Abstract
Pulmonary function (PF) was studied in 69 consecutive patients with hematological diseases, with a minimum of 5 year (range 5-13) follow-up after allogeneic stem cell transplantation (SCT) from an HLA-matched sibling. Fifty-six (81%) patients received total body irradiation (TBI) based myeloablative stem cell transplantation (MT) and 13 (19%) received a non-myeloablative stem cell transplant (NST). Thirty one (45%) patients developed a late decline in PF from baseline, 25 with a restrictive and 6 with an obstructive pattern PF abnormality. Twelve patients (17%) were symptomatic, 8 with a severe restrictive PF defect, but none required supplemental oxygen. The incidence of developing a late PF abnormality was comparable in both MT (24/56) and NST (5/13) (p=0.51). In multivariate analysis, chronic graft-versus-host disease (cGVHD) (relative risk [RR]-16) and pre-transplant DLCO or FEV1 of less than 80% predicted (RR-7) were independently associated with a late decline in PF from baseline. Our results indicate that late PF abnormality is common after both MT and NST. Patients with a low pre-transplant DLCO and or FEV1 who developed cGVHD were most severely affected. Longer follow-up is needed to determine whether PF will continue to decline or reach a plateau and whether more patients with PF abnormality will eventually become symptomatic.
Keywords: pulmonary complications, myeloablative, non-myeloablative, stem cell transplantation
Introduction
Despite the success in treating otherwise fatal disease, allogeneic stem cell transplantation (SCT) is associated with multiple and life-threatening complications from organ damage. Lung injury is a frequent complication after allogeneic stem cell transplantation [1-7]. In our experience in adults with hematological malignancies receiving SCT from HLA-identical siblings, non-infectious pulmonary deaths accounted for 2/3 of transplant related mortality (TRM) occurring mainly in the first six months after SCT [8]. Delayed pulmonary injury can also occur: the lifetime risk of chronic pulmonary dysfunction in long term SCT survivors ranges from 30-60% [1,9-11], depending on donor source and elapsed time from SCT. Chronic pulmonary dysfunction in long term SCT survivors can be of either obstructive or restrictive pattern [1,7,9,12-14]. In addition an association of defective pre-transplant pulmonary function and the development of obstructive PF abnormality after transplant have been recently reported [15]. Pulmonary dysfunction has been linked to chronic graft versus host disease (cGVHD) in children receiving SCT [7] and in adults with unrelated SCT [16]. Reduced intensity SCT has become increasingly popular based on the assumption that lower intensity conditioning regimens will cause less direct organ damage resulting in an improvement in long-term outcomes. Better identification of pre-transplant factors associated with worsening PF are needed to define patients at greatest risk who might benefit from preventive strategies. Here we describe PF abnormalities in a cohort of patients surviving 5 years or more after MT and NST and identify pre and post-transplant risk factors.
Patients, materials, and methods
Study group
69 consecutive patients transplanted between September 1993 and March 2001 (minimum 5 years follow-up after SCT) were included in this analysis. All received SCT from an HLA-identical sibling in 5 successive National, Heart, Lung and Blood Institute (NHLBI) institutional review board-approved protocols (93-H-0212, 97-H-0099, 97-H-0202, 99-H-0046, and 99-H-0050). All patients and donors gave written informed consent according to principles outlined in the Declaration of Helsinki.
Transplant regimens
Conditioning regimens
Two approaches were evaluated: A. MT (n=56): (1) TBI 1360 rads in 8 fractions in 4 days followed by cyclophosphamide (Cy) 60 mg/kg X 2 days and bone marrow transplantation (BMT) (n = 18) or G-CSF mobilized peripheral blood stem cell transplantation (PBSCT) (N= 38). B. (debilitated or older patients) NST (n=13): Cy 120mg/kg + Fludarabine (Flu) 125 mg/m2 and G-CSF mobilized peripheral blood stem cell transplantation (patients with aplastic anemia or patients at increased risk for graft rejection [i.e. patients with heavy transfusion burden or with a evidence of alloimmunization] also received antithymocyte globulin 40mg/kg IV on days -5,-4,-3,-2)
GVHD prophylaxis:
MT patients received cyclosporine alone day -4 to +120 -180. All NST patients received cyclosporine with either methotrexate 5 mg/m2 on days +1, +3, and +6 OR mycophenolate mofetil 1gms PO bid.
Transplantation:
NST patients received an unmanipulated T cell replete PBSCT. T cell depletion was performed in all MT. In the first protocol (93-H-0212), stem cells were collected after bone marrow harvesting and were depleted of T cells by elutriation [17]. In all subsequent protocols the donor underwent granulocyte-colony-stimulating factor (G-CSF)-mobilized peripheral blood apheresis followed by stem cell selection. In protocol 97-H-0099, T cells were depleted by CD34+ selection on the “Ceprate SC” immunoabsorption column, followed by negative selection of T cells using anti-CD2 (CellPro, Bothell, WA). More recent protocols used the Isolex 300i immunomagnetic cell separation system, version 2.5 (Nexell Therapeutics, Irvine, CA), for positive selection of CD34+ cells, followed by negative selection of T cells using an antibody cocktail of anti-CD2, anti-CD6, and anti-CD7, as previously described [18].
