Abstract
Background
Shigellosis or bacillary dysentery, an acute bloody diarrhoea, is a major public health burden in developing countries. In the absence of prompt and appropriate treatment, the infection is often fatal, particularly in young malnourished children. Here, we describe a new diagnostic test for rapid detection, in stool, at the bedside of patients, of Shigella flexneri 2a, the most predominant agent of the endemic form of the disease.
Methodology/Principal Findings
The test is based on the detection of S.flexneri 2a lipopolysaccharide (LPS) using serotype 2a-specific monoclonal antibodies coupled to gold particles and displayed on one-step immunochromatographic dipstick. A concentration as low as 20 ng/ml of LPS is detected in distilled water and in reconstituted stools in under 15 minutes. The threshold of detection corresponds to a concentration of 5×107 CFU/ml of S. flexneri 2a, which provides an unequivocal positive reaction in three minutes in distilled water and reconstituted stools. The specificity is 100% when tested with a battery of Shigella and unrelated strains, in culture. When tested in Vietnam, on clinical samples, the specificity and sensitivity were 99.2 and 91.5%, respectively. A decrease of the sensitivity during the evaluation on stool samples was observed after five weeks at room temperature and was due to moistening of the dipsticks caused by the humidity of the air during the fifth week of the evaluation. This drawback is now overcome by improving the packaging and providing dipsticks individually wrapped in waterproof bags.
Conclusion
This simple dipstick-bases test represents a powerful tool for case management and epidemiological surveys.
Introduction
Shigellosis, an acute bloody diarrhea caused by the Gram negative entero-invasive bacterium Shigella spp, represents a major public health burden in many developing countries [1]. According to a reference study published in 1999, which provided projections derived from literature-based data [2], the annual number of Shigella episodes throughout the world was estimated to be about 164.7 million, with 99% occurring in developing countries. The estimated number of deaths is about 1.1 million. Children under the age of 5 are the main target of the disease, representing 69% of all episodes and 61% of all deaths. Although S. dysenteriae type 1 is associated with the most severe form of the disease and high mortality rate when epidemics occur, most of the deaths are attributable to the endemic form of the disease, which is most often caused by S. flexneri. In the reference study cited above, the median percentages of isolates of S. flexneri, S. sonnei, S. boydii, and S. dysenteriae were 60%, 15%, 6%, and 6% (with 30% of S. dysenteriae isolates being serotype 1) in developing countries, and 16%, 77%, 2%, and 1% in industrialized countries, respectively. In developing countries, the predominant S. flexneri serotype is 2a [2].
A recent study by Kosek et al [3] indicated that the incidence of diarrhoeal diseases remains stable but their mortality rate is tending to decrease. Is Shigella following this tendancy? With regard to mortality, it has indeed very significantly decreased. The main reasons are likely to be the lack of a major S. dysenteriae 1 outbreak for at least 10 years, improvement of mothers' education, better primary care, better nutrition status of children in economically emerging Asian countries, and presumably the large and uncontrolled use of antibiotics. Nevertheless, the steady increase of antibiotic resistance makes the emergence of massive epidemics of S. dysenteriae 1 a possible scenario, particularly in socially unstable areas. Indeed, isolates are now largely resistant to first line antibiotics (ampicillin, chloramphenicol, tetracycline, sulfamides, trimethoprime plus sulfamides, nalidixic acid) and resistance to second line antibiotics including fluoroquinolones becomes increasingly prevalent [1], [4], [5], [6], [7].
With regard to morbidity, a recent multicentric study carried out in six Asian countries [5] showed that Shigella is isolated in at least 5% of the cases of diarrhoea, a remarkably stable value. However, it seems that the incidence of shigellosis is largely underestimated. A major reason is the weakly fastidious nature of Shigella which poorly survives transport, and for which no enrichment medium exists. The traditional identification by culture lacks sensitivity due to the low number of causative micro-organisms, competition with commensal organisms, and deleterious changes in ambient temperature and pH during specimen transport [8], [9], [10]. The detection is also frequently impaired by the use of antibiotics prior to specimen collection. Consequently, only a fraction of the current cases of shigellosis are presumably detected [9]. This was confirmed in a recent study showing that prospective studies using optimized procedures of collection and rapid processing yield incidence data that are several folds higher than those obtained by passive collection of data in natural health cohorts [4].
