Abstract
Lymphocyte production in bone marrow (BM) requires substantial cell division, but the relationship between largely quiescent stem cells and dividing lymphoid progenitors is poorly understood. Therefore, the proliferation and cell cycle status of murine hematopoietic progenitors that have just initiated the lymphoid differentiation program represented the focus of this study. Continuous bromo-2’-deoxyuridine incorporation (BrdU) and DNA/RNA analysis by flow cytometry revealed that a surprisingly large fraction of RAG-1+c-kitHi early lymphoid progenitors (ELP) and RAG-1+c-kitLo pro-lymphocytes (Pro-L) in adult BM were in cell cycle quiescence. In contrast, their counterparts in 14 day fetal liver actively proliferated. Indeed, the growth fraction (cells in G1-S-G2-M phases) of fetal ELP was on average 80% versus only 30% for adult ELP. Following 5-fluorouracil treatment, as many as 60% of the adult ELP-enriched population was in G1-S-G2-M and 34% incorporated BrdU in 6 hours. Transcripts for Bcl-2, p21Cip1/Waf1 and p27 Kip1 cell cycle regulatory genes correlated inversely well with proliferative activity. Interestingly, adult lymphoid progenitors in rebound had the high potential for B lymphopoiesis in culture typical of their fetal counterparts. Thus, lymphocyte production is sustained during adult life by quiescent primitive progenitors that divide intermittently. Some, but not all aspects of the fetal differentiation program are reacquired following chemotherapy.
Introduction
Development and replenishment of the immune system require differentiation from rare hematopoietic stem cells (HSC). This dynamic process can be viewed as a gradual progression from very primitive hematopoietic progenitors with multiple lineage potentials through more restricted progenitors [1], and it is tightly linked to proliferation.
There has been substantial progress in understanding how pre-B cells progressively expand, complete immunoglobulin gene rearrangement and give rise to functional B cells [2]. In contrast, methods have only recently been developed to study cells that have just initiated the lymphoid differentiation program [3] and little information is available about their proliferation. A strain of heterozygous RAG-1/GFP knock-in mice are used to isolate the earliest known lymphoid progenitors from adult bone marrow (BM) and fetal liver[4, 5]. This is possible because GFP fluorescence corresponds to the presence of RAG-1 transcripts in cells that are primitive in terms of transcription factors, surface markers and time required to differentiate into lymphoid cells. These Lin−c-KitHiSca-1+CD27+Flk-2+RAG1+ early lymphoid progenitors (ELP) in adult BM have tremendous potential for generating all lymphoid cell lineages and likely give rise to Lin− c-KitLoSca-1+/−CD27+Flk-2+RAG1+ pro-lymphocytes (Pro-L). Similarly, the RAG1-expressing cells in embryos can be resolved into a series of differentiation stages beginning with c-KitHiSca-1+GFPlo and culminating in c-Kitlo/−GFPhi subsets [5]. The cell cycle status of primitive lymphoid progenitors represented the main focus of this study.
HSC in a state of prolonged cell cycle quiescence has been proposed to support hematopoiesis through clonal succession. That is, one or a small number of HSC clones give rise to mature blood cells as needed, and the remaining HSCs are inactive and do not contribute to hematopoiesis until the proliferative capacity of the cycling HSC clone is exhausted [6, 7, 8]. To address this issue, the proliferation of HSC in adult mice was analyzed in vivo by means of bromo-2’-deoxyuridine (BrdU) incorporation kinetics [9, 10]. By the end of 6 months of continuous BrdU administration, 99% of HSCs had incorporated BrdU during DNA synthesis and, although 75% of HSCs are quiescent in phase G0 at any one time, all HSCs are recruited intermittently into the cell cycle so that 99% of them divide on average every 57 days. Whether cycling HSCs contribute directly to cells entering the lymphopoietic program has not been directly addressed.
The growth, differentiation, and survival of HSCs are regulated by a number of cytokines and chemokines and by the relative basal expression level of cyclins, cyclin-dependent kinases (cdks) and cdk-inhibitors (cdkis) [8]. While stem cell factor, Flt3-ligand, thrombopoietin, interleukin-3, and interleukin-6 promote the growth of human HSC in vitro [11], transforming growth factor-beta (TGF-β1) and monocyte chemoattractant protein-1 (MCP-1) induce cell cycle arrest of HSC12 and primitive hematopoietic progenitor-enriched fractions [13, 14], respectively. Similar information is emerging about extracellular cues that regulate the earliest stages of lymphopoiesis [15].
HSC cyclins are negatively regulated by cdkis [10]. Among the cdkis, p21 is highly expressed in the quiescent HSC-like fraction of BM cells. Moreover, HSCs in the G0 phase are reduced and the total number of HSC increased in p21−/− mice [16], and survival in p21−/− mice treated with the myelotoxic agent 5-fluorouracil (5-FU) is much lower than in littermate controls. These results indicate that p21 is a key molecule that restricts HSC entry into cell cycle, thereby imposing limits on their pool size and preventing their exhaustion. On the other hand, cdki p27 seems to govern the expansion of progenitor cell populations [17, 18, 19].
Many transcription factors such as c-Myb, GATA-2, Gfi-1, Bmi-1 and those of the homeobox (Hox) family have been shown to be additional key players in the proliferation and differentiation of early BM progenitor cells. A recent study using Hoxb4-deficient mice demonstrated reduced proliferative capacity of BM and FL HSC without affecting differentiation or lineage choice [20]. HOXB4, like Notch, has been reported to induce or enhance the expression of c-myc, cyclin D2, cyclin D3, cyclin E, and E2F1 in murine HSCs [21], and modulation of their signaling in hematopoietic precursors could represent an interesting approach to improve cell-based therapy [22]. Key to the long-term success of these strategies will be a molecular understanding of the population dynamics in vivo of HSC and their more committed downstream progenitors [23].
B lymphopoiesis has been depicted as a unidirectional process in which developing cells transit through successive differentiation stages in an irreversible, synchronous manner. Recently, some studies have examined this view by quantification of specific BM precursor B cell populations [24] or by combining kinetic analysis of developing B cell subsets in the BM with mathematical modeling [2, 25]. Asynchronous differentiation models explain BM labeling kinetics and predict reflux between the pre- and immature B cell pools. Additionally, studies in normal, gene deleted, transgenic and mutant mice have shown that the apoptotic index and apoptotic rate are maximal during the pro/preB-cell transition and among immature B lymphocytes in BM [26].
We have now studied the proliferation status of mouse lymphoid progenitors that are recently derived from HSC. The results indicate that ELP in fetal liver proliferate much faster than their counterparts in adult bone marrow. RT-PCR analysis of key cell cycle mediators identified several that could account for the fetal/adult disparity. A BrdU-labeling pattern for ELP and Pro-L in adult BM suggests cells in both compartments are cycling asynchronously and intermittently. Furthermore, adult progenitors acquire some, but not all characteristics of fetal cells during rebound from chemotherapy. Although primitive lymphoid progenitors sustain replenishment of the immune system throughout adult life, they exist in two kinetic states and only a minority is in cell cycle at any one time.
Material and Methods
Mice and cell suspensions
RAG-1/GFP knock-in mice have been described [4, 27] Wild type (WT) C57BL/6 strain mice were purchased from The Jackson Labs (Bar Harbor, ME). Heterozygous F1 RAG-1/GFP mice were generated at the OMRF Laboratory Animal Research Facility by mating homozygous male RAG-1/GFP knock-in mice with WT female mice. Fetal liver cells were obtained at embryonic days 13 to 16 (E13-E16). Adult BM cells were purified from 3-5 month old heterozygous RAG-1/GFP knock-in mice.
