Abstract
The 5-HT7 receptor has been suggested as a new putative target for the treatment of neuropsychiatric disorders, especially depression. This hypothesis is based on the finding that antidepressant drugs have relatively high affinity for the 5-HT7 receptor, and that inactivation or blockade of the receptor leads to an antidepressant-like profile in behavioral models and sleep parameters. Obsessive-compulsive disorder is also believed to involve the serotonergic system and is treated using antidepressants, thus it is of interest to study the possible role of the 5-HT7 receptor in this disorder. We have evaluated the effect of inactivation or pharmacological blockade of the 5-HT7 receptor in three mouse behavioral models that are believed to mimic some of the stereotypic aspects of obsessive-compulsive disorder. In the most well established behavioral model, marble burying, both inactivation and blockade of the 5-HT7 receptor reduced stereotypic behavior in that the number of marbles buried decreased. In two newer, less well characterized models, head dipping and plastic-mesh screen chewing, there was no difference between wild-type mice and mice lacking the 5-HT7 receptor. Taken together the data confirms and expands on previous findings that the 5-HT7 receptor is of importance for behaviors affected by antidepressants, and suggests that the 5-HT7 receptor might be of relevance as a target for the treatment of obsessive-compulsive disorder.
Keywords: serotonin, marble burying, head dipping, screen chewing, SB-269970
The 5-HT7 receptor, one of the most recently discovered receptors for serotonin [12], has been found to modulate behaviors affected by antidepressant drugs. In both the forced swim test and the tail suspension test, inactivation or blockade of the 5-HT7 receptor lead to antidepressant-like behavior [7,13,35]. Furthermore, inactivation or pharmacological blockade of the 5-HT7 receptor lead to changes in rapid eye movement sleep that are opposite to those seen in depression [8,13]. In addition, it has been shown that certain antidepressants might exert some of their effects by acting directly at the 5-HT7 receptor [26].
Obsessive-compulsive disorder (OCD) is a debilitating disease with an overall prevalence of close to 1 % [15]. There is strong evidence in support of a significant role for 5-HT in OCD and antidepressant drugs, mainly selective serotonin reuptake inhibitors (SSRIs), are the pharmacological treatment of choice for OCD [4,15]. However, as many as 40 % of patients do not respond to currently available treatment options [15], so continued research is necessary. Establishing good animal models for OCD has proved to be exceedingly difficult, most clearly demonstrated by the large number of models tried [21,23]. Marble burying is one of the more well-established models for OCD [19,27,28]. New models of supposedly stereotypic behavior, such as the screen chewing and head dipping tests used along with marble burying in the present study, are also continuously being developed and tested [2].
Even though SSRIs supposedly act by blocking the 5-HT transporter, and polymorphisms in the gene for this protein might be related to OCD [5,24], several 5-HT receptor subtypes have also been implicated in OCD. A recent study showed that mice lacking the 5-HT2C receptor show increased compulsive behavior in several behavioral tests [2]. However, the involvement of the 5-HT2C receptor is controversial as both agonists and antagonists have induced or worsened symptoms of OCD [18,20,31,37]. Studies have also suggested that 5-HT2A receptor agonism might have beneficial anti-OCD effects [25,29]. Other evidence suggest an involvement of the 5-HT1A receptor, since agonists at this receptor, specifically, 8-hydroxy-2(di-n-propylamino)tetralin (8-OH-DPAT) have been shown to reduce OCD-like behavior [19,23].
As 8-OH-DPAT is also an agonist at 5-HT7 receptors, and since 5-HT7 receptors influence behaviors modified by antidepressants, it is of interest to study the possible involvement of this receptor in models of OCD. We have investigated the effects of inactivation or blockade of the 5-HT7 receptor on marble burying, screen chewing, and head dipping behavior.
Ten-to-twelve week old male 5-HT7−/− mice and their male 5-HT7+/+ sibling controls were used. The generation of the 5-HT7−/− mouse strain has been described previously [10]. The mice used in this study had been back-crossed on a C57BL/6J background for at least 16 generations.