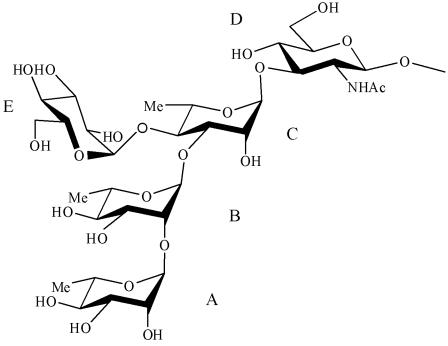
There is therefore a need for updated epidemiological data, particularly in Africa where the pattern of diarrhoeal diseases seems generally more severe, in terms of both morbidity and mortality [11], [12], [13]. For such studies, improved diagnostic tools are needed to complete the currently used classical microbiological methods. In particular, improvement of the methods of enrichment and isolation and novel diagnostic tools are required. They should be robust, quick, reliable (sensitive and specific), efficient on fecal samples and easy to use at patient's bedside. Two approaches have emerged: PCR detection [9], [5], [14], [15], [16], [17] and immunochromatographic techniques, i.e. dipsticks based on the recognition of pathogen-specific antigens by monoclonal antibodies (mAbs). Such dipsticks have already been successfully developed for cholera [18], meningitidis [19] and plague [20]. In this study, we investigated the potential of such dipsticks to detect S. flexneri 2a that remains the most frequently isolated serotype in endemic areas. The dipstick is based on the detection of lipopolysaccharide (LPS), the major bacterial surface antigen. Indeed, Shigella serotypes are defined by the structure of the oligosaccharide repeating unit (RU) that forms the O-antigen (O-Ag), the polysaccharide moiety of LPS [21]. For S. flexneri serotype 2a, the biological RU is a branched pentasaccharide. It is composed of a linear tetrasaccharide backbone made of three L-rhamnose residues, A, B, and C, and a N-acetyl-D-glucosamine residue D, that is common to all S. flexneri, except serotype 6, and of an α-D-glucose residue E, branched at position 4 of rhamnose C that specifies serotype 2a [21], [22].
In this study, we demonstrate that a dipstick based on mAbs recognizing serotype 2a-specific determinants carried by the LPS O-Ag (Figure 1) is a rapid, robust and reliable test to identify S.flexneri 2a in stool samples.
Figure 1.
Structure of the repeating unit of the O-Ag of Shigella flexneri serotype 2a.
Materials and Methods
Development of S. flexneri 2a dipstick
To develop mAbs against the somatic antigen of S. flexneri 2a [22], BALB/c mice were immunized intraperitonally (i.p.) with 107 CFU of killed S. flexneri 2a bacteria, three times at 3-week intervals. Mice eliciting the highest anti-LPS antibody response were given an intraveinous boost injection 3 days before being sacrificed for splenic B cell fusion, according to Kohler and Milstein [23]. Hybridoma culture supernatants were screened for antibody (Ab) production by ELISA using LPS purified from S. flexneri serotype X, Y, 5a, 5b, 2a, 2b, 1a, and 3a, respectively, as previously described [24], [25]. Briefly, LPS purified according to Westphal and Jann [26] was used at a concentration of 5 µg/ml in PBS. As secondary Abs, anti-mouse IgG-, IgM-, or IgA-alkaline phosphatase-labeled conjugate (Sigma-Aldrich) were used at a dilution of 1/5,000. Only the hybridoma cells secreting mIgG reacting specifically with LPS homologous to the strain used for immunization, i.e., recognizing serotype-specific determinants on the LPS O-Ag, were selected. The selected hybridomas, representative of the four murine IgG subclasses, were then cloned by limiting dilution, and injected i.p. into histocompatible mice for ascite production. mIgG were precipitated with 50% ammonium sulfate from ascite fluid, centrifuged, and dialyzed against PBS before being purified using ion-exchange chromatography as previously described [24], [25].
Among the available mAbs specific for S. flexneri serotype 2a, two IgG2a were selected for the development of the diagnostic test. The test is based on a one-step, vertical-flow immunochromatography using mAb-coupled colloidal gold particles [27]. The colloidal gold particles (40 nm diameter) were conjugated to the D15-7 anti-S. flexneri 2a mAb (British Biocell International Cardiff, UK) and lyophilised (A540nm = 2) onto polyester release pads (Accuflow P Schleicher&Shull, Mantes la Ville, France). An automatic thin layer chromatography sampler (CAMAG 5, Muttenz, Switzerland) was used to spray the second selected anti-S.flexneri 2a mAb, E4-1, at a concentration of 2 µg/cm, as a line on nitrocellulose membrane (Immunopore FP, Whatmann International). In addition, a control capture line was obtained by spraying affinity-purified goat anti-mouse IgG (ICN Biomedical, Aurora, Ohio, USA), on a line higher up on the strip, at a concentration of 1 µg/cm. Cellulose filter paper was used for the wicking and sample pads (Cellulose paper 903, Schleicher&Shull).