BrdU treatment of mice and cell cycle analyses
Groups of 35-55 mice for adult BM studies were given an initial intraperitoneal injection of BrdU (100μg/100μl PBS), while groups of 3 pregnant mice for fetal studies were given BrdU intravenously at zero time. This was to establish a satisfactory concentration of label and a valid starting time. In each case BrdU was then administered continuously in drinking water (0.8 mg/ml) for the duration of the experiment. At defined time points, adult or fetal lymphoid progenitors were purified from treated mice by sorting pooled BM or fetal liver samples, respectively, followed by intracellular staining with mAb to BrdU (BrdU flow kit, BD Biosciences). PE rather than FITC labeled anti-BrdU monoclonal antibody was used to visualize BrdU in RAG-1/GFP+ progenitors. The cells were analyzed on a FACScan (BD, San Jose, CA), using Cell Quest and WinMDI 2.8 software programs. Representative analyses are shown in Supplemental Figure 1.
Flow cytometry and cell sorting
Adult BM was flushed from femurs, tibias and humeri with 3% FCS PBS. Cells were enriched by incubation with antibodies to lineage markers Gr1 and CD11b/Mac1 for myeloid cells, CD19 and CD45R/B220 for B lineage cells, and TER-119 for erythroid cells, followed by negative selection using the Bio-Mag cell separation system (Qiagen, Valencia, CA). These partially lineage-depleted cells were further blocked with anti-FcR and stained with APC-anti-ckit antibody and with biotin-anti-lineage markers (Gr-1, Mac-1, CD3, CD8a, CD19, CD45R, DX-5 and TER-119) combined with streptavidin (Sav)-R613. CD11b/Mac1 was not included in the lineage depletion protocol after 5-FU treatment. Sorting on MoFlo (DakoCytomation, Ft. Collins, CO) was performed on the basis of Lin−GFP+c-kithi (ELP) and Lin−GFP+c-kitlo (Pro-L). Sca-1 was used as an additional gating parameter for lymphoid progenitors in our previous studies, but is expressed at artificially high levels on cells in rebound bone marrow. Fetal livers (E13-E16) were minced, and the suspensions were subjected to depletion of TER-119+ cells prior to two-steps cell sorting. In the first step, cells were sorted into GFP−, GFPlo and GFPhi. Background autofluorescence was discriminated from authentic GFP by collecting data in two fluorescence channels without compensation [5]. An illustration of this method is provided as Supplemental Figure 2. Sorted cells were incubated with anti-FcR before staining with APC-anti-c-kit and bio-Sav-R613-anti-Sca-1 or PE-anti-Sca-1 antibodies, and subjected to a second round of sorting. Hematopoietic progenitors were fractionated according to Sca-1 and c-kit expression, as previously described [5].
Treatment with 5-FU
Adult mice were given a single intraperitoneal injection with 5-FU (150 mg/kg body weight) in PBS. BM was recovered at the times indicated.
Cell cycle fractionation with Hoechst and Pyronin Y
A combination of Hoechst 33342 (Hst) and pyronin Y (PY) was used for the differential staining of cellular RNA and DNA, as described elsewhere [28]. Briefly, lymphoid progenitors were fixed in 70% ethanol overnight, then resuspended in a solution of 2μg/ml Hst (Molecular Probes, Eugene, OR) and 4μg/ml PY (Polysciences, Warrington, PA) and measured by flow cytometry on a MoFlo equipped with UV-laser (DakoCytomation, Ft. Collins, CO). Since RNA staining with PY yields a continuous histogram without demarcation between positive and negative cells [29], an arbitrary analysis window comprising about 15% of fetal liver cells displaying minimal PY staining was used to designate G0 fraction in all experiments.
Real-Time PCR analysis of cell cycle gene expression
Lymphoid progenitors were sorted at high purity. Sequences of cell cycle candidate genes were obtained from the UCSC genome browser (http://genome.ucsc.edu/cgi-bin/hgGateway), and specific forward and reverse oligonucleotide primers were designed using Primer 3 program (http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi) (Supplemental Table 1). The Real-Time PCR amplification mixture contained template cDNA, 2x SYBR Green Master Mix (Applied Biosystems, UK) and 2μM-each primer mix. Reactions were 10 min at 95°C followed of 40 cycles of 95°C for 15 sec and 60 sec at 60°C in an ABI Prism 7700 Sequence Detection System (Applied Biosystems, UK). The relative gene expression was calculated using β-actin cDNA as an endogenous control [Relative gene expression = 2(Ct ctrl − Ct gene) x 103].
Telomerase activity detection
Telomerase activity was measured quantitatively in 1x103 lymphoid progenitors by one step Real-Time reaction (Express Biotech International, Thurmont, MD). Briefly, the active telomerase from lysed cells added a varied number of telomeric repeats onto the 3’ end of a substrate oligonucleotide. The extension products were amplified by PCR and then detected by measuring the increase in fluorescence by binding of SYBR green to dsDNA. A standard curve was performed to calculate activity using an oligonucleotide with a sequence identical to telomere primers.
Stromal cell co-cultures
Sorted lymphoid progenitors were co-cultured for up to 3 weeks with Delta-like-1 and GFP retrovirally-transduced OP9 stromal cells (OP9-DL1 and OP9-GFP, kindly provided by Dr. J.C. Zúñiga-Pflücker), as previously described [30] with modifications. During the first week, the α-MEM 10% FCS contained 5x10−5M 2-mercaptoethanol, 2mM L-glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin, plus 2 ng/ml SCF, 5 ng/ml Flt3-L and 2 ng/ml IL-7. During the second and third weeks of co-culture, the IL-7 concentration was increased to 5 ng/ml.
Results
Early lymphoid progenitors and Pro-lymphocytes in adult bone marrow are each heterogeneous with respect to cell cycle kinetics, and a significant fraction are quiescent
BM cells that lack all markers of differentiated blood cells were resolved into primitive and more mature progenitors according to levels of c-kit and RAG-1 in RAG-1/GFP knock-in mice. Stem cells, multipotent progenitors and ELP are in the c-kitHi fraction of lineage marker negative (Lin−) BM, with one of the distinguishing characteristics of ELP being activation of the RAG-1 locus [3, 4]. The c-kitLo fraction of Pro-L (including CLP) generate B and NK lineage cells more quickly in short term cultures.[3 ,4, 31, 32].
Continuous administration of BrdU yielded biphasic labeling curves for both ELP and Pro-L populations (Figure 1A), which were simultaneously sorted for analyses (Supplemental Figures 1 and 2). An initial steep rise in the labeling index, which represented the DNA-labeling of a fraction of rapidly cycling cells, was soon followed by a second slower rise, indicating the gradual entry of labeled cells into a considerably larger cell fraction [33]. Extrapolating from the 36 h interval to the time necessary to reach complete (100%) labeling yielded an apparent average cell cycle time of 11.9 days for the ELP population as a whole and 11.4 days for Pro-L, both long in comparison with that of the Lin− c-kitHi GFP− fraction of BM (5 days). Consistent with these findings, an analysis of DNA/RNA cell content revealed that a majority of ELP and Pro-L are in a G0 state at any given time (Figure 1B). Taken together, these results indicate that ELP and Pro-L in adult BM consist of a mixture of cells representing two kinetic states, a minor set of cycling cells and a major set of quiescent cells which represented approximately two thirds of the total populations of ELP and Pro-L. The slow linear BrdU labeling of the second subset of cells indicates that these ELP and Pro-L do not remain permanently in a dormant G0 state but are slowly turning over. Periodically, G0 cells are triggered to enter cell cycle, after which some or all of their labeled progeny may revert to the G0 state.
Figure 1. Most lymphoid progenitors in adult bone marrow are in a state of cell cycle quiescence.
