikuchi-Fujimoto disease (KFD), was made. She was treated with a course of sulfa drugs for 10 days and started a second course but discontinued it because she broke out in hives. Her symptoms resolved within 2 months.
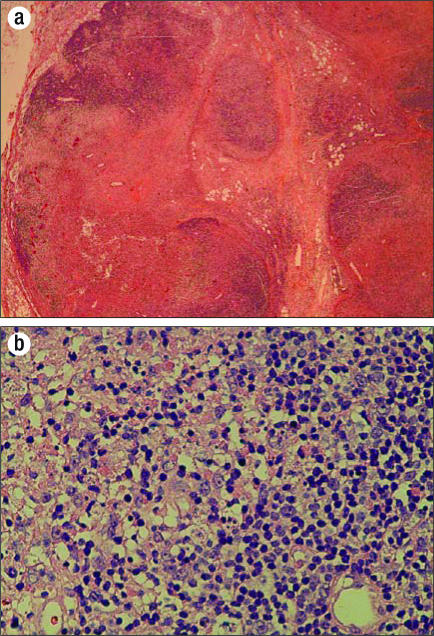
The patient was well until January 2001, when she began having “tenderness” in the posterior aspect of her hairline and noticed an enlarged right occipital lymph node. She took acetaminophen for the episodes of tenderness, which would come and go and last about 24 hours. By April 2001, she began experiencing fatigue, malaise, and fevers to 104°F and was hospitalized. A lymph node biopsy again showed the classic finding of KFD: necrosis containing abundant karyorrhectic nuclear debris (Figure). The presence of histiocytes was confirmed with the CD68 stain. Bacterial, acid-fast bacillus, and fungal stains and cultures of the specimen showed no growth. She was treated with a prednisone taper, and the findings resolved.
Figure.
Lymph node biopsy results. (a) Low-power view shows confluent necrosis with partial preservation of the lymph node architecture. (b) High-power view shows apoptotic cells with nuclear debris, as well as admixed histiocytes and transformed lymphocytes (immunoblasts). Significantly, there is no neutrophilic or eosinophilic inflammation.
Soon after her hospitalization, the patient was referred to a hematologist/oncologist to determine if she had lymphoma. Bone marrow aspirate and biopsy results were normal. Serum protein electrophoresis and urine immunoelectrophoresis results also were normal. Laboratory work did reveal a positive antinuclear antigen titer (1:160) with a speckled pattern. A diagnosis of systemic lupus erythematosus (SLE) was entertained, and the patient was referred to a rheumatologist.
The rheumatologist confirmed the diagnosis of SLE. The patient had the following clinical findings: arthralgias, linear ulcers on the gums, alopecia, and normocytic anemia. In addition, she had a positive antinuclear antigen titer and a positive double-stranded DNA test. Her C-reactive protein level was within normal limits, but she had decreased levels of complement. Her C3 level was 50 mg/dL (normal range, 83–177) and her C4 level was <10 mg/dL (normal range, 12–50). C4 allotyping demonstrated two “null” C4 alleles.
The patient was started on hydroxychloroquine 200 mg twice a day. Her arthralgias and mouth ulcers improved. About 9 months after being diagnosed with SLE, however, she began to experience recurrent episodes of redness and pain in her ears and nose. Her rheumatologist diagnosed relapsing polychondritis. The symptoms would last a few days and then resolve on their own without treatment.
As of December 2006, the patient had gone 5 years without recurrence of lymphadenopathy or symptoms of SLE. Her last flare of relapsing polychondritis was 4 years ago.
DISCUSSION
KFD, or histiocytic necrotizing lymphadenitis, was originally reported in 1972 in Japan. It has been reported in several countries since then. It occurs most commonly in young women (1) with localized lymphadenopathy, most commonly in the cervical region (2). It is associated with fever and leukopenia in up to 50% of patients (3).
The differential diagnosis of fever and cervical lymphadenopathy is broad and often leads to an extensive workup. Our patient was tested for tuberculosis, Epstein-Barr virus, cytomegalovirus, HIV, toxoplasmosis, and syphilis. In addition, she had a bone marrow examination to check for lymphoma. All of these studies were negative or normal. Lymph node biopsy results did facilitate the diagnosis. The characteristic histology of KFD is single or multiple areas within the lymph node that contain necrosis and histiocytic cellular infiltrate. The capsule of the node may be invaded, and perinodal inflammation is common (4). Cultures and stains for organisms are negative.