The immunostrips were then trimmed to a width of 5 mm (Figure 2) and stored in a waterproof bag (50 per bag) at room temperature in Paris (France) or sent to Ho Chi Minh City (Vietnam) to be evaluated. The test was carried out in 5 ml-disposable plastic tubes at room temperature with a sample volume of about 300 µl. After 5–15 min, a positive result appears as two pink lines (upper control line and lower S. flexneri 2a LPS-positive line), and a negative result as a single upper pink control line (Figure 2). S. flexneri strain 2a (ref. NCDC 2747-71) was used as positive control.
Figure 2.
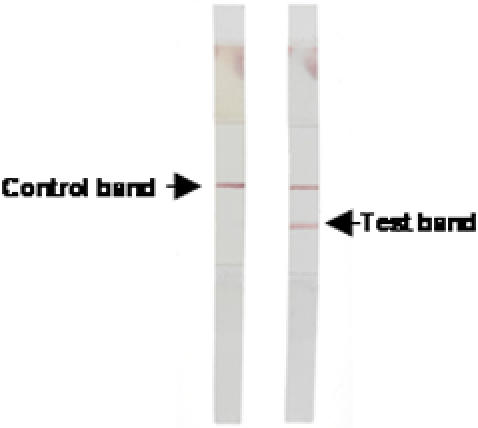
Two dipsticks showing typical negative and positive results after being kept for 15 min in diarrhoea stool samples.
Methodology of the S. flexneri 2a dipstick evaluation
The sensitivity and the specificity of the assays were assessed by one trained technician. The cut-off (detection limit) and the range of detectable LPS concentrations was measured using tenfold dilutions (1 µg/ml to 10 ng/ml) of purified LPS 2a and tenfold dilutions of a S. flexneri 2a suspensions (5×108 to 5×103 bacteria/ml) using saline, and reconstituted stools (10 g of normal stool without Shigella spp suspended in 10 ml of saline). Reliability of immunostrips was assessed with purified LPS (1 µg/ml). To predict the shelf-life of the dipstick, we used the accelerated stability method that consisted in storing the assays for a time at elevated temperature [27]. The shelf life of the strips in the laboratory was assessed by testing three times per week for 10 weeks after storage at 25°C (air-conditioned room) or at 60°C (incubator).
The specificity was assessed using pure cultures of S. flexneri (serotypes 1a, 1b, 2a, 2b, 3a, 3b, 4a, 4b, 5a, 5b, 6, Y, X), S. dysenteriae (serotypes 1-15, and untypable strain 97-10607), S. boydii (serotypes 1 to 20), S. sonnei (strains in phase I, phase II), Salmonella enterica typhimurium (strains 06-2835, 06-2846, 06-2847), S enteritidis (strains 06-2841, 06-2844, 06-2851, 06-2852), S. hadar (strains 06-2533), S. brandenburg (strain 06-2619), S. heidelberg (06-2843), S. oranienburg (strain 06-2634), S. risen (strain 06-2615), S. stanleyville (strain 06-2832), S. typhi (strain 06-2829), S. paratyphi A (strain 06-2633), S. paratyphi B (strain 06-2696), S. meleagridis (strain 06-2850), S. stubra (strain 06-2384), S. huittingfoss (strain 06-2391), enteroagregative Escherichia coli (strains 55989, JM221, O42, 56390 and 384P), diffusely adherent E. coli (strain AL851, AL847, C1845, AL855 and 3043), enterotoxigenic E. coli (strains EDL1496, 440TL, Tx-1, E2539-C1, 469), enteropathogenic E. coli (strains 135/12 (O55:H-), E6468/62 (O86:H34), 11201 (O125:H6), KK111/1 and F88/6848-2 both O26:H11), Vibrio cholerae O1 (strains CNRVC960255, 970002, 970014, 970025, 970067, 960325, 970022, 970053, 970055, 970056), V cholerae O139 (strains CNRVC 930008, 930381, 930210, 930190), V. cholerae non O1 and non O139 (strains CNRVC 930177, 930429, 950689, 950691, 970037, 950769, 910388, 930121, 930297, 930391), V. alginolyticus (strain CIP103336), V. fluvialis (strains CIP103355, CNRVC356), V. parahaemolyticus (strains CIP75.2, CNRVC030478, CNRVC030479, CNRVC000204, CNRVC000208), V. furnissii (strain CIP102972), V. hollisae (strain CIP104354), V. mimicus (strain 101888), Aeromonas caviae (strain CIP76.16), A. enteropelogenes (strain CIP104434), A. hydrophila (strain CIP76.15), A. sobria (strain CIP74.33), Plesiomonas shigelloides (strain CIP63.5), Campylobacter jejuni, Yersinia enterocolitica 1A (6 strains of biotype 1A, 2 strains of biotype 2, 2 strains of biotype 3, and 2 strains of biotype I).