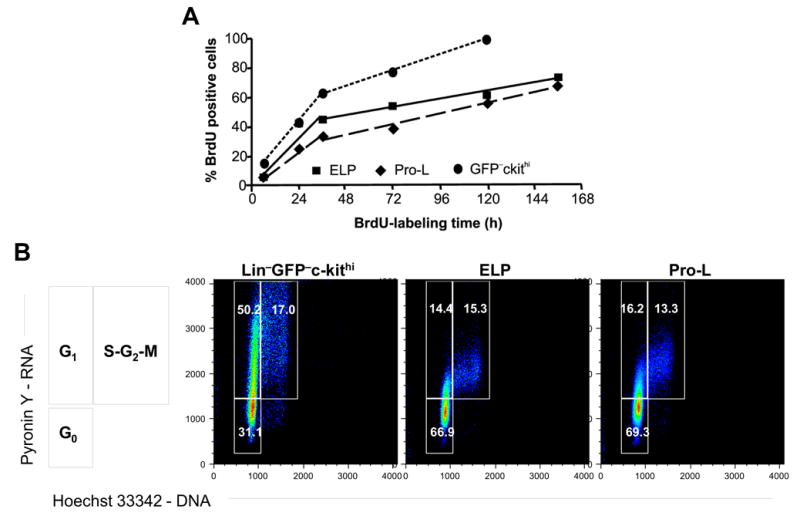
(A) Adult bone marrow ELP, Pro-L and a cohort Lin−GFP−ckithi population were purified from BM pooled from at least 35 BrdU treated mice, followed by intracellular staining for BrdU. Each point on the curves represents a single measurement for one pool. The numbers of mice used per time point were 35 for 6 h, 55 for 24 h, 52 for 36 h, 55 for 72 h, 50 for 120 h, and 50 for 156 h. Linear regression by least squares analysis was used to fit two sets of lines for each cell type. (B) ELP, Pro-L and Lin−GFP−ckithi were stained for Hoechst 33342 and Pyronin Y. The diagram at the side illustrates how actively dividing cells can be resolved on the basis of RNA/DNA staining into G1 or S+G2+M fractions. Cell frequencies in G0 or each cell cycle phase are shown. The data is derived from and representative of two different experiments that were conducted independently from that shown in Figure 1A.
The designation early lymphoid progenitor does not imply homogeneity [1, 4, 3, 34]. Rather, the most primitive lymphoid cells express TdT, RAG-1, a human μ transgene (in transgenic mice) or some combination of these characteristics. Also, levels of GFP vary among cells from RAG-1/GFP mice gated for any given set of cell surface markers. We now report that ELP with the highest levels of RAG-1 incorporate BrdU at a substantially lower rate than ELP with less RAG-1. This analysis was performed after 24, 36, 120 and 156 hours of continual treatment with BrdU (Figure 2 and data not shown). The same parameter revealed similar heterogeneity among Pro-L, the cells showing the highest rate of BrdU uptake having relatively lower levels of RAG-1. Therefore, the kinetic data shown in Figure 1 represent average values for cell subsets that are not homogenous with respect to mitotic and RAG-1 gene activity. Variations in RAG-1 gene transcriptional activity could be inversely linked to proliferation such that Ig gene recombination can safely occur.
Figure 2. RAG-1Lo and RAG-1Hi subsets of lymphoid progenitors are not homogeneous with respect to proliferative activity.
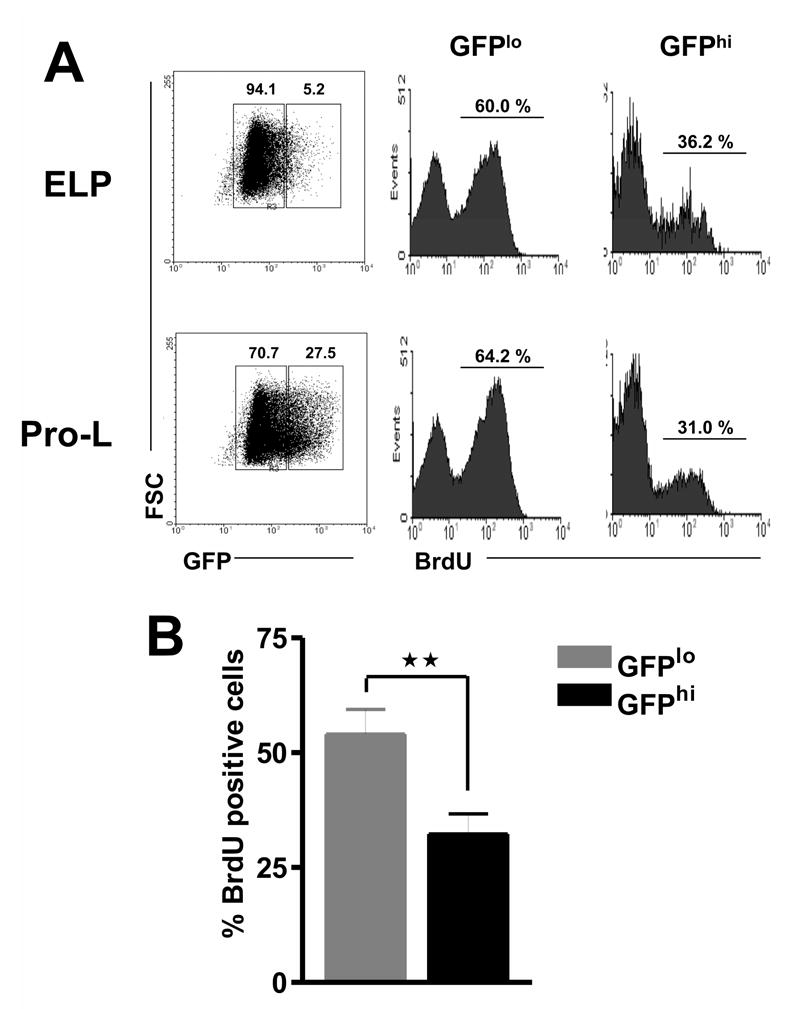
(A). GFP+ ELP and GFP+ Pro-L were sorted from adult bone marrow of mice after 5 days of BrdU treatment. The two subsets were then resolved according to GFP density (left panels) and the incorporation of BrdU was analyzed for each population by means of intracellular staining (right panels). (B). Average percentages for BrdU labeling of RAG-1/GFPlo and RAG-1/GFPhi cells across all intervals are shown. **p = 0.0076
The lymphopoietic series in fetal liver is rapidly dividing
Many studies have revealed fetal/adult differences in lymphopoiesis, raising important questions about the properties of stem cells present at different developmental ages [35]. An analysis of cell cycle status indicated that about 20% of E14 fetal liver c-kitHi Sca-1+ RAG-1/GFPLo ELP were in the S+G2+M phases of the cell cycle and an additional 58% were in G1 (Figure 3A). Similarly, high values for the proportion of cycling cells were observed with all other lymphoid populations in the E14 fetal liver, including cells in the c-KitLo Sca-1− RAG-1/GFPHi category, which are largely CD19+. These observations, consistent with relatively short cell cycle times, demonstrate a high growth fraction of proliferating early lymphoid cells in the fetal liver.
Figure 3. The entire lymphopoietic series in fetal liver is rapidly dividing.
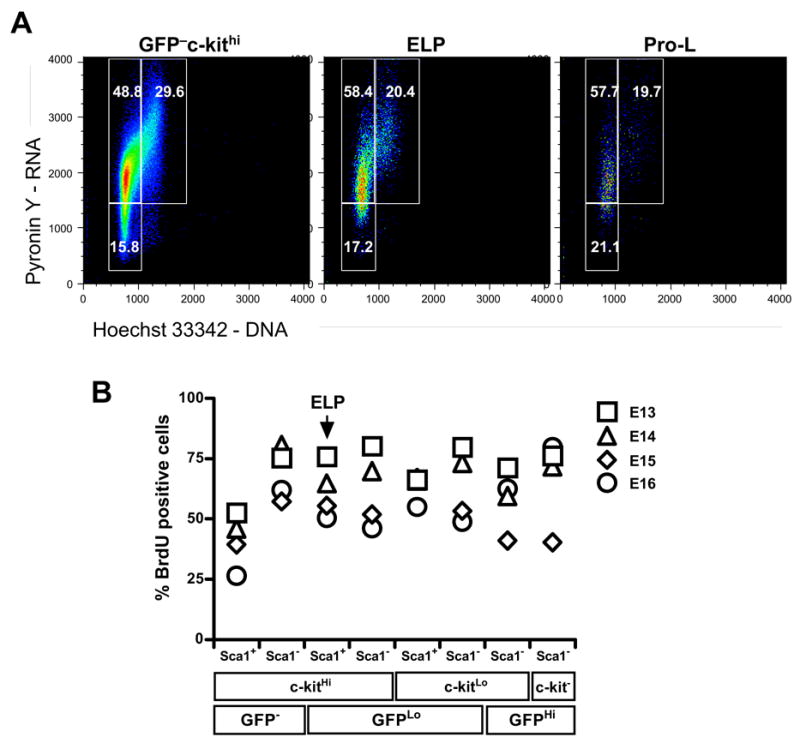
(A) Cell cycle status was determined by flow cytometry of Hoechst 33342 and Pyronin Y stained ELP GFPlockithi, Pro-L GFPlockitlo and GFP−ckithi sorted from E14 fetal liver. (B) Pregnant mice E13-E16 were given BrdU intravenously, and in drinking water for 6 hours. The lympho-hematopoietic progenitors were sorted and stained with mAb to BrdU. The subsets are arranged according to probable degree of maturity, beginning with the stem cell rich fraction on the left and CD19+ expressing cells at the far right. These are representative of results obtained in two independent experiments.