Marble-burying behavior was studied as previously described [19,36]. Twenty-four glass marbles (1.5 cm in diameter) were placed on 5 cm of sawdust bedding along the perimeter of a clear plastic box (44 × 22 × 20 cm). An individual mouse was placed in the box for 30 min. and the number of marbles buried at least two-thirds deep were counted. The effect of (R)-3-(2-(2-(4-methylpiperidin-1-yl)-ethyl)pyrrolidine-1-sulfonyl)phenol (SB-269970; 10 mg/kg) given as a single intraperitoneal injection 30 min. prior to the test was also evaluated. This dose was chosen based on its effectiveness in other behavioral and thermoregulation experiments [11,13]. SB-269970 was dissolved in 0.9% NaCl, which was also used as vehicle control.
Plastic mesh screen chewing and head dipping behavior was tested with minor modifications as previously described [2]. For screen chewing mice were individually housed with free access to food and water. A plastic circular grid-style mat (screen) with a diameter of 7 cm was placed in the cage during a ten-day period. The screen was weighed before and after the experiment. Differences in appearance of the screens indicating different chewing patterns were determined at the end of the experiment. A “spoke” was defined as a freed piece of screen (1.5 – 3.0 mm in length) that radiated from the center of the circular screen. A “spoke site” was defined as a location where a spoke was present or the mouse had chewed a radial segment to less than 1.5 mm.
For head-dipping habituation, mice were individually housed and weighed before testing and on each day of testing. Food intake was assessed by weighing available food. Food was removed before the first trial of a day and returned after the last trial, making food available for 21 h each day. The head dipping was studied using a 30 × 30 cm platform with a single 3.5 cm hole in the center placed on a 20 cm high bucket. The platform was screened from the rest of the room except for a small space where the investigator could observe the behavior. On three consecutive days, each mouse was given a 10-min trial repeated three times at 1-h intervals. The number of head dips was scored. A dip was defined as submerging the head into the hole with both eyes below the surface of the platform. The platform was wiped down with ethanol between individual mice.
All values are expressed as means ± standard errors of mean (S.E.M.). Student’s t-test was used to compare non-paired groups for a single independent variable and two-way ANOVA was used to compare data sets with two independent variables. p < 0.05 was considered significant.
In the marble burying test, in untreated animals there was a 55% reduction in the number of marbles buried by 5-HT7−/− mice compared to 5-HT7+/+ mice (Fig. 1A). A two-way ANOVA showed significant effect for genotype (F(1, 28) = 8.53, p < 0.01), treatment (SB-269970) (F(1, 28) = 12.62, p < 0.01), and for an interaction between the two (F(1, 28) = 5.24, p < 0.05). Thus, 5-HT7−/− mice buried fewer marbles than 5-HT7+/+ mice, and 5-HT7+/+ mice treated with SB-269970 buried fewer marbles than vehicle-treated 5-HT7+/+ mice (Fig. 1B). In other words, inactivation or blockade of the 5-HT7 receptor induced a similar reduction in the number of marbles buried.
Fig. 1.