KFD is known to occur in conjunction with SLE (Table) (5). Some experts even suggest that KFD is one unusual presentation of SLE. Santana et al did a Medline/LILACS (Latin American and Caribbean Health Sciences) search in 2003 and found 35 reported cases in which KFD and SLE occurred together. In the majority of the cases, SLE was diagnosed either after or at the same time as the KFD (6). In the case of our patient, SLE was diagnosed about the same time as her second episode of KFD. It is interesting to note, however, that during her first episode, she did present with arthralgias. It is not known whether SLE serologies were checked at that time.
Table.
Characteristics of patients with systemic lupus erythematosus that occurs simultaneously with Kikuchi-Fujimoto disease: review of the literature∗
| Patient age/gender | Presentation of SLE | Presentation of KFD | Laboratory results |
| 24/F | Malar rash, polyarthritis | Axillary LAP | ANA (+); others: NA |
| 22/F | Fever, Raynaud's, polyarthritis, leukopenia, proteinuria | Cervical LAP | ANA (+); anti-dsDNA (+); low C3/C4 |
| 45/F | Fever, discoid rash | Cervical LAP | ANA (+); anti-dsDNA (+); thrombocytopenia; leukopenia; low C3/C4 |
| 15/F | Fever, malar rash, serositis, polyarthritis | Supraclavicular, cervical LAP | ANA (+); anti-Sm (+); anti-RNP (+); anti-dsDNA (+); low C4 |
| 37/F | Fever, polyarthritis | LAP | ANA (+); anti-dsDNA (+); anti-SSA/SSB (+); lymphopenia |
| 28/M | Fever, seizure, facial swelling | Cervical, axillary LAP | ANA (+); RF (+); proteinuria; low C3/C4 |
| 27/M | Weight loss, night sweats | Cervical, axillary, and inguinal LAP | ANA (+); anti-dsDNA (+); thrombocytopenia; normal C3/C4 |
| 35/F | Fever, malar rash, pleuritis, leukopenia | Cervical LAP | ANA (+); anti-RNP (+); normal C3/C4 |
| 44/M | Fever, myalgia, weight loss | Axillary LAP | ANA (+); anti-dsDNA (+); anti-cardiolipin (+); anti-Ro (+); leukopenia; normal C3/C4 |
| 19/M | Fever, weight loss, right lower leg swelling | Cervical, axillary LAP | ANA (+); anti-Sm (+); anti-RNP (+); anti-cardiolipin (+); VDRL (+); proteinuria (+); Coombs' test (+); thrombocytopenia; low C3/C4 |
∗Modified from Chen HC et al, 2005 (5) with kind permission of Springer Science and Business Media.
LAP indicates lymphadenopathy; ANA, antinuclear antibody; anti-Sm, anti-Smith antibody; anti-RNP, antiribonucleoprotein antibody; SSA, antibodies to Ro; SSB, antibodies to La; NA, not available; RF, rheumatoid factor.
No effective treatment has been established for KFD. It is a benign, self-limited disease that resolves in 1 to 4 months. Patients should be monitored, however, since they may subsequently develop SLE or, in unusual circumstances, develop a recurrence of KFD. Recurrences of the latter are uncommon (7).
In cases in which KFD is diagnosed after or at the same time as SLE, corticosteroids are often used for treatment, often along with hydroxychloroquine (8–10). After treatment with prednisone, our patient received hydroxychloroquine once SLE was diagnosed. She did very well. Her symptoms resolved within 1 month of starting the treatment, and she has not relapsed since.