We then injected pregnant mice with BrdU and performed an incorporation analysis of fetal liver subsets six hours later (Figure 3B). Unlike the case with adult animals, very high labeling indices could be observed during this short interval. Indeed, at 13 days gestation, more than 70% of all lymphoid cells incorporated BrdU in six hours. Thereafter, BrdU labeling indices for most subsets tended to decline with gestational age, but were extremely high at all time points relative to adult BM progenitors. In fact, BrdU incorporation rates for adult ELP were less than fetal ELP even after six days of BrdU injection.
These data suggest that apparent proliferation rates of fetal lymphoid progenitors are substantially higher than those of adult progenitors. Indeed, a 30 fold longer interval was needed to achieve the equivalent degree of labeling with adult cells. Furthermore, two-parameter flow cytometry revealed that the growth fraction of fetal progenitors is correspondingly high with an average of only 20% in the G0 phase of the cell cycle at any one time (Figure 3A). We conclude that few of these cells are in a state of cell cycle quiescence prior to birth.
Adult lymphoid progenitors enter rapid cycle during rebound from 5-FU treatment
Fetal, but not adult, stem cells express Flk-2, Mac-1 and CD34 [5, 36, 37]. It is interesting that stem cells regenerating in the BM of adult mice following chemotherapy are CD11b/Mac-1+ and CD34+ [38,39]. There are also fetal/adult differences with respect to the markers displayed on lymphoid progenitors, and we wondered if proliferative characteristics of fetal cells could be also induced on those within BM. RAG-1/GFP mice were treated with a single injection of 5-FU and then examined for up to 8 days, resulting in a transient depletion in lymphoid progenitors. RAG-1/GFP+ cells could be identified among the Lin− fraction of 5-FU treated BM, and numbers of these cells recovered dramatically from day 4 after 5-FU treatment, when rebound from chemotherapy is said to begin (Figure 4A) [38].
Figure 4. Transient depletion of lymphoid progenitors along with loss of c-kit expression following 5-FU chemotherapy.
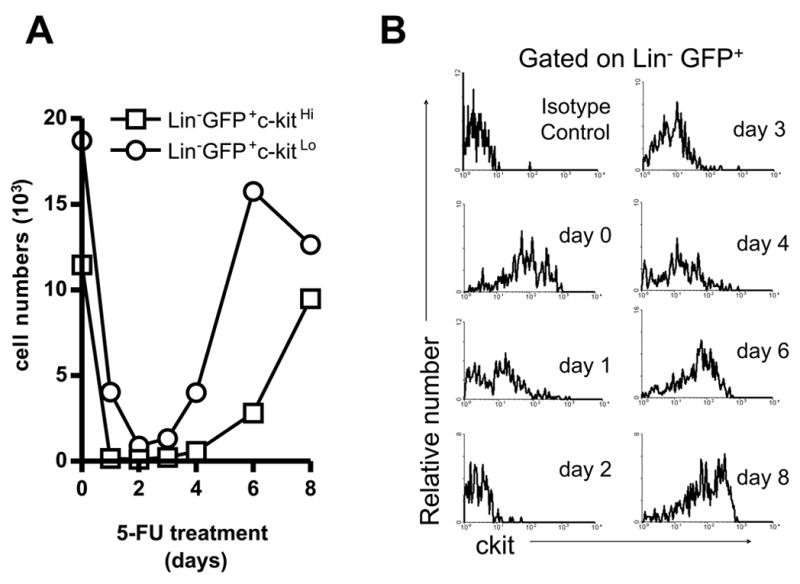
Mice were treated with a single dose of 5-FU and ELP and Pro-L were sorted after the indicated intervals. Absolute numbers of each cell type recovered from four bones are given in (A). Temporal changes in the expression of c-kit on Lin−GFP+ cells after treatment is shown in (B). The top left panel shows the results of staining with an isotype control antibody.
As previously reported, BM hematopoietic cells at early times post 5-FU treatment expressed only low levels of the c-kit receptor for stem cell factor (Figure 4B) [38]. Therefore, in subsequent experiments conducted at 4 days post treatment we did not use c-Kit density to discriminate ELP from Pro-L. A minority expressed detectable amounts of IL-7Rα (data not shown) and we assume that most progenitors at that time would be recently generated, and thus equivalent to ELP. Very small numbers of Lin− GFP+ c-kit+ cells displayed low levels of the CD11b myeloid marker after 5-FU treatment, but this was not comparable to ELP or Pro-L present in fetal liver (Supplemental Figure 3). Furthermore, the CD34 marker characteristic of fetal stem/progenitor cells was not re-expressed on adult lymphoid progenitors in rebound (Supplemental Figure 3).
To assess proliferation, the animals were injected with and then fed BrdU for 6h before analysis. Approximately one third of recovering adult progenitors incorporated the label (Figure 5A), and the percentage of progenitors in the inactive G0 fraction was also greatly reduced (Figure 5B). It is noteworthy that BrdU incorporation indices were lower than the cell cycle activity determined by Pyronin Y/Hoechst staining. The latter method may be more sensitive, and especially when only six hours are allowed for tissue equilibration and uptake of BrdU. Telomerase is important for maintaining stem cell chromosome integrity, and we evaluated this enzyme in ELP. Telomerase activity in fetal cells was four fold higher than in adult cells and markedly up-regulated in progenitors recovered during marrow rebound (Figure 5C).
Figure 5. Lymphoid progenitors rebounding from chemotherapy are, like fetal progenitors, in active cell cycle.
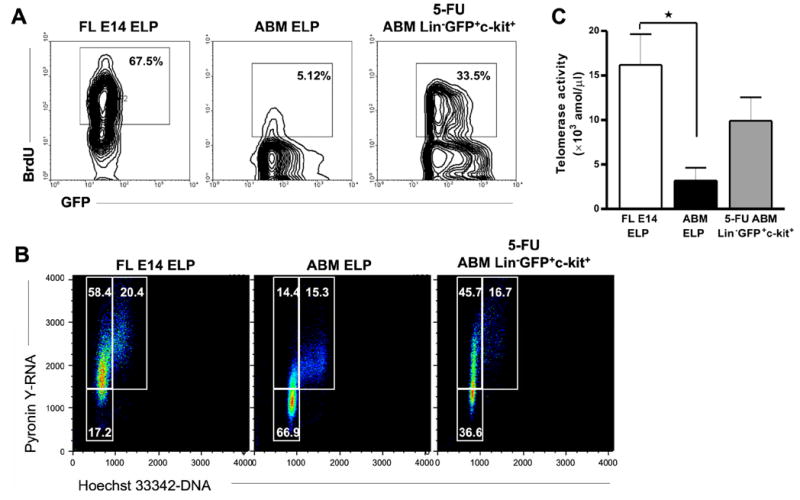
After 90 hours of 5-FU treatment, mice were given BrdU i.p. and in drinking water for 6 hours. ABM was recovered at 96 h and the purified 5-FU Lin−GFP+c-kit+ lymphoid progenitors and control ELP were stained with anti-BrdU to examine proliferation rates (A), or stained for Hoechst 33342 and Pyronin Y (B). In parallel, BrdU was given to pregnant mice for six hours and FL E14 ELP were analyzed. Telomerase activity was measured quantitatively in FL E14 ELP, ABM ELP and ABM 5-FU Lin−GFP+c-kit+ by Real-Time PCR (C). The graph shows median values (attomol/μl) with bars representing standard errors. *p=0.025.