Donor lymphocyte infusion:
In MT in the absence of GVHD or complete molecular remission of CML, T cells were added back between days 30-100. NST patients received DLI for disease progression or for persistent mixed T-cell chimerism following cyclosporine tapering.
Supportive care:
Standard prophylaxis against infection included fluconazole to day 100, bactrim for 6 months after transplantation, and weekly surveillance for cytomegalovirus antigenemia, as described previously [18,19]. Acute GVHD was managed with high-dose steroids. Steroid-refractory patients received treatment with daclizumab (anti-CD25 monoclonal antibodies) alone or in combination with infliximab (anti-tumor necrosis factor), as described previously [20].
Pulmonary function tests
Baseline pulmonary function (PF) was obtained in all patients 5 to 21 days before SCT and then post-transplant at 3 months, 6 months, 1 year, and thereafter annually when possible. In patients with a concurrent acute respiratory illness, PF’s were deferred till recovery from illness or return to base line status. Ventilatory capacity was measured by forced vital capacity, forced expiratory volume in the first second (FEV1), the FEV1/forced vital capacity ratio, and peak expiratory flow. Lung volume measurements included vital capacity (VC), total lung capacity (TLC), residual volume, and the residual volume/total lung capacity ratio. Diffusion capacity for carbon monoxide (DLCO) was determined by using a carbon monoxide single-breath technique with correction for hemoglobin concentration. PF were expressed as a percentage of the predicted values in healthy controls with corresponding age, sex, and smoking habits. Eligibility criteria for enrollment into protocols included DLCO ≥60% of predicted.
Definitions
Stringent criteria for the definition of abnormal PF’s were chosen because of inherent variability in the tests employed. Pre-transplant abnormal PF was defined as DLCO, FEV1, or both ≤80% predicted. Post-transplant PF abnormality, was defined as restrictive when there was significant decline from baseline in DLCO and TLC and obstructive pattern with decline in FEV1 and FEV1/FVC ratio. We defined a mild to moderate in post-transplant PF abnormality as a 5-15 % decline in DLCO, FEV1 or TLC and severe as a >15% decline, compared to pre-transplant values. Symptomatic PF abnormality was defined as decline in PF from baseline (as defined above), with any combination of two or more respiratory symptoms-shortness of breath, wheezing, fatigue, and cough unrelated to acute illness lasting for more than 4 weeks duration. Smokers were defined as patients who regularly smoked ≥ 1 pack of cigarettes within 12 months before PBSCT for a minimum of 2 years.
Statistical methods
Summary statistics, such as proportions, means, standard deviations, 95% confidence intervals, medians, and ranges, were used to describe the patient characteristics, pre-transplant variables, and post-transplant outcomes. Kaplan-Meier curves were used to display the distributions of survival and events among subgroups of patients, and Cox proportional hazard models with univariate or multivariate covariates were used to evaluate the effects of covariates, such as smoking, pre-transplant abnormal PF, , type of transplantation, CD34 cell dose, and acute/chronic GVHD on survival times. Variables entered into the multivariate models were age, smoking, pre-transplant abnormal PF, aGVHD and cGVHD. Statistical associations between pre-transplant variables were investigated by using correlation analysis, including Pearson correlation coefficients and Spearman rank correlation coefficients, and multiple regression analysis. Statistical tests based on t tests, χ2 tests, and F tests were used to evaluate the statistical significance of covariates in multiple regression models or Cox proportional hazard models. Data analysis was performed with SPSS 14 for Windows (SPSS Inc., Chicago, IL) software.
Results
Patients
A total of 199 patients with hematological disorders (malignant-191; benign-8) received allogeneic SCT between September 1993 and March 2001 in NHLBI protocols. Of these, 83 patients survived beyond 5 years and 69 patients were available for PF and clinical assessment beyond 5 year post-transplantation. In this cohort of 69 patients all survived at the time of analysis. Patient characteristics of the 69 patients included in this analysis are summarized in Table 1. The median follow-up of the study population was 7.5 years (range, 5-13). The median age of patients at transplantation was 37 years (range 10-68). The median ages (range) of patients receiving MT and NST was 37 (10-55) and 34 (15-68) years respectively. Sixty-five patients (94%) had hematological malignancies and 4 had benign hematological disorders (severe aplastic anemia-2; paroxysmal nocturnal hemoglobinuria-2). Before transplant 11(16%) patients were smokers. Pre-transplant PF abnormalities (DLCO and or FEV1 ≤80% predicted) were observed in 19 (27.5%) patients. There was no difference between patients with normal and abnormal PF groups. Fifty six (81%) patients received MT and 13 (19%) received NST. The median CD34+ cell dose infused was 5.26x106/kg (range 0.98-21.1).
Table 1.