Sensitivity and specificity of the dipsticks were evaluated in a clinical setting in Ho Chi Minh City, an area of dysentery endemicity, during a period of high incidence of the disease [5]. The study has been approved by the Scientific and Ethical Committee of Pasteur Institute in Ho Chi Minh City. The dipsticks were shipped from France to Vietnam at ambient temperature in grip seal bags by air mail. We compared the results obtained with stool cultures for enteropathogenic bacteria and dipsticks performed in a blind study by two different technicians. A total of 191 stool samples from infants hospitalized for dysentery were collected and tested in the Paediatric Hospitals I and II at Ho Chi Minh City. The dipstick test was performed on freshly collected stools. A volume of about 200 µl of stools (preferentially blood, mucus, rectal sputum when present) were transferred with a disposable pipette in a haemolysis glass tube of 5 ml containing 300 µl of distilled water. The sample was homogenised by several pipetting. The immunostrip was then introduced in the test tube and the test was read after 15 min.
Stool samples were transported to the microbiology laboratory of the Pasteur Institute of Ho Chi Minh City for diagnosis by classical methods. In brief, stool samples were cultured for Shigella spp and other enteric pathogens on Hektoen enteric agar and Bromocresol Purple Lactose agar [28]. Suspected colonies resembling Shigella were identified biochemically and serotyped. S. flexneri 2a colonies were identified by slide agglutination with anti-II and anti-3,(4) sera (Eurobio, France), according to the International Enterobacteriaceae Grouping Subcommittee [29].
Samples that were positive by the dipsticks but negative by culture were stored at −20°C and later examined by a PCR targeting the ipaH gene of Shigella spp [30], [31].
Results
The objective of the study was to develop and evaluate a dipstick test for the rapid diagnosis of S. flexneri 2a infection using stool samples at the bedside of the patient. The evaluation was performed firstly by using purified LPS of S. flexneri 2a in distilled water and then in reconstituted stools at different concentrations. The dipsticks were then tested on various concentrations of S. flexneri 2a in culture and in reconstituted stools. The dipsticks were also tested with various species of bacteria in cultures, and finally on diarrhoeal stools.
In distilled water, the lower detection threshold of the dipstick for S. flexneri 2a was 20 ng/ml of LPS. The same threshold was observed in stools reconstituted with different amount of purified S. flexneri 2a LPS. In addition, both in distilled water and in reconstituted stools containing different concentrations of S. flexneri 2a, an unequivocal positive reaction was obtained in 10 minutes with 5×107 CFU/ml of S. flexneri 2a. The specificity of the dipstick was 100% for all bacterial cultures. Similar results were obtained using dipsticks stored for 7 days at 60°C and 10 weeks at 25°C. No prozone effect (i. e. no signal detected for high concentrations) was observed by using a range of LPS concentrations extending from 10 ng/ml to 1 µg/ml.
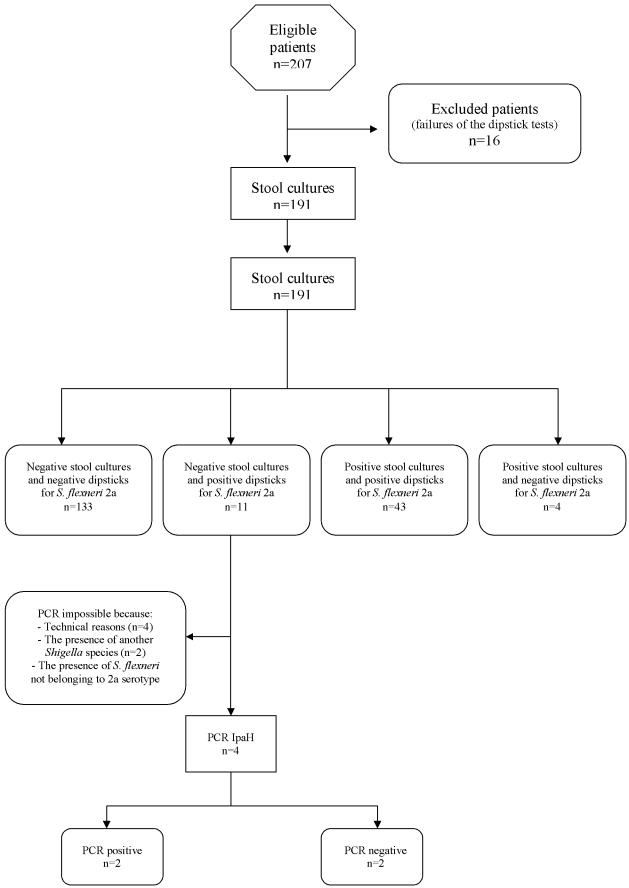
Of the 191 stool samples from patients displaying symptoms of dysentery (Figure 3), 43 were both dipstick-and culture-positive, 11 were dipstick-positive but culture-negative, 4 were dipstick-negative but culture-positive, and 133 were negative by both culture and dipstick (Table 1). Specificity (133/144) on the field was therefore 99.2% (95% CI 97.8%–100%), the sensitivity (43/47) was 91.5% (95% CI 83.6%–99.4%), positive predictive value (43/54) was 79.6% (95% CI 69%–90.3%) and negative predictive value (133/137) 97% (95% CI 89.6%–100%).