Mitotic activity was also assessed by measuring transcripts for key cell cycle mediators (Figure 6). Transcripts for Cyclin A2, Cyclin D1, and CDK4 were much lower in adult than fetal progenitors and the pattern was not reversed following chemotherapy. CDK2 and Gfi-1 were low in fetal cells and again unchanged by 5-FU treatment. In contrast, levels of Cyclin D2, c-myc, Bcl-2, Ink4d/p19, Cip1Waf1/p21, Kip1/p27, HoxB4 and TGFβ2R levels in lymphoid progenitors from rebound adult BM closely resembled those from fetal liver.
Figure 6. Changes in cell cycle regulated gene expression during rebound from chemotherapy correspond to the fetal pattern in early lymphoid progenitors.
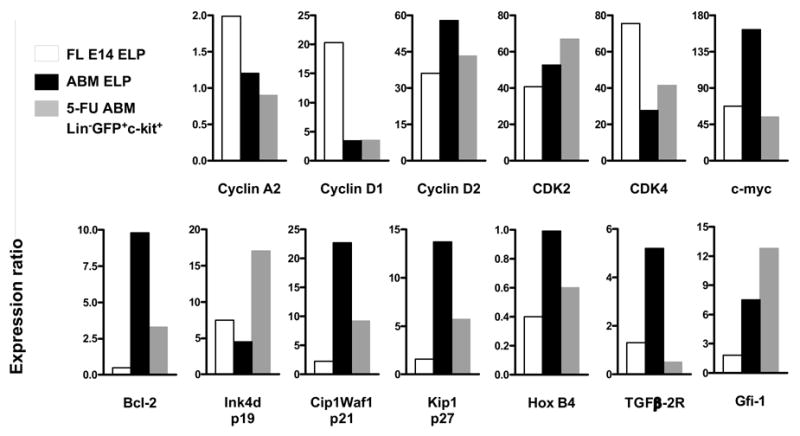
Lymphoid progenitors from FL E14, ABM and 5-FU-treated ABM were sorted, and transcripts for cell cycle regulators were detected by cDNA-based real time amplification. The data are expressed relative to levels of β-actin cDNA.
Thus, some, but not all aspects of the fetal differentiation program could be reacquired by adult lymphoid progenitors during rebound from chemotherapy. Levels of eight of 13 cell cycle regulators paralleled reentry into rapid division, but there was no corresponding re-expression of the fetal cell surface markers CD11b and CD34.
Rebounding adult lymphoid progenitors are not harmed by chemotherapy and have some functional properties typical of their fetal counterparts
Stromal cell co-cultures were used to compare the differentiation potential of fetal, adult and rebound lymphoid progenitors (Figure 7). All three populations produced B220/CD45R+ CD19+ GFP+ CD11b− B lineage lymphocytes in three week cultures on OP-9 stromal cells (Figure 7A), while T lineage cells were made when the same cells received a Notch signal by culture on OP9-DL1 stromal cells (Figure 7B). In the latter circumstance, recovering lymphoid progenitors had a tendency to make TCRγ/δ+ rather than TCRβ+ cells. Yields of lymphocytes were extremely dependent on the source of the progenitors (Figure 7C). Approximately two logs more B lineage cells were produced from fetal or rebound progenitors on OP-9 as compared to those freshly isolated from normal adult marrow. The situation was quite different with respect to T lineage cell differentiation where only fetal progenitors yielded high numbers of T cells within three weeks of culture on OP9-DL1.
Figure 7. Lymphoid progenitors in rebound from chemotherapy resemble their fetal counterparts with respect to B, but not T differentiation potential.
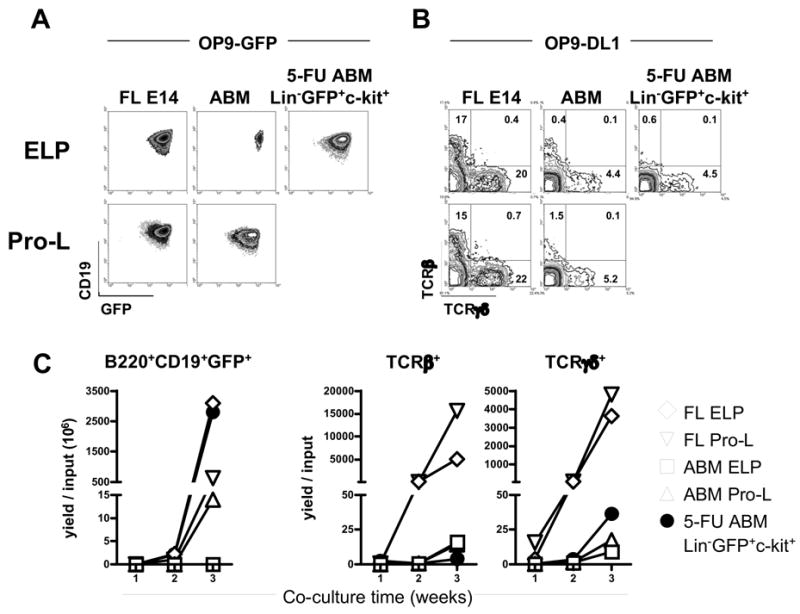
Lymphoid progenitors from FL E14, non-treated adult mice or 4 days 5-FU-treated mice were cultured for three weeks on OP9-GFP or OP9-DL1 stromal cells. (A) B lineage GFP+ CD19+ B220+ cells were stained after harvesting from OP9-GFP co-cultures. Plots show the populations gated on CD45R/B220. (B) T lineage TCRβ+ and TCRγδ+ cells were assessed in OP9-DL1 co-cultures. (C) The yields per input progenitor for each lineage are depicted in the time-course graphs.
Thus, chemotherapy by 5-FU did not alter the ability of adult lymphoid progenitors in rebound to generate T lineage cells. Remarkably, B lymphopoiesis from rebound adult BM progenitors was as robust as that typical of fetal cells.
Discussion
Environmental cues in a specialized bone marrow niche may determine what fraction of stem cells gives rise to proliferating lymphoid progenitors, and a subset of those replenishes the immune system. The population dynamics of T lymphocyte lineage cells in the thymus have been extensively studied, and many aspects of B lymphocyte formation have been similarly investigated [2, 40-44]. However, no comparable information was available about the most primitive lymphoid progenitors, and our understanding of fetal/adult differences in lymphopoiesis was incomplete. Here we have exploited Rag-1/GFP knock-in mice4 and BrdU incorporation by DNA-synthesizing cells to explore those important issues. Although more mitotically active than stem cells, the most primitive cells in the lymphoid series divide only once every 12 days. Changes that occur in ELP during recovery from 5-FU treatment may reflect processes that are important during transplantation and chemotherapy.
ELP begin the lymphoid differentiation program and express several lymphocyte related genes at low levels but have greatly reduced myeloid potential relative to stem cells. They are part of the Lin− Sca-1+ c-KitHi CD27+ Flk-2+ fraction of BM that has been variously referred to as multipotent progenitors (MPP) or lymphocyte-primed multipotent progenitors (LMPP) [15, 37]. While the RAG-1/GFP knock-in model used here allows isolation of viable, functional ELP, cells prepared this way may be very closely related to GFP− ones expressing other lymphoid genes such as TdT [4, 45]. In any case, ELP are potent progenitors for T, B, NK and plasmacytoid dendritic cell lineages [4, 32, 46]. They likely give rise to Lin− Sca-1+ c-KitLo pro-lymphocytes that include most cells designated common lymphoid progenitors (CLP) on the basis of IL-7Rα expression [31].