Patients characteristics and univariate analysis.
| Factor | No of patients (%) | PF abnormality n (%) | p value | Symptomatic PF abnormality n (%) | p value | |
|---|---|---|---|---|---|---|
| Pre-transplant variables | ||||||
| Age (median 37 years) | 0.195 | 0.13 | ||||
| <37 | 34 | 13 (38.2) | 2 (5.8) | |||
| ≥37 | 35 | 18 (51.4) | 10 (28.5) | |||
| Gender | 0.495 | 0.038 | ||||
| Female | 30 (43) | 14 (46.6) | 2 (6.6) | |||
| Male | 39 (57) | 17 (43.5) | 10 (25.6) | |||
| Race | 0.772 | 0.885 | ||||
| Asian | 11 (16) | 6 (54.5) | 2 (18.1) | |||
| Black | 5 (7) | 3 (60) | 1 (20) | |||
| Hispanic | 24 (35) | 10 (41.6) | 3 (12.5) | |||
| White | 29 (42) | 12 (41.3) | 6 (20.6) | |||
| Smoking | 0.001 | <0.0001 | ||||
| No | 58 (84) | 21 (36.2) | 2 (3.4) | |||
| Yes | 11 (16) | 10 (90.9) | 10 (90.9) | |||
| Pre-transplant abnormal PF | <0.0001 | 0.002 | ||||
| No | 50 (72) | 15 (30) | 4 (8) | |||
| Yes | 19 (28) | 16 (84.2) | 8 (42.1) | |||
|
Transplant-related variables | ||||||
| Type of transplant | 0.419 | 0.402 | ||||
| Myeloablative (MST) | 56 (81) | 26(46.4) | 9(16) | |||
| Non-Myeloablative (NST) | 13(19) | 5(38.4) | 3(23) | |||
| CD 34 dose (median 5.26 million cells/ kg) | 0.195 | 0.397 | ||||
| <5.26 | 34 | 13 (38.2) | 5 (14.7) | |||
| ≥5.26 | 35 | 18 (51.4) | 7(20) | |||
| Acute GVHD | 0.127 | 0.02 | ||||
| No | 44(64) | 17(38.6) | 4 (9) | |||
| Yes | 25 (36) | 14 (56) | 8 (32) | |||
| Chronic GVHD | <0.0001 | 0.019 | ||||
| No | 26 (38) | 2 (7.6) | 1 (3.8) | |||
| Yes | 43 (62) | 29 (67.4) | 11 (25.5) | |||
PF- pulmonary function.
GVHD
Among long term survivors, 25 (36.2%) patients had a history of aGVHD (grade II-IV) and 43 patients (62.3%) patients had cGVHD (limited-39; extensive-4) at some point post-transplant. Of 17 patients with abnormal PF pre-transplant, 14 developed limited and 3 extensive cGVHD. Of 26 patients who had normal pre-transplant PF, 25 developed limited and one patient developed extensive cGVHD.
At the time of this analysis, only 3 (4.3%) patients remained on immunosuppressive therapy for cGVHD. The incidence of cGVHD was 58% after MT and 76% after NST. Of 19 patients with abnormal PF pre-transplant (DLCO and or FEV1 of <80% predicted), 17 developed cGVHD vs. 26 of 50 with normal pre-transplant PF developing cGVHD (p=0.0049).
PF abnormality
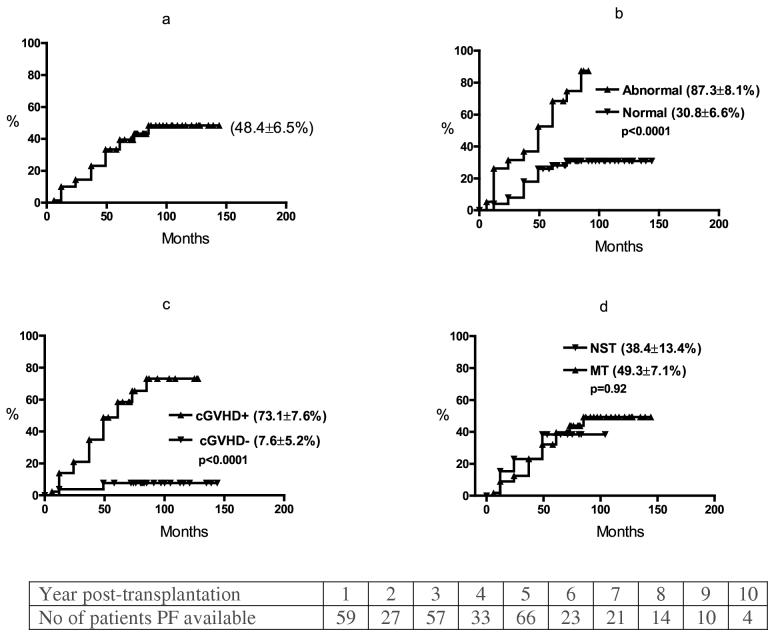
Details of PF abnormality are summarized in Table 2. Thirty one (45%) patients had a decline in PF during long term follow-up, 25 (36%) with a restrictive pattern and 6 (9%) with an obstructive pattern. In 22 patients the abnormalities were mild to moderate in severity (5-15 % decline in % predicted values) and severe (≥15%) in 9 patients. Twelve of these 31 patients (17% of all 69 patients) were symptomatic (Table 3), 8 with a severe restrictive and 4 with obstructive pattern post-transplant PF abnormality. None required supplemental oxygen. All but one had a history of cGVHD. The median time to post-transplant PF decline from baseline was 37 months (range 6-85) (Figure 1a) and median time to symptomatic PF abnormality was 49 months (12-85) .
