Abstract
Neurons in the superficial gray layer (SGS) of the superior colliculus receive visual input and excite intermediate layer (SGI) neurons that play a critical role in initiating rapid orienting movements of the eyes, called saccades. In the present study, two types of experiments demonstrate that a population of SGI neurons gives rise to a reciprocal pathway that inhibits neurons in SGS. First, in GAD67-GFP knockin mice, GABAergic SGI neurons that expressed GFP fluorescence were injected with the tracer biocytin to reveal their axonal projections. Axons arising from GFP-positive neurons in SGI terminated densely in SGS. Next, SGI neurons in rats and mice were stimulated by using the photolysis of caged glutamate, and in vitro whole-cell patch-clamp recordings were used to measure the responses evoked in SGS cells. Large, synaptically mediated outward currents were evoked in SGS neurons. These currents were blocked by gabazine, confirming that they were GABAA receptor-mediated inhibitory postsynaptic currents. This inhibitory pathway from SGI transiently suppresses visual activity in SGS, which in turn could have multiple effects. These effects could include reduction of perceptual blurring during saccades as well as prevention of eye movements that might be spuriously triggered by the sweep of the visual field across the retina.
Keywords: patch-clamp, photostimulation, sensorimotor, superior colliculus, visuomotor
Neurons in the superficial layer (SGS) of the superior colliculus receive input from the retina and the visual cortex (1) and provide a powerful excitatory input to premotor neurons in the intermediate layer (SGI) that play a critical role in initiating rapid orienting movements of the eyes, called saccades (2–4). The activity of many of these superficial layer neurons is suppressed during saccades (5). By reducing the activity in the pathway from the superficial to the premotor cells in the intermediate layer, this suppression may prevent unwanted saccades that might otherwise be triggered by the rapid movement of the visual field that occurs during a saccade. SGS cells also project to nuclei in the dorsal thalamus that relay visual information to the cortex (1), and the reduction of activity in these relay nuclei may contribute to the attenuation of visual perception that occurs during the course of saccades (6–9).
Experiments designed to identify the neural mechanisms responsible for saccadic suppression suggest that neither retinal input nor proprioceptive input from the periphery is responsible. For example, superficial layer neurons can respond to visual stimuli moving at the velocity of saccades (5), which suggests that the retinal input is suppressed after reaching SGS. Moreover, the observation that the suppression of activity in SGS can occur during eye movements in the dark indicates that the mechanism is not dependent on changes in the level of retinal activity (5). Similarly, the proposal that the suppression is mediated by input from receptors in extraocular eye muscles or tendons that detect the eye movements (10, 11) is contradicted by experiments demonstrating that suppression begins before the onset of the saccade and occurs even when the eyes are paralyzed by retrobulbar injections of lidocaine HCl (12, 13).
An alternative hypothesis proposes that the saccade premotor neurons provide an inhibitory corollary discharge that suppresses the visual activity resulting from the eye movement (7, 13, 14). The present experiments provide both anatomical and physiological support for this hypothesis. Specifically, the results of two types of experiments comprise evidence that a GABAA receptor-mediated inhibitory pathway to SGS arises in SGI. First, GAD67-GFP knockin mice, in which GABAergic neurons express green fluorescent protein (GFP), have GABAergic neurons in the intermediate layers that project to the superficial layer. Second, in vitro whole-cell patch-clamp recordings from both rats and mice reveal powerful inhibitory currents in superficial layer cells evoked by photostimulation of cells in the intermediate layer. These results are consistent with a circuit model proposing that axon collaterals from the premotor cells in SGI activate neighboring GABAergic cells that in turn inhibit the visuosensory cells in SGS.
Results
GAD67-GFP Knockin Mice Reveal Intermediate Layer GABAergic Neurons That Project to the Superficial Layers.
For the identification of SGI GABAergic neurons, we used GAD67-GFP knockin mice, in which GABAergic neurons express GFP fluorescence (15). The GFP fluorescent SGI neurons were first located by using an epifluorescence microscope (Fig. 1) and whole-cell patch-clamp recordings were obtained from these cells under visual control. During these recordings, the tracer biocytin was diffused into the cells from the patch pipette internal solution. The biocytin was used to verify the location and morphology of the GFP-positive neurons and to trace their axonal projections (Fig. 2).
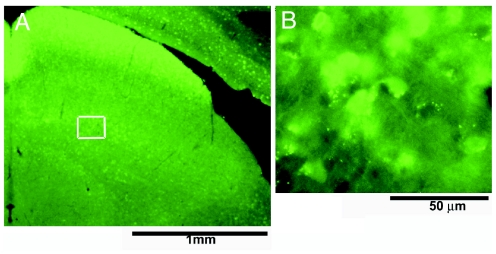
Fig. 1.