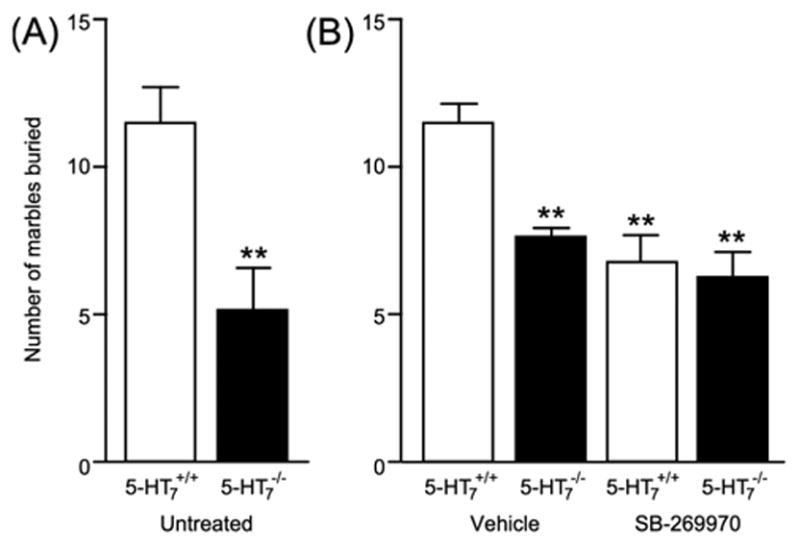
In the marble burying test (A) 5-HT7−/− mice buried fewer marbles than 5-HT7+/+ mice. A similar reduction in burying behavior (B) could be induced in 5-HT7+/+ mice by a single injection of the selective 5-HT7 receptor antagonist SB-269970 (10 mg/kg) given intraperitoneally 30 min. before the test. The antagonist had no effect in 5-HT7−/− mice. Data is presented as mean ± S.E.M. n = 8 animals per group. ** p < 0.01, student’s t-test (A) or two-way ANOVA followed by Newman-Keuls post-hoc analysis (B).
The results from the screen chewing test are summarized in Table 1. There were no differences that reached significance in the amount chewed or in the chewing pattern between 5-HT7+/+ and 5-HT7−/− mice.
Table 1.
There was no difference between 5-HT7+/+ mice and 5-HT7−/− mice in any of the parameters measured in the screen chewing test.
| Genotype | n | Amount chewed (g) | Spoke sites | Spokes | Remaining spokes (%) |
|---|---|---|---|---|---|
| 5-HT7+/+ | 16 | 0.16 ± 0.05 | 10.25 ± 5.39 | 2.31 ± 1.85 | 96.45 ± 1.87 |
| 5-HT7−/− | 16 | 0.10 ± 0.06 | 8.38 ± 5.98 | 3.44 ± 2.99 | 97.09 ± 2.08 |
Data is presented as mean ± S.E.M.
A two-way ANOVA of the head dipping data showed a significant effect for session (F(8,240) = 19.55, p < 0.0001), but no effect for genotype (F(1,240) = 1.33, p = 0.26) and no significant interaction between the two (F(8,240) = 1.11, p = 0.35). Thus, there was a significant habituation seen in the behavior that was similar in both 5-HT7+/+ and 5-HT7−/− mice (Fig. 2). The habituation was most pronounced during the first day of testing.
Fig. 2.
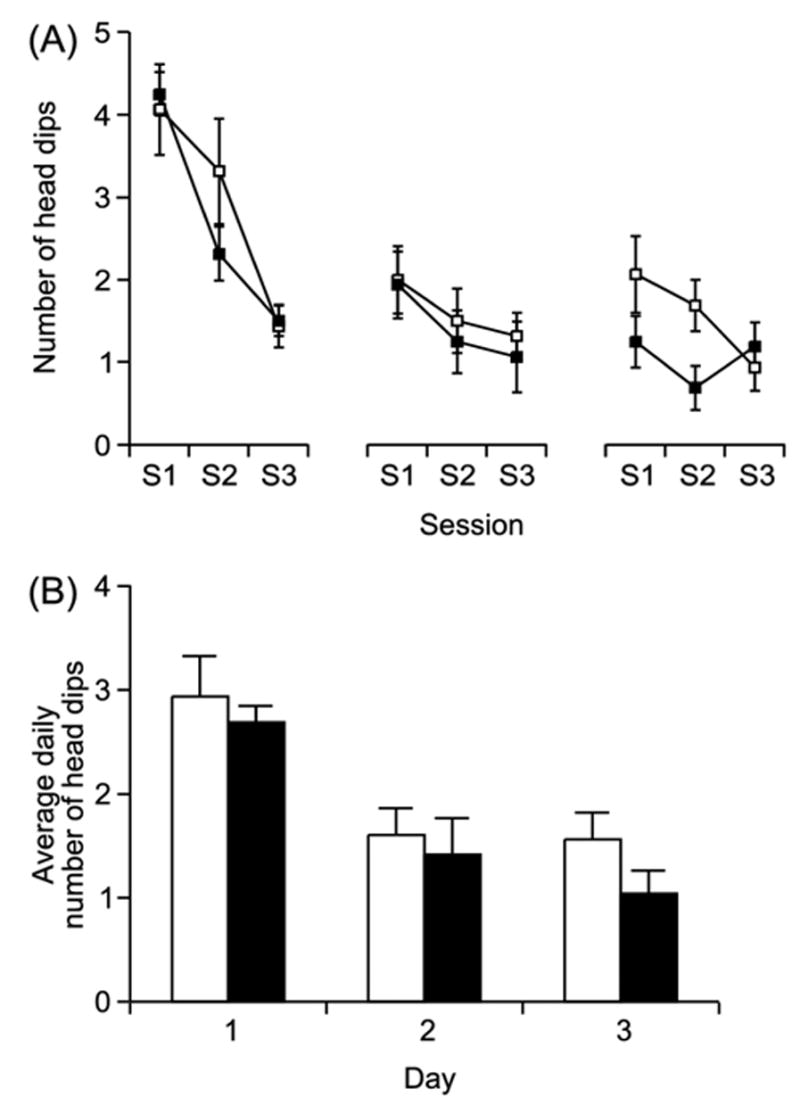
There was no difference between 5-HT7+/+ mice (open symbols) and 5-HT7−/− mice (filled symbols) in the head dipping test. (A) shows data for each of the three daily sessions and (B) shows the daily average for the three consecutive days of testing. Both genotypes show a similar habituation, which was most marked during the first day of testing. Data is presented as mean ± S.E.M. n = 16 animals per genotype.