Table 2.
Details of post-transplant PF abnormality (compared to baseline value).
| Patients with decline in PF, n=31 | ||
|---|---|---|
| n of patients | ||
| Symptomatic | 12 | |
| Type of PF abnormality | ||
| Restrictive | 25 | |
| Obstructive | 6 | |
| Severity of PF abnormality | ||
| Mild to moderate (5-15% decline)* | 22 | |
| Severe (>15% decline)* | 9 |
PF- pulmonary function;
Decline in DLCO and or FEV1 and or TLC from baseline.
Table 3.
Details of patients with symptomatic PF abnormalities
| N | Diagno sis | Age (years) | Age (years) | Sex* | Race↑ | Smoking | Pre-SCT Ab PFT | Type of SCT | CD-34 dose | aGVHD(II-IV) | cGVHD | Severity of PFT Ab† | Type of PFT Ab | Time to Symptom atic PFT Ab (months) | Survival (years) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | MDS | 66 | M | W | Y | Y | NST | 5.81 | N | Y | 1 | O | 15 | 12 | 6 |
| 2 | CML | 46 | M | W | Y | Y | MT | 11.50 | Y | Y | 2 | R | 16 | 12 | 5 |
| 3 | CML | 56 | F | W | Y | N | NST | 6.58 | Y | Y | 1 | R | 28 | 24 | 7 |
| 4 | CML | 43 | M | A | Y | Y | MT | 6.75 | N | Y | 2 | R | 26 | 24 | 5 |
| 5 | CML | 31 | M | A | Y | Y | NST | 3.70 | Y | Y | 1 | R | 52 | 49 | 7 |
| 6 | CML | 39 | M | H | Y | N | MT | 1.48 | N | Y | 2 | R | 54 | 49 | 9 |
| 7 | CLL | 49 | F | W | N | N | MT | 5.66 | Y | Y | 2 | R | 50 | 49 | 7 |
| 8 | CML | 36 | M | H | Y | N | MT | 2.88 | N | N | 1 | R | 51 | 49 | 10 |
| 9 | CML | 53 | M | W | Y | Y | MT | 5.42 | Y | Y | 1 | R | 49 | 37 | 7 |
| 10 | CML | 55 | M | W | N | Y | MT | 2.88 | Y | Y | 1 | O | 54 | 49 | 10 |
| 11 | CML | 39 | M | B | Y | Y | MT | 6.11 | Y | Y | 2 | O | 80 | 73 | 10 |
| 12 | CML | 39 | M | H | Y | Y | MT | 2.69 | Y | Y | 1 | O | 90 | 85 | 8 |
M-male; F-female; W-white, A-asian, H-hispanic, B-black; Y-yes, N-no; SCT-stem cell transplantation; Ab-abnormality; PF- pulmonary function tests; NST-non-myeloablative conditioning; MT-myeloablative conditioning;
1-mild to moderate (5-15% decline in DLCO and or FEV1), 2-severe (>15% decline in DLCO and or FEV1 O-predominantly obstructive, R-predominantly restrictive PFT abnormality
Figure 1.
Time to a significant decline in PF (from baseline)-a. all patients; b-pre-transplant abnormal PF (DLCO and or FEV1 <80% predicted vs. normal PF pre-transplantation; c-cGVHD vs. no cGVHD; d-myeloablative (MT) vs. non-myeloablative (NST) stem cell transplantation.
Pretransplantation factors affecting late PF
Table 4 shows PF changes over 7 years post-SCT period in patients with abnormal pre-transplant PF with ≤80% predicted value in either DLCO or FEV1 or both at baseline. The nature of the pre-transplant abnormality (obstructive or restrictive pattern) did not influence the evolution towards a global deterioration in DLCO, FEV1 and TLC irrespective of whether the initial abnormality was only in DLCO or FEV1 (Table 4). We therefore defined abnormal pretransplant PF as ≤80% predicted value in either DLCO, or FEV1, or both. In univariate analysis the pre-transplantation variables, smoking and abnormal pre-transplant PF were associated with a significant decline in PF from baseline (Table 1, Figures 1b, ). Age, gender, and race did not significantly affect the risk of late PF abnormality (Table 1). Ten of 11 smokers developed late decline in PF from baseline compared to only 21 of 58 non-smokers (p ≤0.001). Male sex was significantly associated with symptomatic PF abnormality (10/11 smokers were male). In multivariate analysis (Table 5), among pre-transplant factors, abnormal pre-transplant PF was predictive for asymptomatic and symptomatic late decline in PF from baseline (RR 7, p=0.02 for both).
Table 4.