The etiology of KFD is unknown. Certain causative organisms have been proposed. These include Epstein-Barr virus, human T-cell leukemia virus type 1, human herpesvirus type 6, B19 parvovirus, cytomegalovirus, Brucella, Yersinia enterocolitica, and parainfluenza virus (11). An autoimmune mechanism has also been proposed because KFD is seen in conjunction with SLE. One theory involves molecular mimicry, in which infectious agents that closely resemble a host peptide affect the ability of T cells to detect self from nonself (12). An example of this is the cross-reaction between Borrelia burgdorferi antigen and a peptide from human lymphocyte function–associated antigen 1 that leads to the chronic arthritis seen in Lyme disease (13). Another theory regarding autoimmunity is that apoptotic cells are the source of the autoantigens of SLE. Apoptotic cells express many of the nuclear autoantigens of SLE on their surface. In patients with defective clearance of these cells (i.e., complement deficiency), these cells may become a nidus for autoimmune disease (14).
Relapsing polychondritis, a disorder of cartilage and connective tissue, is another problem that our patient developed. Patients most commonly present with unilateral or bilateral ear inflammation with sparing of the noncartilaginous parts of the ear (15). The next most common presentation is joint involvement, followed by nasal and ocular involvement (16). Relapsing polychondritis is also thought to have an autoimmune etiology, with autoantibodies attacking the patient's cartilage (17).
Complement levels may play a role in the constellation of diseases seen in our patient. The complement system is a set of proteins that aids in phagocytosis, chemotaxis, opsonization, and the clearance of immune complexes. There are three different paths in the complement system: the classic pathway, the alternative pathway, and the mannose-binding lectin pathway (18). Each pathway is activated differently. The classic pathway is activated by binding to immune complexes (19). C4 is an important component of the classic pathway, and so a deficiency in it results in defective immune-complex clearance.
Our patient was found to have persistently low C4 levels. Allotyping had demonstrated two “null” alleles at her C4 locus. C4 is involved in the early part of the classical complement pathway. Homozygous complement deficiency, especially of the early components of the classical pathway, has been strongly associated with the development of some autoimmune disorders, in particular SLE (20).
We believe that low C4 levels in our patient perhaps contributed to impaired clearance of immune complexes and may have predisposed her to SLE, KFD (which may have been an unusual presentation of her SLE), and perhaps even to relapsing polychondritis. After her initial treatment with low-dose prednisone and hydroxychloroquine, she gradually became asymptomatic and has done well for the last several years.
It was helpful to know that the patient produced less than the expected amount of C4 because many authorities suggest that low C4 levels may suggest either persistent disease activity or a “flare” of the disease. Knowing that her baseline levels were less than those seen in the general population helped to prevent “overtreatment.” Unfortunately, allotyping is no longer performed in any US laboratories.
We feel that this patient's KFD is probably an unusual manifestation of SLE. In addition, the patient's atypical course may be related to her inherited chronically low C4 levels. To our knowledge, previous cases of this syndrome were not evaluated for complement deficiency.
References
- 1.Lopez C, Oliver M, Olavarria R, Sarabia MA, Chopite M. Kikuchi-Fujimoto necrotizing lymphadenitis associated with cutaneous lupus erythematosus: a case report. Am J Dermatopathol. 2000;22(4):328–333. doi: 10.1097/00000372-200008000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Kaur S, Thami GP, Kanwar AJ. Kikuchi's disease, skin and systemic lupus erythematosus. Br J Dermatol. 2002;146(1):167–168. doi: 10.1046/j.1365-2133.2002.4513_4.x. [DOI] [PubMed] [Google Scholar]