Figure 3.
STARD flow diagram of the evaluation study in Vietnam.
Table 1. Detection of S. flexneri 2a in 191 fresh stool samples by dipstick test versus conventional culture.
| Bacteriological culture | N° of specimens with S. flexneri 2a dipstick test result | ||
| Positive | Negative | Total | |
| Positive | 43 | 4 | 47 |
| Negative | 11 | 133 | 144 |
| Total | 54 | 137 | 191 |
Among the 11 samples which were positive by the dipsticks, but negative by bacteriological culture for S. flexneri 2a, S. sonnei was isolated from 2 patients and S. flexneri not belonging to the 2a serogroup was isolated from one patients. For these three cases, it was not possible to conclude if there were co-infections because no PCR specific for S. flexneri 2a was available. In one sample, Salmonella enterica was isolated; the ipaH PCR was positive and the patient was presumably co-infected with S. flexneri 2a. In two samples, the PCR was negative; the presence of PCR inhibitors in these stool samples was suspected, but false-positive results with the dipsticks cannot be excluded. One sample was ipaH PCR positive; the prior use of antibiotics by this patient before the sampling was documented and may have lessened the excretion of viable S. flexneri 2a in the stool sample. For technical reasons (insufficient amount of stools, problems of storage), PCR was not done on four samples.
The four stool samples which were negative by the dipstick test were positive for S. flexneri 2a by bacteriological culture. One of the reasons for such a result might be that the amount of LPS in these stool samples was lower than the threshold level. Another explanation could be the decrease of the sensitivity of the conjugated mAb due to moisture, because these dipsticks were used after a delay of five weeks at ambient temperature during the rainy season. Positive controls worked well but appeared weaker than previously, even when the test was carried out with pure culture of S. flexneri 2a (at least 5×108 CFU/ml).
The blindness of the study was broken to control the serotype of all the strains of Shigella flexneri in case of discordant results; all the serotypes were confirmed after the second serogrouping.
From the 133 dipstick-and culture-negative samples, 21 cultures were positive for S. enterica (including one co-infection with S. sonnei), 20 for S. sonnei, 1 for S. dysenteriae 2, and 9 for S. flexneri not belonging to serotype 2a.
Discussion
Severe and milder forms of shigellosis are developed by patients living in endemic areas. Dysenteric patients have a more severe form of shigellosis with a clinical spectrum ranging from watery diarrhea to diarrhea with mucus and frank bloody diarrhea. Bloody diarrhea is associated with the rupture of the intestinal epithelial barrier, followed by the invasion and destruction of the intestinal mucosa, resulting in the proliferation of the pathogens faster than that occuring in patients with a milder form of the disease [32]. Patients who have the most severe form of shigellosis also shed an higher number of micro organisms [32]. A direct relationship between bacterial load (i. e. LPS concentration in stools), detection by culture, and disease severity has also been reported by Thiem et al [9]. Consequently, it is essential to develop an efficient dipstick test displaying a low detection threshold, and detecting the somatic antigen without prozone effect to avoid false-negative results in samples containing high concentrations of S. flexneri 2a LPS antigen.
We report here such a tool. The S. flexneri 2a dipstick we developed was found to be highly specific when tested on bacterial cultures, with a detection threshold comparable to those of dipsticks developed to diagnose cholera (107 CFU/ml of V. cholerae O1 and 50 ng/ml of LPS) [18], [33]. The S. flexneri 2a dipstick detected somatic antigen at a wide range of concentrations, in 5–15 minutes, without prozone effect.
The evaluation on stool samples of patients living in endemic area confirms the good specificity of the S. flexneri 2a dipstick test. Regarding the sensitivity of 91.5% measured during the “on field evaluation” as compared to that using laboratory testing, the most likely explanation for the discrepancies observed for four samples that appeared false negative by S. flexneri 2a dipstick is certainly the condition in which the dipsticks were preserved locally. This problem of decreasing of sensitivity due to humidity was also observed with other dipsticks developed for other pathogens [20]. This drawback is now overcome by improvement of the individual dipstick packaging, making them easily transportable and adapted to the local environmental conditions. Although the study is not completed, we already have new data with two other evaluations that are undergoing in Santiago (Chile) and Djibouti (with the French Army) by using dipsticks individually wrapped in waterproof bags. The results we obtained demonstrate that the problem has been resolved. After about 5 weeks of storage of the dispsticks at room temperature, the tests have been performed on positive and negative stools stored at −20°C in Santiago. We observed that the concordance was 100% (data not shown). Another evaluation is currently being performed in Dhaka with a special attention to this problem.