The major finding of the present study was that inactivation or blockade of the 5-HT7 receptor reduced marble burying behavior, a mouse behavioral model linked to OCD and anxiety. A similar reduction in the number of marbles buried was observed in mice lacking the 5-HT7 receptor as in wild-type mice given a single injection of the selective 5-HT7 receptor antagonist SB-269970 (10 mg/kg). The similarity in effect seen between inactivation and blockade is in line with previous studies evaluating models of depression [7,13,35]. In both the forced swim test and the tail suspension test an antidepressant-like effect has been observed after inactivation of the 5-HT7 receptor [7,13] and also after treatment with a selective antagonist [13,35].
It has previously been found that agonism at the 5-HT1A receptor also reduces marble burying behavior [19,23]. This is of interest in relation to previously described relationships between 5-HT1A and 5-HT7 receptors. As 5-HT1A receptors inhibit adenylyl cyclase [3] and 5-HT7 receptors activate it [22], it could easily be hypothesized that agonism at the 5-HT1A receptor and antagonism at the 5-HT7 receptor produced the same effect. Indeed, the present results would support such a hypothesis. However, several previous studies found that antagonism or inactivation of both receptor subtypes produce the same result. This has been the case in thermoregulation where blocking or inactivating 5-HT1A or 5-HT7 receptors inhibit the hypothermic effect induced by 5-HT [6,11,17]. A similar relationship is also seen in models of depression in which mice lacking the 5-HT1A receptor and mice lacking the 5-HT7 receptor both show decreased immobility in the tail suspension test and the forced swim test [7,13,14,30,32]. Further studies are required to fully describe the functional relationship between 5-HT1A and 5-HT7 receptors and their effects on second messengers at the systems level.
The 5-HT7 receptor has previously been suggested as a putative target for the treatment of depression as blockade or inactivation of the receptor leads to an anti-depressant like profile in animal models of depression and in sleep parameters [7,8,13]. The present results support that notion as antidepressants have been shown to reduce marble burying [16,29]. The mechanism behind such an antidepressant-like profile remains unclear, but might include the localization of the receptors to areas such as the hippocampus and amygdala [12], and/or their possible regulation of the activity of serotonergic neurons by interactions with glutamatergic [9] or GABAergic [34] neurons in the raphe nuclei.