- 3.Norris AH, Krasinskas AM, Salhany KE, Gluckman SJ. Kikuchi-Fujimoto disease: a benign cause of fever and lymphadenopathy. Am J Med. 1996;101(4):401–405. doi: 10.1016/S0002-9343(96)00231-8. [DOI] [PubMed] [Google Scholar]
- 4.Loachim HL, Ratech H. Loachim's Lymph Node Pathology. 3rd ed. New York: Lippincott Williams & Wilkins; 2002. [Google Scholar]
- 5.Chen HC, Lai JH, Huang GS, Gao HW, Chen CH, Kuo SY, Chang DM. Systemic lupus erythematosus with simultaneous onset of Kikuchi-Fujimoto's disease complicated with antiphospholipid antibody syndrome: a case report and review of the literature. Rheumatol Int. 2005;25(4):303–306. doi: 10.1007/s00296-004-0507-4. [DOI] [PubMed] [Google Scholar]
- 6.Santana A, Lessa B, Galrao L, Lima I, Santiago M. Kikuchi-Fujimoto's disease associated with systemic lupus erythematosus: case report and review of the literature. Clin Rheumatol. 2005;24(1):60–63. doi: 10.1007/s10067-004-0923-6. [DOI] [PubMed] [Google Scholar]
- 7.Litwin MD, Kirkham B, Henderson DR, Milazzo SC. Histiocytic necrotising lymphadenitis in systemic lupus erythematosus. Ann Rheum Dis. 1992;51(6):805–807. doi: 10.1136/ard.51.6.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vila LM, Mayor AM, Silvestrini IE. Therapeutic response and long-term follow-up in a systemic lupus erythematosus patient presenting with Ki-kuchi's disease. Lupus. 2001;10(2):126–128. doi: 10.1191/096120301673275701. [DOI] [PubMed] [Google Scholar]
- 9.Meyer O, Kahn MF, Grossin M, Ribard P, Belmatoug N, Morinet F, Fournet JC. Parvovirus B19 infection can induce histiocytic necrotizing lymphadenitis (Kikuchi's disease) associated with systemic lupus erythematosus. Lupus. 1991;1(1):37–41. doi: 10.1177/096120339100100107. [DOI] [PubMed] [Google Scholar]
- 10.Tumiati B, Casoli P, Perazzoli F, Cavazza A. Necrotizing lymphadenitis in systemic lupus erythematosus. Kikuchi's disease or a Kikuchi's-like disease? J Clin Rheumatol. 1999;5:121–125. doi: 10.1097/00124743-199906000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Menasce LP, Banerjee SS, Edmondson D, Harris M. Histiocytic necrotizing lymphadenitis (Kikuchi-Fujimoto disease): continuing diagnostic difficulties. Histopathology. 1998;33(3):248–254. doi: 10.1046/j.1365-2559.1998.00469.x. [DOI] [PubMed] [Google Scholar]
- 12.Marrack P, Kappler J, Kotzin BL. Autoimmune disease: why and where it occurs. Nat Med. 2001;7(8):899–905. doi: 10.1038/90935. [DOI] [PubMed] [Google Scholar]
- 13.Gross DM, Forsthuber T, Tary-Lehmann M, Etling C, Ito K, Nagy ZA, Field JA, Steere AC, Huber BT. Identification of LFA-1 as a candidate auto-antigen in treatment-resistant Lyme arthritis. Science. 1998;281(5377):703–706. doi: 10.1126/science.281.5377.703. [DOI] [PubMed] [Google Scholar]
- 14.Botto M. Links between complement deficiency and apoptosis. Arthritis Res. 2001;3(4):207–210. doi: 10.1186/ar301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coppola M, Yealy DM. Relapsing polychondritis: an unusual cause of painful auricular swelling. Ann Emerg Med. 1992;21(1):81–85. doi: 10.1016/s0196-0644(05)82246-2. [DOI] [PubMed] [Google Scholar]
- 16.Balsa A, Expinosa A, Cuesta M, MacLeod TI, Gijon-Banos J, Maddison PJ. Joint symptoms in relapsing polychondritis. Clin Exp Rheumatol. 1995;13(4):425–430. [PubMed] [Google Scholar]
- 17.Herman JH, Carpenter BA. Immunobiology of cartilage. Semin Arthritis Rheum. 1975;5(1):1–40. doi: 10.1016/0049-0172(75)90021-9. [DOI] [PubMed] [Google Scholar]
- 18.Campbell RD. The molecular genetics of components of the complement system. Baillieres Clin Rheumatol. 1988;2(3):547–575. doi: 10.1016/s0950-3579(88)80028-1. [DOI] [PubMed] [Google Scholar]
- 19.Schumaker VN, Zavodszky P, Poon PH. Activation of the first component of complement. Annu Rev Immunol. 1987;5:21–42. doi: 10.1146/annurev.iy.05.040187.000321. [DOI] [PubMed] [Google Scholar]
- 20.Lachmann PJ. Complement—friend or foe? Br J Rheumatol. 1987;26(6):409–415. doi: 10.1093/rheumatology/26.6.409. [DOI] [PubMed] [Google Scholar]