Serial PF measurements in patients with abnormal pre-transplant PF.
| Pre-transplant PF | Pre-SCT* | 1 year* | 3 year* | 5 year* | 6 year* | 7 year* | |
|---|---|---|---|---|---|---|---|
| DLCO <80, (n=11) | n= | 11 | 10 | 8 | 10 | 6 | 5 |
| DLCO | 73 ± 2.0 | 77 ± 2.4 | 70 ± 3.5 | 66 ± 3.2 | 60 ± 3.3 | 61 ± 5.3 | |
| FEV1 | 88 ± 4.8 | 85 ± 4.7 | 85 ± 5.0 | 83 ± 5.2 | 69 ± 7.7 | 69 ± 10.6 | |
| TLC | 86 ± 2.5 | 83 ± 3.5 | 84 ± 4.9 | 80 ± 5.9 | 71 ± 6.7 | 71 ± 9.2 | |
| FEV1 <80, (n=8) | n= | 8 | 8 | 6 | 7 | 3 | 4 |
| DLCO | 90 ± 8.9 | 74 ± 6.3 | 76 ± 6.1 | 68 ± 10.4 | 61 ± 1.3 | 59 ± 0.3 | |
| FEV1 | 73 ± 1.7 | 76 ± 5.7 | 74 ± 3.4 | 69 ± 3.2 | 65 ± 13.7 | 57 ± 4.1 | |
| TLC | 87 ± 2.2 | 83 ± 4.8 | 83 ± 3.5 | 78 ± 7.1 | 70 ± 12.9 | 70 ± 8.2 |
Mean ± standard error of mean, percentage predicted value; ; n- number of patients.
Table 5.
Multivariate analysis: factors affecting late decline in PF from baseline.
| Factor | Relative risk | 95 % CI | p | |
|---|---|---|---|---|
| PF abnormality | ||||
| Pre-transplant abnormal PF | ||||
| No | 1 | |||
| Yes | 7 | 1.3-42 | 0.02 | |
| Chronic GVHD | ||||
| No | 1 | |||
| Yes | 16 | 2.9- 68 | 0.001 | |
| Symptomatic PF abnormality | ||||
| Pre-transplant abnormal PF | ||||
| No | 1 | |||
| Yes | 7 | 1.3-39 | 0.02 | |
| Chronic GVHD | ||||
| No | 1 | |||
| Yes | 18 | 3.0-114 | 0.002 | |
PF-pulmonary function; CI-confidence interval.
Transplant-related factors affecting late PF abnormality
The incidence of late PF abnormality was comparable in MT (26/56) and NST (5/13) (p=0.51) (Table 1). In univariate analysis, cGVHD was significantly associated with a higher incidence of late PF PF abnormality (Figure 1c, ) and aGVHD was significantly associated with symptomatic PF abnormality. Neither CD34 dose nor type of transplant (MT vs. NST) (Figure 1d, ) (Table 1) influenced asymptomatic or symptomatic late PF abnormality. In multivariate analysis (Table 5), only cGVHD was found to be predictive for asymptomatic and symptomatic late decline in PF from baseline (RR-16, p=0.001, RR 18, p=0.002 respectively).
Serial measurements of PF in different risk categories (Table 6)
Table 6.
Pre-transplant PF and cGVHD- serial PF measurements.
| Pre-SCT* | 1 year* | 3 year* | 5 year* | 6 year* | 7 year* | ||
|---|---|---|---|---|---|---|---|
| Normal PF † & no cGVHD (n=24) | n= | 24 | 23 | 23 | 22 | 7 | 9 |
| DLCO | 94 ± 2.5 | 91 ± 3.6 | 95 ± 3.1 | 94 ± 2.1 | 91 ± 4.1 | 89 ± 3.2 | |
| FEV1 | 103 ± 2.0 | 103 ± 3.0 | 103 ± 3.0 | 103 ± 2.8 | 107 ± 7.0 | 109 ± 4.3 | |
| TLC | 94 ± 2.0 | 97 ± 3.0 | 96 ± 3.1 | 97 ± 2.4 | 99 ± 6.0 | 97 ± 4.3 | |
| Normal PF † & cGVHD(n=26) | n= | 26 | 21 | 21 | 25 | 10 | 5 |
| DLCO | 102 ± 3.4 | 91 ± 3.1 | 89 ± 3.7 | 81 ± 3.5 | 81 ± 4.2 | 80 ± 6.3 | |
| FEV1 | 99 ± 2.2 | 101 ± 3.3 | 97 ± 3.6 | 95 ± 4.1 | 94 ± 4.9 | 98 ± 4.4 | |
| TLC | 96 ± 2.3 | 99 ± 3.5 | 93 ± 3.5 | 95 ± 3.3 | 83 ± 4.2 | 82 ± 3.8 | |
| Abnormal PF† & cGVHD(n=17) | n= | 17 | 14 | 12 | 16 | 6 | 7 |
| DLCO | 84 ± 4.6 | 76 ± 3.8 | 75 ± 3.8 | 68 ± 4.8 | 60 ± 3.3 | 61 ± 3.7 | |
| FEV1 | 85 ± 3.4 | 83 ± 4.4 | 83 ± 4.6 | 79 ± 4.1 | 69 ± 7.7 | 67 ± 7.3 | |
| TLC | 85 ± 1.9 | 84 ± 3.5 | 83 ± 3.9 | 80 ± 4.3 | 71 ± 6.7 | 74 ± 6.7 |
Mean ± standard error of mean, percentage predicted value; ; n- number of patients;
pre-transplant PF; two patients with abnormal pre-transplant PF and no cGVHD not shown.