Neither of these two lymphocyte progenitor populations is homogeneous, and at any given time they existed in two kinetic states. Additionally, both ELP and Pro-L had a range of GFP levels, and this inversely corresponded to degrees of BrdU incorporation. That is, the GFP brightest progenitors labeled more slowly than the dim ones, making it tempting to propose an inverse relationship between activation of the RAG-1 locus and proliferation. There is a coordination of RAG-2 protein with the cell cycle, in parallel with fluctuations in the activity of cyclinA/CDK2 [47, 48]. RAG-2 preferentially accumulates in G0/G1, declines before cells enter S phase, and remains low throughout the S, G2 and M phases. Although levels of RAG-1 show less fluctuation, it has been suggested that its E3 ligase activity may target cell cycle regulatory proteins [49]. Our experience with the RAG-1/GFP knock-in mice indicates that endogenous RAG-1 is initially expressed in strict concordance with GFP levels [5], but decay of the GFP protein is thought to be slower than the RAG-1 protein [4, 50]. Regardless, initiation and progression of progenitor cells through the earliest stages of lymphopoiesis are unlikely to be synchronous.
Cells with low RNA content maintain a state of dormancy in G0. As the cells enter G1, they accumulate RNA mainly in the form of ribosomal RNA until reaching S phase [28, 29]. Many LTHC-IC (long-term hematopoietic cell-initiating culture) in humans and stem cells in mice are present in the G0 fraction of adult BM [16, 29, 39]. Cycling and other HSC behavior is thought to be regulated by coordination of environmental signals and intrinsic programs. The environmental cues may be provided by a niche composed of specialized cell populations located in unique sites. Arai and colleagues recently demonstrated that Lin− Sca-1+ c-KitHi Tie-2+ BM in G0 efflux dye and adhere to osteoblasts at the sub-endosteal bone surface. This and other recent studies are beginning to reveal signaling pathways, cytokines and adhesion molecules that may regulate cell quiescence in the postulated stem cell niche [51-53].
Surprisingly, a substantial majority of primitive lymphoid progenitors were in G0 and additional ones were in early G1 (Figure 1B). While some of these cells are in proximity to the endosteal surface of the bone [54], that is not the case for a majority of the population and signals for quiescence might be delivered in other sites. That possibility accords with a recent report that quiescent HSC can be found it tissues outside BM [55]. In any case, the slow population turnover for ELP and Pro-L contrasts with the rapid proliferation of the large pro-B and pre-B cells that derive from them [2, 43, 44]
It is not known whether fetal lymphocyte progenitors are intrinsically different from those that arise as the hematopoietic system is replenished during adult life [1, 5]. BrdU labeling now shows that fetal lymphocyte progenitors divide considerably more rapidly than their adult counterparts, the rate of BrdU labeling gradually declining with gestational age (Figure 3A). ELP and Pro-L populations in adult BM have low incidences, and their numbers remain constant without gross disturbance of the steady state. The primitive GFPloc-kithiSca1+ subset of fetal progenitors does not numerically increase in parallel with embryo size, whereas the more mature GFPhic-kitloSca1− fraction expands explosively from embryonic day 15 [56]. Therefore, rapid differentiation, cell death and/or export to other tissues must balance production of the earliest lymphopoietic cells.
Many previous studies have used 5-FU treatment to deplete actively proliferating marrow cells following which several characteristics of fetal stem cells are reactivated. Our analysis centered on four days post treatment because that is when c-kit+ RAG-1+ lymphoid progenitors began to emerge. This is consistent with previous descriptions of the recovery of hematopoietic stem cells [38;57]. CD11b/Mac-1 and CD34 are present on fetal and rebound HSC, but not those in BM of normal mature animals [36, 38]. Changes in marker expression might reflect either the recent regeneration of lymphohematopoietic cells post chemotherapy or abnormalities in the marrow microenvironment. Although lymphoid progenitors did not appear to reacquire these two fetal markers during rebound, they were in active cell cycle and had at least one function characteristic of fetal cells.
Substantial information is available about proteins that govern progression through the cell cycle, and we sought explanations for fetal/adult/rebound disparities by extensive real-time RT-PCR analyses. Of particular interest were mediators that were dramatically elevated or depressed in fetal ELP and rebound lymphoid progenitors as compared to those taken from adult marrow. These include Cyclin D2, c-myc, Bcl-2, Ink4d/p19, Cip1Waf1/p21, Kip1/p27, HoxB4 and TGFβ2R. Of that group, Bcl-2, Cip1Waf1/p21, and Kip1/p27 have been described as inhibitors of cell cycle progression [8, 16, 58], and thus match the pattern of quiescence we observed for adult ELP. The last two of these have been implicated in cell cycle regulation in HSC and multipotent progenitors, respectively [8]. Therefore, further investigation of these molecules might provide a mechanistic explanation for fetal/adult differences in proliferative activity.
While early lymphoid progenitors are not stem cells, they can sustain lymphocyte production in the thymus for at least 6 weeks [4]. Furthermore, memory lymphocytes can expand and survive for long periods after participation in immune responses [59]. Telomerase is thought to be important for maintaining chromosome integrity in cells with such replicative potential, and its activity is known to be higher in fetal than adult life [60, 61]. Our results with ELP correspond to that pattern, and it is interesting that telomerase activity increased during rebound from chemotherapy.
Some components of the immune system, and especially CD4+ T lymphocytes are slow to recover following the marrow ablation treatment used for chemotherapy and transplantation [62, 63]. However, we found no alteration in the T lineage differentiation potential for post 5-FU treatment lymphoid progenitors. It could be that performance in the stromal cell co-culture system we employed does not accurately reflect the ability to migrate to and colonize the thymus [30]. However, other culture assays for progenitors of NK and pDC also did not reveal an obvious influence of chemotherapy on differentiation potential (not shown). In striking contrast, formation of B lineage lymphocytes from rebound progenitors was considerably greater than that from normal adult BM and equivalent to that observed with fetal cells.
As this manuscript was being completed, mitotic properties were described for hematopoietic cells defined with different criteria [64]. Some 50% of MPP, a category that includes ELP, were found to be in G0. The MPP fraction also includes Lin-c-KitHiRAG-1- cells that we found to be 30% quiescent. Thus, RAG-1+ ELP (67% in G0) are less mitotically active than otherwise similar cells. CLP comprise most of the Pro-L fraction and Passegue, et al. reported that 74% were in G0 + G164, that is in general agreement with our finding of 85% for Pro-L.
Overall, this analysis provides important information about the earliest events in lymphopoiesis and raises a number of interesting questions. For example, do ELP and stem cells share a common niche in BM and depend on the same signals to maintain quiescence? We also need to learn if and how mitotic activity of lymphoid progenitors relates to their ability to differentiate and rapidly restore the immune system.
Supplementary Material
Acknowledgments
The authors are grateful to Drs. Michael Cancro and Linda Thompson for critical reading of this manuscript and suggestions. We also thank Robert Welner for valuable discussion, Jacob Bass and Diana Hamilton for expert sorting, Tara Khamphanthala for technical assistance, and Shelli Wasson for professional editorial assistance.
Footnotes
This work was supported by grants AI 20069 and AI 58162 from the National Institutes of Health. P.W.K. holds the William H. and Rita Bell Endowed Chair in Biomedical Research.