Marble burying has also been used as a model for anxiety as anxiolytic agents such as diazepam have been shown to reduce burying behavior [28]. Indeed, antidepressants, particularly SSRIs, are effective in the treatment of anxiety [1]. The degree of reduction in the number of marbles buried observed in the present study is similar to that observed with benzodiazepines in C57BL/6J mice [27]. Even greater reduction in the number of marbles buried can be achieved with other anxiolytics, e. g. buspirone [27]. However, the possible role of 5-HT7 receptors in anxiety is not clearly understood. One study found no change in mice lacking the 5-HT7 receptor in the light-dark transfer test, a model of anxiety [33], and another study saw no change in the elevated plus maze in such mice [7]. In contrast, a third study found that the selective 5-HT7 receptor antagonist SB-269970 had an anxiolytic effect in rats in the elevated plus maze, although with an inverted U-shaped dose-response curve and with an effect smaller than observed with diazepam in the same study [35]. Further studies are needed to more clearly define the role, if any, of 5-HT7 receptors in anxiety.
Certain drugs, however not the most commonly used anxiolytics or antidepressants, will reduce marble burying behavior at least partly by reducing spontaneous locomotor activity [27]. It is unlikely that this is the case in the present study, as it has been previously shown that locomotor activity is not altered in 5-HT7−/− mice [33] and that SB-269970 at any dose tested, including the one used in the present study, does not alter locomotor activity in C57BL/6J mice [35].
The lack of effect observed in the present study in the screen chewing and head dipping tests is currently difficult to interpret. These tests have to our knowledge only been used in one previous study [2] and it is therefore difficult to design a positive control. Their correlation, if any, to the marble burying test has never been evaluated. In view of the lack of difference between 5-HT7+/+ and 5-HT7−/− mice in screen chewing and head dipping we did not evaluate the possible modulation by SB-269970 of these behaviors, as there in our hands has always been a correlation between effects observed after inactivation and blockade of the 5-HT7 receptor. Nevertheless, the tests have been suggested as models for compulsive-like behavior, especially in view of the distinct chewing pattern observed in mice lacking the 5-HT2C receptor in the screen chewing test [2]. A definitive conclusion on the possible relevance of these tests for animal behavior in general and OCD in particular awaits future studies.
In conclusion, the present study show that inactivation of the 5-HT7 receptor leads to decreased burying behavior in the marble burying test, a model linked to OCD and anxiety, a finding that is also mimicked in wild-type mice after treatment with a selective 5-HT7 receptor antagonist. The results strengthen and extend the hypothesis that antagonism of the 5-HT7 receptor might be a valuable approach for the treatment of disorders, such as major depression and OCD, currently treated with antidepressants.