The test was applied to the diagnosis of bacillary dysentery to watery stools, frank bloody stools, as well as stools with mucus and rectal mucous discharge. Although the diarrheic stools contain high concentrations of S. flexneri 2a somatic antigen in the severe forms of the illness (i.e. true dysenteric syndrome), we thought that it was important to dilute the sample in distilled water to increase antigen-antibody interactions and to improve the flow of immune complexes through the dipstick. Otherwise these could be inhibited by the viscosity and the density of the rectal sputum or of the mucus.
The reference test–isolation, biochemical and seroagglutination of Shigella–which can be done only in the laboratory is specific but lacks sensitivity. This may explain why S. flexneri 2a was missed in eleven stools that were dipstick-positive. Co-infections involving probably S. flexneri 2a and other enteropathogenic bacteria were observed in 4 samples (2 with S. sonnei, 1 with S. flexneri not belonging to serogroup 2a, and 1 with S. enterica). This is explained by the fact that during routine work, only five lactose negative colonies were studied per stool, a condition which is not in favour of the diagnosis of infections with multiple enteropathogenic bacteria. Indeed the coproculture is generally stopped when an enteric bacterium in relation to the clinical symptoms is isolated, but enteric infections with multiple pathogens are frequent in developing countries in endemic tropical area [28]. Reasons for the low sensitivity of traditional culture methods also include the low number of causative Shigella strains in several cases, competition from other commensal microorganisms, and inappropriate changes in ambient temperature and pH during specimen transport [10]. The growth, and thus the detection, of the bacteria is further impaired by the use of antibiotics prior to specimen collection (one documented case in this study). However, the coproculture remains “indispensable” to complete the diagnosis in particular for determining antibioresistance and for characterization of the strains.
No specific PCR was available to confirm the presence of S. flexneri 2a in the case of negative results of the coproculture, but two infections with S. flexneri 2a (including one co-infection with S. enterica) were highly suspected because the dipsticks and the PCR assay based on the amplification of the invasion plasmid antigen H (ipaH) gene sequence were positive.
Because prompt diagnosis of diarrhoea is of key importance to initiate effective therapy and to decide proper epidemiological measures, multivalent dipsticks are needed. Therefore, we are currently developing other dipsticks for Shigella spp (generic diagnosis) and the most prevalent serotypes (S. dysenteriae 1, 2 and 3; S. flexneri 1b, 2b, 3a, 6b; S. sonnei), but also for Salmonella enterica, diarrheogenic Escherichia coli (EIEC, EPEC, EHEC, EAEC), Campylobacter spp, E. histolytica, Giardia lamblia. These tools will complete those actually available for Rotavirus and V. cholerae O1 and O139.The use of PCR assay based on the amplification of the invasion plasmid antigen H (ipaH) gene sequence can overcome some of the shortcomings of culture methods, but the method itself has not yet received global acceptance due to difficulties in the implementation by some laboratories located in developing countries.
In this study, we demonstrated the proof of principle of a one-step, vertical-flow immunochromatography test based upon mAbs recognizing serotype-specific determinants carried by the O-Ag as a rapid, robust and reliable test to identify S. flexneri 2a from stool samples. This new diagnosis test, which requires minimal technical skill efficiently completes the classical microbiological methods.
The importance of an efficient bacillary dysentery surveillance system continues to be stressed by World Health Organization with regard to improving risk assessment of potential bacillary dysentery outbreaks [1]. To fullfill this need, further studies are under way to develop: i) a single test able to diagnose all Shigella spp (whatever the serotype) and EIEC, ii) a multiplex dipstick for several prevalent serotypes of Shigella spp (ie. S. dysenteriae 1, S. flexneri, S. sonnei, etc.) that will be evaluated in different settings in endemic countries including: on the field, in dispensary (remote area), and in public health laboratories.
Acknowledgments
We thank the patients of Ho Chi Minh City who participated in the study and the staff of the Paediatric Hospitals I and II of Ho Chi Minh City who made this study possible.
We thank the Collection de l'Institut Pasteur for kind gift of the strains of Vibrionaceae. We are grateful to Francine Grimont, Elisabeth Carniel, Jean Michel Fournier, Marie Laure Quilici and Chantal LeBouguénec for kind gift of the strains used in our specificity study. We thank Muriel Vray for statistical analysis and John Rohde for careful reading the manuscipt. We are also grateful to Edith Fournié-Amazouz for her excellent technical help. We also thank Valeria Prado and Jérôme Maslin for their contribution.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by the Institut Pasteur, Paris (grant ACIP and PTR 179). Part of the work was funded by a grant from TOTAL SA.