References
- 1.Baba Y, Pelayo R, Kincade PW. Relationships between hematopoietic stem cells and lymphocyte progenitors. Trends Immunol. 2004;25:645–649. doi: 10.1016/j.it.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 2.Mehr R, Shahaf G, Sah A, et al. Asynchronous differentiation models explain bone marrow labeling kinetics and predict reflux between the pre- and immature B cell pools. Int Immunol. 2003;15:301–312. doi: 10.1093/intimm/dxg025. [DOI] [PubMed] [Google Scholar]
- 3.Pelayo R, Welner RS, Nagai Y, et al. Life before the pre-B cell receptor checkpoint: specification and commitment of primitive lymphoid progenitors in adult bone marrow. Sem Immunol. 2006;18:2–11. doi: 10.1016/j.smim.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 4.Igarashi H, Gregory SC, Yokota T, et al. Transcription from the RAG1 locus marks the earliest lymphocyte progenitors in bone marrow. Immunity. 2002;17:117–130. doi: 10.1016/s1074-7613(02)00366-7. [DOI] [PubMed] [Google Scholar]
- 5.Yokota T, Kouro T, Hirose J, et al. Unique properties of fetal lymphoid progenitors identified according to RAG1 gene expression. Immunity. 2003;19:365–375. doi: 10.1016/s1074-7613(03)00231-0. [DOI] [PubMed] [Google Scholar]
- 6.Snodgrass R, Keller G. Clonal fluctuation within the haematopoietic system of mice reconstituted with retrovirus-infected stem cells. EMBO J. 1987;6:3955–3960. doi: 10.1002/j.1460-2075.1987.tb02737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Capel B, Hawley R, Covarrubias L, et al. Clonal contributions of small numbers of retrovirally marked hematopoietic stem cells engrafted in unirradiated neonatal W/Wv mice. Proc Natl Acad Sci U S A. 1989;86:4564–4568. doi: 10.1073/pnas.86.12.4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ezoe S, Matsumura I, Satoh Y, et al. Cell cycle regulation in hematopoietic stem/progenitor cells. Cell Cycle. 2004;3:314–318. [PubMed] [Google Scholar]
- 9.Bradford GB, Williams B, Rossi R, et al. Quiescence, cycling, and turnover in the primitive hematopoietic stem cell compartment. Exp Hematol. 1997;25:445–453. [PubMed] [Google Scholar]
- 10.Cheshier SH, Morrison SJ, Liao X, et al. In vivo proliferation and cell cycle kinetics of long-term self-renewing hematopoietic stem cells. Proc Natl Acad Sci U S A. 1999;96:3120–3125. doi: 10.1073/pnas.96.6.3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakauchi H, Sudo K, Ema H. Quantitative assessment of the stem cell self-renewal capacity. Ann N Y Acad Sci. 2001;938:18–24. doi: 10.1111/j.1749-6632.2001.tb03570.x. [DOI] [PubMed] [Google Scholar]
- 12.Fan X, Valdimarsdottir G, Larsson J, et al. Transient disruption of autocrine TGF-β signaling leads to enhanced survival and proliferation potential in single primitive human hemopoietic progenitor cells. J Immunol. 2002;168:755–762. doi: 10.4049/jimmunol.168.2.755. [DOI] [PubMed] [Google Scholar]
- 13.Cashman JD, Eaves CJ, Sarris AH, et al. MCP-1, not MIP-1α, is the endogenous chemokine that cooperates with TGF-β to inhibit the cycling of primitive normal but not leukemic (CML) progenitors in long-term human marrow cultures. Blood. 1998;92:2338–2344. [PubMed] [Google Scholar]
- 14.Cashman JD, Clark-Lewis I, Eaves AC, et al. Differentiation stage-specific regulation of primitive human hematopoietic progenitor cycling by exogenous and endogenous inhibitors in an in vivo model. Blood. 1999;94:3722–3729. [PubMed] [Google Scholar]
- 15.Adolfsson J, Mansson R, Buza-Vidas N, et al. Identification of Flt3+ lympho-myeloid stem cells lacking erythro-megakaryocytic potential a revised road map for adult blood lineage commitment. Cell. 2005;121:295–306. doi: 10.1016/j.cell.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 16.Cheng T, Rodrigues N, Shen H, et al. Hematopoietic stem cell quiescence maintained by p21cip1/waf1. Science. 2000;287:1804–1808. doi: 10.1126/science.287.5459.1804. [DOI] [PubMed] [Google Scholar]
- 17.Taniguchi T, Endo H, Chikatsu N, et al. Expression of p21Cip1/Waf1/Sdi1 and p27Kip1 cyclin-dependent kinase inhibitors during human hematopoiesis. Blood. 1999;93:4167–4178. [PubMed] [Google Scholar]
- 18.Steinman R, Yaroslavskiy B, Goff JP, et al. Cdk-inhibitors and exit from quiescence in primitive haematopoietic cell subsets. Br J Haematol. 2004;124:358–365. doi: 10.1046/j.1365-2141.2003.04780.x. [DOI] [PubMed] [Google Scholar]
- 19.Yaroslavskiy B, Watkins S, Donnenberg AD, et al. Subcellular and cell-cycle expression profiles of CDK-inhibitors in normal differentiating myeloid cells. Blood. 1999;93:2907–2917. [PubMed] [Google Scholar]
- 20.Brun AC, Bjornsson JM, Magnusson M, et al. Hoxb4-deficient mice undergo normal hematopoietic development but exhibit a mild proliferation defect in hematopoietic stem cells. Blood. 2004;103:4126–4133. doi: 10.1182/blood-2003-10-3557. [DOI] [PubMed] [Google Scholar]
- 21.Satoh Y, Matsumura I, Tanaka H, et al. Roles for c-Myc in self-renewal of hematopoietic stem cells. J Biol Chem. 2004;279:24986–24993. doi: 10.1074/jbc.M400407200. [DOI] [PubMed] [Google Scholar]
- 22.Nakano T. Hematopoietic stem cells: Generation and manipulation. Trends Immunol. 2003;24:589–594. doi: 10.1016/j.it.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 23.Bruno L, Rocha B, Rolink A, et al. Intra- and extra-thymic expression of the pre-T cell receptor alpha gene. Eur J Immunol. 1995;25:1877–1882. doi: 10.1002/eji.1830250713. [DOI] [PubMed] [Google Scholar]
- 24.Lu L, Smithson G, Kincade P, et al. Two models of murine B lymphopoiesis: a correlation. Eur J Immunol. 1998;28:1755–1761. doi: 10.1002/(SICI)1521-4141(199806)28:06<1755::AID-IMMU1755>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 25.Asquith B, Debacq C, Macallan DC, et al. Lymphocyte kinetics: the interpretation of labelling data. Trends Immunol. 2002;23:596–601. doi: 10.1016/s1471-4906(02)02337-2. [DOI] [PubMed] [Google Scholar]
- 26.Lu L, Osmond DG. Apoptosis and its modulation during B lymphopoiesis in mouse bone marrow. Immunol Rev. 2000;175:158–174. [PubMed] [Google Scholar]
- 27.Kuwata N, Igarashi H, Ohmura T, et al. Cutting edge: Absence of expression of RAG1 in peritoneal B-1 cells detected by knocking into RAG1 locus with green fluorescent protein gene. J Immunol. 1999;163:6355–6359. [PubMed] [Google Scholar]
- 28.Darzynkiewica Z, Juan G, Srour EF. Current Protocols in Cytometry. John Wiley & Sons, Inc; 2005. Differential Staining of DNA and RNA; pp. 7.3.1–7.3.16. [DOI] [PubMed] [Google Scholar]
- 29.Gothot A, Pyatt R, McMahel J, et al. Functional heterogeneity of human CD34(+) cells isolated in subcompartments of the G0 /G1 phase of the cell cycle. Blood. 1997;90:4384–4393. [PubMed] [Google Scholar]
- 30.Huang J, Garrett KP, Pelayo R, et al. Propensity of adult lymphoid progenitors to progress to DN2/3 stage thymocytes with Notch receptor ligation. J Immunol. 2005;175:4858–4865. doi: 10.4049/jimmunol.175.8.4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kondo M, Weissman IL, Akashi K. Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell. 1997;91:661–672. doi: 10.1016/s0092-8674(00)80453-5. [DOI] [PubMed] [Google Scholar]