Acknowledgments
This work was supported by NIH grant MH73923 (PBH) and by NARSAD (PBH). We thank Patria Danielson for excellent technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Borsini F, Podhorna J, Marazziti D. Do animal models of anxiety predict anxiolytic-like effects of antidepressants? Psychopharmacology (Berl) 2002;163:121–141. doi: 10.1007/s00213-002-1155-6. [DOI] [PubMed] [Google Scholar]
- 2.Chou-Green JM, Holscher TD, Dallman MF, Akana SF. Compulsive behavior in the 5-HT2C receptor knockout mouse. Physiol Behav. 2003;78:641–649. doi: 10.1016/s0031-9384(03)00047-7. [DOI] [PubMed] [Google Scholar]
- 3.De Vivo M, Maayani S. Characterization of the 5-hydroxytryptamine1a receptor-mediated inhibition of forskolin-stimulated adenylate cyclase activity in guinea pig and rat hippocampal membranes. J Pharmacol Exp Ther. 1986;238:248–253. [PubMed] [Google Scholar]
- 4.Dell’Osso B, Nestadt G, Allen A, Hollander E. Serotonin-norepinephrine reuptake inhibitors in the treatment of obsessive-compulsive disorder: A critical review. J Clin Psychiatry. 2006;67:600–610. doi: 10.4088/jcp.v67n0411. [DOI] [PubMed] [Google Scholar]
- 5.Frisch A, Michaelovsky E, Rockah R, Amir I, Hermesh H, Laor N, Fuchs C, Zohar J, Lerer B, Buniak SF, Landa S, Poyurovsky M, Shapira B, Weizman R. Association between obsessive-compulsive disorder and polymorphisms of genes encoding components of the serotonergic and dopaminergic pathways. Eur Neuropsychopharmacol. 2000;10:205–209. doi: 10.1016/s0924-977x(00)00071-7. [DOI] [PubMed] [Google Scholar]
- 6.Guscott MR, Egan E, Cook GP, Stanton JA, Beer MS, Rosahl TW, Hartmann S, Kulagowski J, McAllister G, Fone KC, Hutson PH. The hypothermic effect of 5-CT in mice is mediated through the 5-HT7 receptor. Neuropharmacol. 2003;44:1031–1037. doi: 10.1016/s0028-3908(03)00117-5. [DOI] [PubMed] [Google Scholar]
- 7.Guscott M, Bristow LJ, Hadingham K, Rosahl TW, Beer MS, Stanton JA, Bromidge F, Owens AP, Huscroft I, Myers J, Rupniak NM, Patel S, Whiting PJ, Hutson PH, Fone KC, Biello SM, Kulagowski JJ, McAllister G. Genetic knockout and pharmacological blockade studies of the 5-HT7 receptor suggest therapeutic potential in depression. Neuropharmacology. 2005;48:492–502. doi: 10.1016/j.neuropharm.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 8.Hagan JJ, Price GW, Jeffrey P, Deeks NJ, Stean T, Piper D, Smith MI, Upton N, Medhurst AD, Middlemiss DN, Riley GJ, Lovell PJ, Bromidge SM, Thomas DR. Characterization of SB-269970-A, a selective 5-HT7 receptor antagonist. Br J Pharmacol. 2000;130:539–548. doi: 10.1038/sj.bjp.0703357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harsing LG, Prauda I, Barkoczy J, Matyus P, Juranyi Z. A 5-HT7 heteroreceptor-mediated inhibition of [3H]serotonin release in raphe nuclei slices of the rat: evidence for a serotonergic-glutamatergic interaction. Neurochem Res. 2004;29:1487–1497. doi: 10.1023/b:nere.0000029560.14262.39. [DOI] [PubMed] [Google Scholar]
- 10.Hedlund PB, Danielson PE, Thomas EA, Slanina K, Carson MJ, Sutcliffe JG. No hypothermic response to serotonin in 5-HT7 receptor knockout mice. Proc Natl Acad Sci USA. 2003;100:1375–1380. doi: 10.1073/pnas.0337340100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hedlund PB, Kelly L, Mazur C, Lovenberg T, Sutcliffe JG, Bonaventure P. 8-OH-DPAT acts on both 5-HT1A and 5-HT7 receptors to induce hypothermia in rodents. Eur J Pharmacol. 2004;487:125–132. doi: 10.1016/j.ejphar.2004.01.031. [DOI] [PubMed] [Google Scholar]