GFP-positive neurous in SGI. (A) Low-magnification photomicrograph of the superior colliculus in a GAD67-GFP mouse. (B) High-magnification photomicrograph of neurons in SGI (from A Inset) that express GFP fluorescence. This GFP fluorescence allows selective, visually guided sampling of GABAergic neurons that, because of their small size and number, otherwise are rarely encountered.
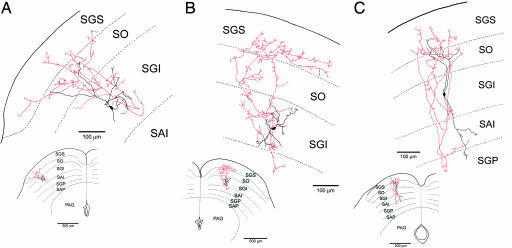
Fig. 2.
Camera lucida drawings of GAD67-GFP-positive neurons that were filled with biocytin during whole-cell patch-clamp recordings. The axons project mainly dorsally to the SGS (A and B) or to both SGS and SGP (C). Red lines indicate axon collaterals; black, the cell soma and dendrites. SO, optic layer; SAI, stratum album intermedium or intermediate white layer; SAP, stratum album profundum or deep white layer; PAG, periaqueductal gray layer.
A total of 164 GFP-positive SGI neurons from 25 GAD67-GFP knockin mice were recorded from in these experiments, and the morphology of 134 of these neurons was sufficiently revealed by the intracellular staining with biocytin to allow tracing their axons. Among these 134 recovered neurons, we found 32 with axon collaterals that projected to SGS. Within this group, the axons of 19 neurons projected primarily to the SGS (Fig. 2 A and B), and those of 13 neurons projected to both SGS and the deep gray layer (SGP) (Fig. 2C). The projection from SGI to SGS was columnar in the sense that the SGS terminal field of the axon was most dense in a region immediately superficial to the SGI cell soma that gave rise to the projection. However, this organization is coarse in the sense that the axon arbors from single cells branch widely and can include 20–30% of the mediolateral extent of SGS (Fig. 2).
Photostimulation in the Intermediate Layer Evokes Inhibitory Postsynaptic Currents (IPSCs) in the Superficial Layers.
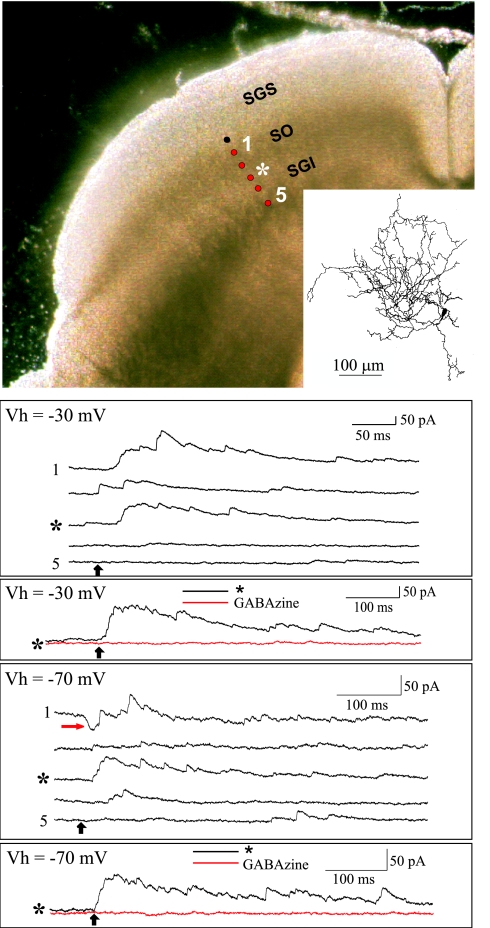
In a separate set of experiments, SGI neurons were activated by the photolysis of caged glutamate while the evoked responses of SGS cells were recorded in the whole-cell patch-clamp mode (4). Twenty-one SGS neurons from rats and 4 neurons from mice were analyzed. In the rat, 16 of the 21 neurons generated prominent IPSCs in response to SGI stimulation; 5 of these also generated small excitatory postsynaptic currents (EPSCs). Large IPSCs were evoked in three of the four neurons in mice and, of these, one also exhibited small EPSCs. These results are documented in Fig. 3. The photograph in Fig. 3 Upper Left was taken during the course of the recording experiment and illustrates the living slice cut in the coronal plane. The Inset is a drawing of the patch-clamped cell, which was filled with biocytin during the recording to reveal its dendritic morphology. The black circle in the figure indicates the location of the patch-clamped cell in SGS, and the red circles show the photostimulation sites (1–5 indicate stimulation sites 100–500 μm from the soma).
Fig. 3.