- 32.Kouro T, Kumar V, Kincade PW. Relationships between early B- and NK-lineage lymphocyte precursors in bone marrow. Blood. 2002;100:3672–3680. doi: 10.1182/blood-2002-02-0653. [DOI] [PubMed] [Google Scholar]
- 33.Osmond DG, Hales P. Methodology of Lymphocyte Kinetics. In: Herzenberg LA, editor. The Integrated Immune System. Weir's Handbook of Experimental Immunology. IV. Cambridge: Blackwell Science, Inc; 1996. pp. 209.1–209.22. [Google Scholar]
- 34.Pelayo R, Welner R, Perry SS, et al. Lymphoid progenitors and primary routes to becoming cells of the immune system. Curr Opin Immunol. 2005;17:100–107. doi: 10.1016/j.coi.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 35.Kincade PW, Owen JJT, Igarashi H, et al. Nature or Nurture? Steady state lymphocyte formation in adults does not recapitulate ontogeny. Immunol Rev. 2002;187:116–125. doi: 10.1034/j.1600-065x.2002.18710.x. [DOI] [PubMed] [Google Scholar]
- 36.Ogawa M. Changing phenotypes of hematopoietic stem cells. Exp Hematol. 2002;30:3–6. doi: 10.1016/s0301-472x(01)00770-6. [DOI] [PubMed] [Google Scholar]
- 37.Christensen JL, Weissman IL. Flk-2 is a marker in hematopoietic stem cell differentiation: a simple method to isolate long-term stem cells. Proc Natl Acad SciU S A. 2001;98:14541–14546. doi: 10.1073/pnas.261562798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Randall TD, Weissman IL. Phenotypic and functional changes induced at the clonal level in hematopoietic stem cells after 5-fluorouracil treatment. Blood. 1997;89:3596–3606. [PubMed] [Google Scholar]
- 39.Sato T, Laver JH, Ogawa M. Reversible expression of CD34 by murine hematopoietic stem cells. Blood. 1999;94:2548–2554. [PubMed] [Google Scholar]
- 40.Porritt HE, Rumfelt LL, Tabrizifard S, et al. Heterogeneity among DN1 prothymocytes reveals multiple progenitors with different capacities to generate T cell and non-T cell lineages. Immunity. 2004;20:735–745. doi: 10.1016/j.immuni.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 41.Ceredig R, Rolink T. A positive look at double-negative thymocytes. Nat Rev Immunol. 2002;2:888–897. doi: 10.1038/nri937. [DOI] [PubMed] [Google Scholar]
- 42.Laurent J, Bosco N, Marche PN, et al. New insights into the proliferation and differentiation of early mouse thymocytes. Int Immunol. 2004;16:1069–1080. doi: 10.1093/intimm/dxh108. [DOI] [PubMed] [Google Scholar]
- 43.Rolink A, Karasuyama H, Haasner D, et al. Two pathways of B-lymphocyte development in mouse bone marrow and the roles of surrogate L chain in this development. Immunol Rev. 1994;137:185–201. doi: 10.1111/j.1600-065x.1994.tb00665.x. [DOI] [PubMed] [Google Scholar]
- 44.Lu L, Osmond DG. Apoptosis during B lymphopoiesis in mouse bone marrow. J Immunol. 1997;158:5136–5145. [PubMed] [Google Scholar]
- 45.Medina KL, Garrett KP, Thompson LF, et al. Identification of very early lymphoid precursors in bone marrow and their regulation by estrogen. Nature Immunol. 2001;2:718–724. doi: 10.1038/90659. [DOI] [PubMed] [Google Scholar]
- 46.Pelayo R, Hirose J, Huang J, et al. Derivation of two categories of plasmacytoid dendritic cells in murine bone marrow. Blood. 2005;105:4407–4415. doi: 10.1182/blood-2004-07-2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lin WC, Desiderio S. Cell cycle regulation of V(D)J recombination-activating protein RAG-2. Proc Natl Acad Sci U S A. 1994;91:2733–2737. doi: 10.1073/pnas.91.7.2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jiang H, Chang FC, Ross AE, et al. Ubiquitylation of RAG-2 by Skp2-SCF links destruction of the V(D)J recombinase to the cell cycle. Mol Cell. 2005;18:699–709. doi: 10.1016/j.molcel.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 49.Sadofsky MJ. Recombination-activating gene proteins: more regulation, please. Immunol Rev. 2004;200:83–89. doi: 10.1111/j.0105-2896.2004.00164.x. [DOI] [PubMed] [Google Scholar]
- 50.Igarashi H, Kuwata N, Kiyota K, et al. Localization of recombination activating gene 1/green fluorescent protein (RAG1/GFP) expression in secondary lymphoid organs after immunization with T-dependent antigens in rag1/gfp knockin mice. Blood. 2001;97:2680–2687. doi: 10.1182/blood.v97.9.2680. [DOI] [PubMed] [Google Scholar]
- 51.Arai F, Hirao A, Ohmura M, et al. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004;118:149–161. doi: 10.1016/j.cell.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 52.Suda T, Arai F, Hirao A. Hematopoietic stem cells and their niche. Trends Immunol. 2005;26:426–433. doi: 10.1016/j.it.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 53.Li L, Xie T. STEM CELL NICHE: Structure and Function. Annu Rev Cell Dev Biol. 2005;21:605–631. doi: 10.1146/annurev.cellbio.21.012704.131525. [DOI] [PubMed] [Google Scholar]
- 54.Hirose J, Kouro T, Igarashi H, et al. A developing picture of lymphopoiesis in bone marrow. Immunol Rev. 2002;189:28–40. doi: 10.1034/j.1600-065x.2002.18904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kiel MJ, Yilmaz OH, Iwashita T, et al. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 56.Yokota T, Tavian M, Hirose J, et al. Tracing the First Wave of Lymphopoiesis in Mice. Development. 2006;133:2041–2051. doi: 10.1242/dev.02349. [DOI] [PubMed] [Google Scholar]
- 57.Nishio N, Hisha H, Ogata H, et al. Changes in markers, receptors and adhesion molecules expressed on murine hemopoietic stem cells after a single injection of 5-fluorouracil. Stem Cells. 1996;14:584–591. doi: 10.1002/stem.140584. [DOI] [PubMed] [Google Scholar]
- 58.O'Reilly LA, Harris AW, Tarlinton DM, et al. Expression of a bcl-2 transgene reduces proliferation and slows turnover of developing B lymphocytes in vivo. J Immunol. 1997;159:2301–2311. [PubMed] [Google Scholar]
- 59.Lanzavecchia A, Sallusto F. Progressive differentiation and selection of the fittest in the immune response. Nat Rev Immunol. 2002;2:982–987. doi: 10.1038/nri959. [DOI] [PubMed] [Google Scholar]
- 60.Blasco MA, Funk W, Villeponteau B, et al. Functional characterization and developmental regulation of mouse telomerase RNA. Science. 1995;269:1267–1270. doi: 10.1126/science.7544492. [DOI] [PubMed] [Google Scholar]
- 61.Blasco MA. Telomerase beyond telomeres. Nat Rev Cancer. 2002;2:627–633. doi: 10.1038/nrc862. [DOI] [PubMed] [Google Scholar]
- 62.Fry TJ, Mackall CL. Immune reconstitution following hematopoietic progenitor cell transplantation: challenges for the future. Bone Marrow Transplant. 2005;35 (Suppl 1):S53–S57. doi: 10.1038/sj.bmt.1704848. [DOI] [PubMed] [Google Scholar]
- 63.Hakim FT, Memon SA, Cepeda R, et al. Age-dependent incidence, time course, and consequences of thymic renewal in adults. J Clin Invest. 2005;115:930–939. doi: 10.1172/JCI22492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Passegue E, Wagers AJ, Giuriato S, et al. Global analysis of proliferation and cell cycle gene expression in the regulation of hematopoietic stem and progenitor cell fates. J Exp Med. 2005;202:1599–1611. doi: 10.1084/jem.20050967. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