- 12.Hedlund PB, Sutcliffe JG. Functional, molecular and pharmacological advances in 5-HT7 receptor research. Trends Pharmacol Sci. 2004;25:481–486. doi: 10.1016/j.tips.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 13.Hedlund PB, Huitron-Resendiz S, Henriksen SJ, Sutcliffe JG. 5-HT7 receptor inhibition and inactivation induce antidepressantlike behavior and sleep pattern. Biol Psychiatry. 2005;58:831–837. doi: 10.1016/j.biopsych.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 14.Heisler LK, Chu HM, Brennan TJ, Danao JA, Bajwa P, Parsons LH, Tecott LH. Elevated anxiety and antidepressant-like responses in serotonin 5-HT1A receptor mutant mice. Proc Natl Acad Sci USA. 1998;95:15049–15054. doi: 10.1073/pnas.95.25.15049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heyman I, Mataix-Cols D, Fineberg NA. Obsessive-compulsive disorder. BMJ. 2006;333:424–429. doi: 10.1136/bmj.333.7565.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirano K, Kimura R, Sugimoto Y, Yamada J, Uchida S, Kato Y, Hashimoto H, Yamada S. Relationship between brain serotonin transporter binding, plasma concentration and behavioural effect of selective serotonin reuptake inhibitors. Br J Pharmacol. 2005;144:695–702. doi: 10.1038/sj.bjp.0706108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hjorth S. Hypothermia in the rat induced by the potent serotoninergic agent 8-OH- DPAT. J Neural Transm. 1985;61:131–135. doi: 10.1007/BF01253058. [DOI] [PubMed] [Google Scholar]
- 18.Hollander E, DeCaria CM, Nitescu A, Gully R, Suckow RF, Cooper TB, Gorman JM, Klein DF, Liebowitz MR. Serotonergic function in obsessive-compulsive disorder. Behavioral and neuroendocrine responses to oral m-chlorophenylpiperazine and fenfluramine in patients and healthy volunteers. Arch Gen Psychiatry. 1992;49:21–28. doi: 10.1001/archpsyc.1992.01820010021003. [DOI] [PubMed] [Google Scholar]
- 19.Ichimaru Y, Egawa T, Sawa A. 5-HT1A-receptor subtype mediates the effect of fluvoxamine, a selective serotonin reuptake inhibitor, on marble-burying behavior in mice. Jpn J Pharmacol. 1995;68:65–70. doi: 10.1254/jjp.68.65. [DOI] [PubMed] [Google Scholar]
- 20.Khullar A, Chue P, Tibbo P. Quetiapine and obsessive-compulsive symptoms (OCS): case report and review of atypical antipsychotic-induced OCS. J Psychiatry Neurosci. 2001;26:55–59. [PMC free article] [PubMed] [Google Scholar]
- 21.Korff S, Harvey BH. Animal models of obsessive-compulsive disorder: rationale to understanding psychobiology and pharmacology. Psychiatr Clin North Am. 2006;29:371–390. doi: 10.1016/j.psc.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 22.Lovenberg TW, Baron BM, de Lecea L, Miller JD, Prosser RA, Rea MA, Foye PE, Racke M, Slone AL, Siegel BW, Danielson PE, Sutcliffe JG, Erlander MG. A novel adenylyl cyclase-activating serotonin receptor (5-HT7) implicated in the regulation of mammalian circadian rhythms. Neuron. 1993;11:449–458. doi: 10.1016/0896-6273(93)90149-l. [DOI] [PubMed] [Google Scholar]
- 23.Matsushita M, Egashira N, Harada S, Okuno R, Mishima K, Iwasaki K, Nishimura R, Fujiwara M. Perospirone, a novel antipsychotic drug, inhibits marble-burying behavior via 5-HT1A receptor in mice: implications for obsessive-compulsive disorder. J Pharmacol Sci. 2005;99:154–159. doi: 10.1254/jphs.fp0050144. [DOI] [PubMed] [Google Scholar]
- 24.McDougle CJ, Epperson CN, Price LH, Gelernter J. Evidence for linkage disequilibrium between serotonin transporter protein gene (SLC6A4) and obsessive compulsive disorder. Mol Psychiatry. 1998;3:270–273. doi: 10.1038/sj.mp.4000391. [DOI] [PubMed] [Google Scholar]