References
- 1.World Health Organization. Shigellosis: disease burden, epidemiology and case management. Weekly epidemiological record. 2005;80:93–100. [Google Scholar]
- 2.Kotloff KL, Winickoff JP, Ivanoff B, Clemens JD, Swerdlow DL, et al. Global burden of Shigella infections: implications for vaccine development and mplementation of control strategies. Bull World Health Organ. 1999;77(8):651–666. [PMC free article] [PubMed] [Google Scholar]
- 3.Kosek M, Bern C, Guerrant RL. The global burden of diarrhoeal disease, as estimated from studies published between 1992 and 2000. Bull World Health Organ. 2003;81(3):197–204. [PMC free article] [PubMed] [Google Scholar]
- 4.Chompook P, Samosornsuk S, von Seidlein L, Jitsanguansuk S, Sirima N, et al. Estimating the burden of shigellosis in Thailand: 36-month population-based surveillance study. Bull WHO, 2005;83:739–746. [PMC free article] [PubMed] [Google Scholar]
- 5.von Seidlein L, Kim DR, Ali M, Lee H, Wang X, et al. A Multicentre Study of Shigella Diarrhoea in Six Asian Countries: disease burden, clinical manifestations, and microbiology. PLoS Med. 2006;12;3(9) doi: 10.1371/journal.pmed.0030353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diniz-Santos DR, Santana JS, Barretto JR, Andrade MG, Silva LR. Epidemiological and microbiological aspects of acute bacterial diarrhea in children from Salvador, Bahia, Brazil. Braz J Infect Dis. 2005;9(1):77–83. doi: 10.1590/s1413-86702005000100013. [DOI] [PubMed] [Google Scholar]
- 7.Vu Nguyen T, Le Van P, Le Huy C, Nguyen Gia K, Weintraub A. Etiology and epidemiology of diarrhea in children in Hanoi, Vietnam. Int J Infect Dis. 2006;10(4):298–308. doi: 10.1016/j.ijid.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 8.Shears P. Shigella infection. Ann Trop Med Parasitol. 1996;90:105–114. doi: 10.1080/00034983.1996.11813034. [DOI] [PubMed] [Google Scholar]
- 9.Thiem DV, Sethabutr O, von Seidlein L, Van Tung T, Gia Canh D, et al. Detection of Shigella by a PCR assay targeting the ipaH gene suggests increased prevalence of shigellosis in Nha Trang, Vietnam. J Clin Microbiol. 2004;42:2031–2035. doi: 10.1128/JCM.42.5.2031-2035.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taylor WI, Schelhart D. Effect of temperature on transport and plating media for enteric pathogens. J Clin Microbiol. 1975;2:281–286. doi: 10.1128/jcm.2.4.281-286.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee LA, Dogore R, Redd SC, Dogore E, Metchock B, et al. Severe illness in African children with diarrhoea: implications for case management strategies. Bull World Health Organ. 1995;73:779–785. [PMC free article] [PubMed] [Google Scholar]
- 12.Wierzba TF, Abdel-Messih IA, Abu-Elyazeed R, Putnam SD, Kamal KA, et al. Clinic-based surveillance for bacterial- and rotavirus-associated diarrhea in Egyptian children. Am J Trop Med Hyg. 2006;74:148–153. [PubMed] [Google Scholar]
- 13.Legros D, Paquet C, Dorlencourt F, Saoult E. Risk factors for death in ihospitalized dysentery patients in Rwanda. Trop Med Int Health. 1999;4:428–432. doi: 10.1046/j.1365-3156.1999.00413.x. [DOI] [PubMed] [Google Scholar]
- 14.Frankel G, Riley L, Giron JA, Valmassoi J, Friedmann A, et al. Detection iof Shigella in feces using DNA amplification. J Infect Dis. 1990;161:1252–1256. doi: 10.1093/infdis/161.6.1252. [DOI] [PubMed] [Google Scholar]
- 15.Gaudio PA, Sethabutr O, Echeverria P, Hoge CW. Utility of a polymerase chain reaction diagnostic system in a study of the epidemiology of shigellosis among dysentery patients, family contacts, and well controls living in a shigellosis-endemic area. J Infect Dis. 1997;176:1013–1018. doi: 10.1086/516531. [DOI] [PubMed] [Google Scholar]
- 16.Houng HS, Sethabutr O, Echeverria P. A simple polymerase chain reaction technique to detect and differentiate Shigella and enteroinvasive Escherichia coli in human feces. Diagn Microbiol Infect Dis. 1997;28:19–25. doi: 10.1016/s0732-8893(97)89154-7. [DOI] [PubMed] [Google Scholar]
- 17.Sethabutr O, Echeverria P, Hoge CW, Bodhidatta L, Pitarangsi C. Detection of Shigella and enteroinvasive Escherichia coli by PCR in the stools of patients with dysentery in Thailand. J Diarrhoeal Dis Res. 1994;12:265–269. [PubMed] [Google Scholar]