Table 6 shows the results of serial PF measurements for the variables identified as significant in multivariate analysis. Twenty-four patients with no abnormal PF before transplant, who did not develop cGVHD maintained the same level of function over the observation period, with no emergence of restrictive or obstructive defects. In contrast, 26 patients with no abnormality in PF pre-transplant who developed cGVHD showed a marked progressive decline in DLCO and TLC but no fall in FEV1, indicating that cGVHD caused predominantly restrictive pulmonary damage. It was notable that the PF abnormalities continued despite no clinical evidence of active cGVHD-only 2 of 32 patients with late PF decline were receiving immunosuppressive therapy 5 years after SCT. It was not possible to measure the exact time to PF decline after development of cGVHD because of the infrequent timing of PF measurements. Most profoundly affected was the group of 17 patients with abnormal PF pre-transplant who developed cGVHD. Only two patients with abnormal pre transplant lung function did not develop cGVHD (PF data not shown) suggesting that abnormal PF might predispose to cGVHD.
Discussion
This study emphasizes the importance of long-term follow up of SCT recipients to identify delayed defects in pulmonary function and to determine causative factors for pulmonary damage. It is disturbing to observe that delayed lung injury is a significant problem after SCT-in our cohort of 69 patients between 5-13 years post SCT, 17% were symptomatic due to pulmonary insufficiency, and 45% had PF deterioration. Furthermore, pulmonary damage appeared to be a slowly progressive process. Continued follow-up of SCT patients one and two decades from SCT will be required to fully characterize the natural history of this problem.
Only patients who had no abnormal PF pretransplant who did not develop cGVHD appeared to be spared from progressive deterioration in PF. Studies have shown that poor PF before transplantation is associated with higher transplant related mortality and morbidity [15,21]. Somewhat predictably, pulmonary defects acquired pre-transplant were an important factor for increasing the risk of post-transplantation pulmonary defects. It is important to note that the cohort of patients who entered the transplant with DLCO of ≤80% predicted had further deterioration in all PF parameters and 4/11 patients developed symptomatic PF abnormalities. Similarly patients with FEV1 of ≤80% predicted pre-transplant had deterioration in all PF parameters and 5/8 patients developed symptomatic PF abnormalities. Thus in selecting patients for SCT with diminished lung function, the possibility of further deterioration should be considered.
We next sought to identify transplant-related factors that might predict delayed PF defects. Radiation damage to the lung during TBI has been described in animal models [22,23] and there is an extensive literature linking restrictive PF defects with TBI [1,24-26]. Factors which affect radiation lung damage are the total dose, the dose fractionation employed and the acute dose rate per fraction. The TBI schedule used in our patients followed a standard approach where 12-13.6 cGy is administered in 8 fractions of 150-170 cGy over 4 days. More recently lung shielding was introduced to reduce the total lung dose to 6 cGy. In a previous study we identified pulmonary failure as the most common cause of non-relapse mortality after SCT but did not find any significant improvement in outcome in patients receiving reduced lung doses [8]. In this analysis we had the opportunity to compare outcomes of patients receiving TBI with a smaller cohort of patients receiving a reduced intensity regimen of fludarabine and cyclophosphamide. Surprisingly, there was no difference in the incidence of late PF abnormalities between the TBI recipients and the non-TBI group (46.4% vs 38.4% respectively, p=0.419). However the lack of difference between the two cohorts cannot reliably be ascribed to the absence of an adverse effect of TBI, since the non-irradiated reduced intensity conditioning recipients represent an older patient population with different pre-transplant treatments.
Obstructive and restrictive forms of PF abnormalities have been reported in long-term survivors of allo-SCT, with incidence ranges from 20-50% [7,9,12,26]. Collagen deposition and the development of fibrosis either in the interstitial (in restrictive pattern) or peribronchial space (in obstructive pattern) are believed to contribute to the characteristic PF abnormality. An obstructive pattern PF is classically linked to cGVHD [27,28], however we found more patients developing a predominantly restrictive pattern PF abnormality.
In multivariate analysis, cGVHD was the only transplant-related factor that emerged as a variable significantly affecting PF. cGVHD was in fact more common in the non-TBI recipients (76% vs 58%). The impact of cGVHD on development of PF defects was striking-with a 16-18 fold increase in relative risk of PF abnormalities for any degree of cGVHD.
Clinical and experimental data suggest that the progression to a chronic profibrotic pulmonary toxicity involves the secretion of cytokines and chemokines [23,29]. Several studies have shown association between GVHD and pulmonary injury [2,6,27,28,30]. In a murine model, TNF-α has been shown to be with involved chronic lung injury [31]. Though GVHD has been linked to the pulmonary complications after SCT, the pathogenesis and epidemiology of chronic lung injury from cGVHD remains ill defined. In our study it sufficed to have a history of any degree of severity of cGVHD to increase the probability of a delayed PF defect, suggesting either that cGVHD sets up a process of long-term damage, or that a subclinical form of isolated pulmonary cGVHD persists for many years. Measures to prevent late pulmonary damage without preventing the protective effect of cGVHD on leukemic relapse are warranted. The most severely affected subset of patients were those with both pre-transplant pulmonary damage and cGVHD. The association of pretransplant PF defects with cGVHD (17 of 19 patients) was striking and strongly suggests that pre-existing lung injury is a provocative factor for cGVHD. However, the prolonged intervals between PF measurements prevented analysis of the relationship between onset of cGVHD and decline in PF.