- 25.McDougle CJ, Epperson CN, Pelton GH, Wasylink S, Price LH. A double-blind, placebo-controlled study of risperidone addition in serotonin reuptake inhibitor-refractory obsessive-compulsive disorder. Arch Gen Psychiatry. 2000;57:794–801. doi: 10.1001/archpsyc.57.8.794. [DOI] [PubMed] [Google Scholar]
- 26.Mullins UL, Gianutsos G, Eison AS. Effects of antidepressants on 5-HT7 receptor regulation in the rat hypothalamus. Neuropsychopharmacol. 1999;21:352–367. doi: 10.1016/S0893-133X(99)00041-X. [DOI] [PubMed] [Google Scholar]
- 27.Nicolas LB, Kolb Y, Prinssen EPM. A combined marble burying-locomotor activity test in mice: a practical screening test with sensitivity to different classes of anxiolytics and antidepressants. Eur J Pharmacol. 2006;547:106–115. doi: 10.1016/j.ejphar.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 28.Njung’e K, Handley SL. Evaluation of marble-burying behavior as a model of anxiety. Pharmacol Biochem Behav. 1991;38:63–67. doi: 10.1016/0091-3057(91)90590-x. [DOI] [PubMed] [Google Scholar]
- 29.Njung’e K, Handley SL. Effects of 5-HT uptake inhibitors, agonists and antagonists on the burying of harmless objects by mice; a putative test for anxiolytic agents. Br J Pharmacol. 1991;104:105–112. doi: 10.1111/j.1476-5381.1991.tb12392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parks CL, Robinson PS, Sibille E, Shenk T, Toth M. Increased anxiety of mice lacking the serotonin1A receptor. Proc Natl Acad Sci USA. 1998;95:10734–10739. doi: 10.1073/pnas.95.18.10734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramasubbu R, Ravindran A, Lapierre Y. Serotonin and dopamine antagonism in obsessive-compulsive disorder: effect of atypical antipsychotic drugs. Pharmacopsychiatry. 2000;33:236–238. doi: 10.1055/s-2000-8360. [DOI] [PubMed] [Google Scholar]
- 32.Ramboz S, Oosting R, Amara DA, Kung HF, Blier P, Mendelsohn M, Mann JJ, Brunner D, Hen R. Serotonin receptor 1A knockout: an animal model of anxiety-related disorder. Proc Natl Acad Sci USA. 1998;95:14476–14481. doi: 10.1073/pnas.95.24.14476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roberts AJ, Krucker T, Levy CL, Slanina KA, Sutcliffe JG, Hedlund PB. Mice lacking 5-HT7 receptors show specific impairments in contextual learning. Eur J Neurosci. 2004;19:1913–1922. doi: 10.1111/j.1460-9568.2004.03288.x. [DOI] [PubMed] [Google Scholar]
- 34.Roberts C, Thomas DR, Bate ST, Kew JN. GABAergic modulation of 5-HT7 receptor-mediated effects on 5-HT efflux in the guinea-pig dorsal raphe nucleus. Neuropharmacology. 2004;46:935–941. doi: 10.1016/j.neuropharm.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 35.Wesolowska A, Nikiforuk A, Stachowicz K, Tatarczynska E. Effect of the selective 5-HT(7) receptor antagonist SB 269970 in animal models of anxiety and depression. Neuropharmacology. 2006;51:578–586. doi: 10.1016/j.neuropharm.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 36.Woods-Kettelberger A, Kongsamut S, Smith CP, Winslow JT, Corbett R. Animal models with potential applications for screening compounds for the treatment of obsessive-compulsive disorder. Expert Opin Investig Drugs. 1997;6:1369–1381. doi: 10.1517/13543784.6.10.1369. [DOI] [PubMed] [Google Scholar]
- 37.Zohar J, Mueller EA, Insel TR, Zohar-Kadouch RC, Murphy DL. Serotonergic responsivity in obsessive-compulsive disorder. Comparison of patients and healthy controls. Arch Gen Psychiatry. 1987;44:946–951. doi: 10.1001/archpsyc.1987.01800230026006. [DOI] [PubMed] [Google Scholar]