- 18.Nato F, Boutonnier A, Rajerison M, Grosjean P, Dartevelle S, et al. One-step immunochromatographic dipstick tests for rapid detection of Vibrio cholerae O1 and O139 in stool samples. Clin Diagn Lab Immunol. 2003;10:476–478. doi: 10.1128/CDLI.10.3.476-478.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chanteau S, Dartevelle S, Mahamane AE, Djibo S, Boisier P, et al. New Rapid Diagnostic Tests for Neisseria meningitidis Serogroups A, W135, C, and Y. PLoS Med. 2006;Sep 5;3(9) doi: 10.1371/journal.pmed.0030337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chanteau S, Rahalison L, Ralafiarisoa L, Foulon J, Ratsitorahina M, et al. Development and testing of a rapid diagnostic test for bubonic and pneumonic plague. Lancet, 2003;361:211–216. doi: 10.1016/S0140-6736(03)12270-2. [DOI] [PubMed] [Google Scholar]
- 21.Lindberg AA, Karnell A, Weintraub A. The lipopolysaccharide of Shigella bacteria as a virulence factor. Rev Infect Dis. 199;13 Suppl 4:S279–284. doi: 10.1093/clinids/13.supplement_4.s279. [DOI] [PubMed] [Google Scholar]
- 22.Phalipon A, Costachel C, Grandjean C, Thuizat A, Guerreiro C, et al. Characterization of functional oligosaccharide mimics of the Shigella flexneri serotype 2a O-antigen: implications for the development of a chemically defined glycoconjugate vaccine. J Immunol. 2006;176:1686–1694. doi: 10.4049/jimmunol.176.3.1686. [DOI] [PubMed] [Google Scholar]
- 23.Kohler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- 24.Barzu S, Nato F, Rouyre S, Mazie JC, Sansonetti P, et al. Characterization of B-cell epitopes on IpaB, an invasion-associated antigen of Shigella flexneri: identification of an immunodominant domain recognized during natural infection. Infect Immun. 1993;6:3825–3831. doi: 10.1128/iai.61.9.3825-3831.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Phalipon A, Arondel J, Nato F, Rouyre S, Mazie JC, et al. Identification and characterization of B-cell epitopes of IpaC, an invasion-associated protein of Shigella flexneri. Infect Immun. 1992;60:1919–1926. doi: 10.1128/iai.60.5.1919-1926.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Westphal O, Jann J. Bacterial lipopolysaccharide extraction with phenol-water and further application of the procedures. Meth Carbohydr Chem. 1965;5:83–91. [Google Scholar]
- 27.Paek SH, Lee SH, Cho JH, Kim YS. Development of rapid one-step immunochromatographic assay. Methods. 2000;22:53–60. doi: 10.1006/meth.2000.1036. [DOI] [PubMed] [Google Scholar]
- 28.Germani Y, Minssart P, Vohito M, Yassibanda S, Glaziou P, et al. Etiologies of acute, persistent, and dysenteric diarrheas in adults in Bangui, Central African Republic, in relation to human immunodeficiency virus serostatus. Am J Trop Med Hyg. 1998;59:1008–1014. doi: 10.4269/ajtmh.1998.59.1008. [DOI] [PubMed] [Google Scholar]
- 29.Brenner DJ. Recommendation on recent proposals for the classification of Shigella, International journal of Syst bacterial. 1984;34:87. [Google Scholar]
- 30.Sethabutr O, Venkatesan M, Murphy GS, Eampokalap B, Hoge CW, et al. Detection of Shigellae and enteroinvasive Escherichia coli by amplification of the invasion plasmid antigen H DNA sequence in patients with dysentery. J Infect Dis. 1993;167:458–461. doi: 10.1093/infdis/167.2.458. [DOI] [PubMed] [Google Scholar]
- 31.Toma C, Lu Y, Higa N, Nakasone N, Chinen I, et al. Multiplex PCR assay for identification of human diarrheagenic Escherichia coli. J Clin Microbiol. 2003;41:2669–2671. doi: 10.1128/JCM.41.6.2669-2671.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sansonetti PJ, Tran Van Nhieu G, Egile C. Rupture of the intestinal epithelial barrier and mucosal invasion by Shigella flexneri. Clin Infect Dis. 1999;28:466–475. doi: 10.1086/515150. [DOI] [PubMed] [Google Scholar]
- 33.Bhuiyan NA, Qadri F, Faruque AS, Malek MA, Salam MA, et al. Use of dipsticks for rapid diagnosis of cholera caused by Vibrio cholerae O1 and O139 from rectal swabs. J Clin Microbiol. 2003;41:3939–3941. doi: 10.1128/JCM.41.8.3939-3941.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]