In conclusion, our results emphasize the importance of cGVHD as a key factor in progressive pulmonary damage after all forms of allogeneic SCT. However, it should be noted that other factors not recognized in this analysis could have contributed to the changes in PF observed. Furthermore, this data is retrospective and our findings need to be further validated in prospective and larger studies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Yanik G, Cooke KR. The lung as a target organ of graft-versus-host disease. Semin Hematol. 2006;43:42–52. doi: 10.1053/j.seminhematol.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 2.Crawford SW, Longton G, Storb R. Acute graft-versus-host disease and the risks for idiopathic pneumonia after marrow transplantation for severe aplastic anemia. Bone Marrow Transplant. 1993;12:225–231. [PubMed] [Google Scholar]
- 3.Crawford SW, Hackman RC. Clinical course of idiopathic pneumonia after bone marrow transplantation. Am Rev Respir Dis. 1993;147:1393–1400. doi: 10.1164/ajrccm/147.6_Pt_1.1393. [DOI] [PubMed] [Google Scholar]
- 4.Clark JG, Hansen JA, Hertz MI, et al. NHLBI workshop summary. Idiopathic pneumonia syndrome after bone marrow transplantation. Am Rev Respir Dis. 1993;147:1601–1606. doi: 10.1164/ajrccm/147.6_Pt_1.1601. [DOI] [PubMed] [Google Scholar]
- 5.Kantrow SP, Hackman RC, Boeckh M, Myerson D, Crawford SW. Idiopathic pneumonia syndrome: changing spectrum of lung injury after marrow transplantation. Transplantation. 1997;63:1079–1086. doi: 10.1097/00007890-199704270-00006. [DOI] [PubMed] [Google Scholar]
- 6.Afessa B, Litzow MR, Tefferi A. Bronchiolitis obliterans and other late onset non-infectious pulmonary complications in hematopoietic stem cell transplantation. Bone Marrow Transplant. 2001;28:425–434. doi: 10.1038/sj.bmt.1703142. [DOI] [PubMed] [Google Scholar]
- 7.Schultz KR, Green GJ, Wensley D, et al. Obstructive lung disease in children after allogeneic bone marrow transplantation. Blood. 1994;84:3212–3220. [PubMed] [Google Scholar]
- 8.Savani BN, Montero A, Wu C, et al. Prediction and prevention of transplant-related mortality from pulmonary causes after total body irradiation and allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2005;11:223–230. doi: 10.1016/j.bbmt.2004.12.328. [DOI] [PubMed] [Google Scholar]
- 9.Cerveri I, Fulgoni P, Giorgiani G, et al. Lung function abnormalities after bone marrow transplantation in children: has the trend recently changed? Chest. 2001;120:1900–1906. doi: 10.1378/chest.120.6.1900. [DOI] [PubMed] [Google Scholar]
- 10.Krowka MJ, Rosenow EC, III, Hoagland HC. Pulmonary complications of bone marrow transplantation. Chest. 1985;87:237–246. doi: 10.1378/chest.87.2.237. [DOI] [PubMed] [Google Scholar]
- 11.Chan CK, Hyland RH, Hutcheon MA. Pulmonary complications following bone marrow transplantation. Clin Chest Med. 1990;11:323–332. [PubMed] [Google Scholar]
- 12.Clark JG, Schwartz DA, Flournoy N, et al. Risk factors for airflow obstruction in recipients of bone marrow transplants. Ann Intern Med. 1987;107:648–656. doi: 10.7326/0003-4819-107-5-648. [DOI] [PubMed] [Google Scholar]
- 13.Dudek AZ, Mahaseth H. Hematopoietic stem cell transplant-related airflow obstruction. Curr Opin Oncol. 2006;18:115–119. doi: 10.1097/01.cco.0000208782.61452.08. [DOI] [PubMed] [Google Scholar]
- 14.Marras TK, Chan CK, Lipton JH, et al. Long-term pulmonary function abnormalities and survival after allogeneic marrow transplantation. Bone Marrow Transplant. 2004;33:509–517. doi: 10.1038/sj.bmt.1704377. [DOI] [PubMed] [Google Scholar]
- 15.Chien JW, Maris MB, Sandmaier BM, et al. Comparison of lung function after myeloablative and 2 Gy of total body irradiation-based regimens for hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2005;11:288–296. doi: 10.1016/j.bbmt.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 16.Bacigalupo A, Lamparelli T, Barisione G, et al. Thymoglobulin Prevents Chronic Graft-versus-Host Disease, Chronic Lung Dysfunction, and Late Transplant-Related Mortality: Long-Term Follow-Up of a Randomized Trial in Patients Undergoing Unrelated Donor Transplantation. Biol Blood Marrow Transplant. 2006;12:560–565. doi: 10.1016/j.bbmt.2005.12.034. [DOI] [PubMed] [Google Scholar]
- 17.Mavroudis DA, Read EJ, Molldrem J, et al. T cell-depleted granulocyte colony-stimulating factor (G-CSF) modified allogenic bone marrow transplantation for hematological malignancy improves graft CD34+ cell content but is associated with delayed pancytopenia. Bone Marrow Transplant. 1998;21:431–440. doi: 10.1038/sj.bmt.1701120. [DOI] [PubMed] [Google Scholar]
- 18.Solomon SR, Nakamura R, Read EJ, et al. Cyclosporine is required to prevent severe acute GVHD following T-cell-depleted peripheral blood stem cell transplantation. Bone Marrow Transplant. 2003;31:783–788. doi: 10.1038/sj.bmt.1703928. [DOI] [PubMed] [Google Scholar]
- 19.Nakamura R, Battiwalla M, Solomon S, et al. Persisting posttransplantation cytomegalovirus antigenemia correlates with poor lymphocyte proliferation to cytomegalovirus antigen and predicts for increased late relapse and treatment failure. Biol Blood Marrow Transplant. 2004;10:49–57. doi: 10.1016/j.bbmt.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 20.Srinivasan R, Chakrabarti S, Walsh T, et al. Improved survival in steroid-refractory acute graft versus host disease after non-myeloablative allogeneic transplantation using a daclizumab-based strategy with comprehensive infection prophylaxis. Br J Haematol. 2004;124:777–786. doi: 10.1111/j.1365-2141.2004.04856.x. [DOI] [PubMed] [Google Scholar]
- 21.Kotloff RM, Ahya VN, Crawford SW. Pulmonary complications of solid organ and hematopoietic stem cell transplantation. Am J Respir Crit Care Med. 2004;170:22–48. doi: 10.1164/rccm.200309-1322SO. [DOI] [PubMed] [Google Scholar]
- 22.Down JD, Mauch P, Warhol M, Neben S, Ferrara JL. The effect of donor T lymphocytes and total-body irradiation on hemopoietic engraftment and pulmonary toxicity following experimental allogeneic bone marrow transplantation. Transplantation. 1992;54:802–808. doi: 10.1097/00007890-199211000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Shankar G, Cohen DA. Idiopathic pneumonia syndrome after bone marrow transplantation: the role of pre-transplant radiation conditioning and local cytokine dysregulation in promoting lung inflammation and fibrosis. Int J Exp Pathol. 2001;82:101–113. doi: 10.1111/j.1365-2613.2001.iep0082-0101-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fanfulla F, Locatelli F, Zoia MC, et al. Pulmonary complications and respiratory function changes after bone marrow transplantation in children. Eur Respir J. 1997;10:2301–2306. doi: 10.1183/09031936.97.10102301. [DOI] [PubMed] [Google Scholar]
- 25.Gopal R, Ha CS, Tucker SL, et al. Comparison of two total body irradiation fractionation regimens with respect to acute and late pulmonary toxicity. Cancer. 2001;92:1949–1958. doi: 10.1002/1097-0142(20011001)92:7<1949::aid-cncr1714>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 26.Beinert T, Dull T, Wolf K, et al. Late pulmonary impairment following allogeneic bone marrow transplantation. Eur J Med Res. 1996;1:343–348. [PubMed] [Google Scholar]
- 27.Holland HK, Wingard JR, Beschorner WE, Saral R, Santos GW. Bronchiolitis obliterans in bone marrow transplantation and its relationship to chronic graft-v-host disease and low serum IgG. Blood. 1988;72:621–627. [PubMed] [Google Scholar]
- 28.Ringden O, Remberger M, Ruutu T, et al. Increased risk of chronic graft-versus-host disease, obstructive bronchiolitis, and alopecia with busulfan versus total body irradiation: long-term results of a randomized trial in allogeneic marrow recipients with leukemia. Nordic Bone Marrow Transplantation Group. Blood. 1999;93:2196–2201. [PubMed] [Google Scholar]
- 29.Sime PJ, Marr RA, Gauldie D, et al. Transfer of tumor necrosis factor-alpha to rat lung induces severe pulmonary inflammation and patchy interstitial fibrogenesis with induction of transforming growth factor-beta1 and myofibroblasts. Am J Pathol. 1998;153:825–832. doi: 10.1016/s0002-9440(10)65624-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duncker C, Dohr D, Harsdorf S, et al. Non-infectious lung complications are closely associated with chronic graft-versus-host disease: a single center study of incidence, risk factors and outcome. Bone Marrow Transplant. 2000;25:1263–1268. doi: 10.1038/sj.bmt.1702429. [DOI] [PubMed] [Google Scholar]
- 31.Miyazaki Y, Araki K, Vesin C, et al. Expression of a tumor necrosis factor-alpha transgene in murine lung causes lymphocytic and fibrosing alveolitis. A mouse model of progressive pulmonary fibrosis. J Clin Invest. 1995;96:250–259. doi: 10.1172/JCI118029. [DOI] [PMC free article] [PubMed] [Google Scholar]