Examples of synaptic currents evoked in SGS cells by photostimulation in SGI. (Upper Left) The photomicrograph is of a coronal slice through the superior colliculus and shows the location of the patch-clamped cell (black dot) and the photostimulation sites (red dots). Sites 1–5 were 100–500 μm from the recorded cell, respectively. (Inset) Camera lucida drawing of the patch-clamped cell. (Top traces) Outward currents evoked by photostimulation at each of the sites indicated by the red dots while the cell was clamped at −30 mV. (Middle traces) Currents evoked by stimulation at site 3 (*), which was 300 μm from the recorded cell and in SGI, were blocked by gabazine. (Bottom five traces) Currents evoked while the cell was clamped at −70 mV. Both EPSCs (red arrow) and IPSCs were evoked when the photostimulation site was in the optic layer (site 1), but the predominant responses were IPSCs when the stimulus was located in SGI (site 3, *). (Bottom trace) Currents evoked by stimulation at site 3 (*) while the cell was clamped at −70 mV. These outward currents were blocked by the addition of gabazine (red trace), but few if any EPSCs were unmasked.
In the figure, the upper 5 traces illustrate outward synaptic currents that were evoked by stimulation (black arrow) at sites 1 to 5 while the SGS cell was voltage-clamped at −30 mV to increase the amplitude of the IPSCs and reduce the amplitude of EPSCs that might otherwise mask the IPSCs (see Materials and Methods). Stimulation sites 3–5 were in SGI. In the middle trace, the outward currents evoked by stimulation at site 3 (300 μm from the soma) were blocked by gabazine, confirming that the currents evoked in the SGS cell were GABAA receptor-mediated IPSCs. The lower 5 traces are currents evoked when the SGS cell was clamped at −70 mV. Both EPSCs (red arrow) and IPSCs were evoked when the photostimulation site was in SO (site 1), but the predominant responses were IPSCs when the stimulus was located in SGI (site 3, *). The lowest tracing in Fig. 3 shows currents evoked by stimulation at site 3 (*) while the cell was clamped at −70 mV to increase the amplitude of EPSCs. These outward currents were blocked by the addition of gabazine (red trace), but few if any EPSCs were unmasked in the absence of the IPSCs, confirming that the pathway from SGI to SGS is predominantly inhibitory.
Six cells were filled well enough with biocytin to allow reconstruction of their dendritic fields. Although the morphology of these cells varied, most resembled SGS wide-field cells that previous studies have shown project to SGI (ref. 3 and Fig. 3). Similar cells project to visual relay nuclei in the dorsal thalamus (1), which suggests that the pathway from SGI to SGS also may attenuate the transmission of visual signals to the cortex.
Discussion
These experiments provide both anatomical and physiological evidence for an inhibitory pathway that arises in a premotor layer, SGI, and terminates in a visuosensory layer, SGS. By filling the GFP-positive cells with biocytin, it was possible to trace their intracollicular projections. Of the cells filled with biocytin, ≈25% gave rise to axons that formed terminal arbors in SGS. These anatomical observations were confirmed and extended by the physiological experiments that demonstrated that photostimulation in SGI evoked GABAA receptor-mediated inhibitory currents in SGS. The photostimulation method was crucial for these experiments because of its high spatial resolution and because the synaptic currents evoked with this method are not confounded by activation of fibers of passage (4). Thus, the inhibitory input to SGS was assuredly evoked by stimulating cells in SGI. The widespread extent of the axonal terminal arbors in SGS together with the observation that a large number of broadly tuned premotor neurons is active during a saccade (16) argues that the suppression of activity in SGS during a saccade is global.
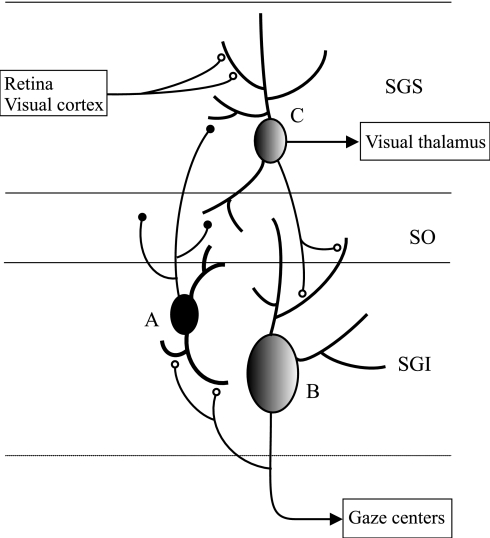
Saccade suppression is one example of diverse mechanisms that have been proposed to account for how an organism is able to differentiate between self- and externally induced stimulation. Fig. 4 presents a circuit model for saccadic suppression based on the present results that might also apply to other sensorimotor systems and to other organisms. According to the model, a population of GABAergic neurons in SGI (cell A in Fig. 4) receives excitatory input from the local collaterals that arise from the axons of premotor cells (such as cell B) in SGI and/or from the descending projections (not shown) from the visuosensory cells in SGS (cell C). Both local collaterals and descending projections from SGS to SGI have been demonstrated in previous studies (2–4, 17, 18). The input to the GABAergic cells from the premotor cells might help ensure that the duration of the saccadic suppression approximates the duration of visual stimulation that occurs during the saccade (8, 12, 19). According to the model in Fig. 4, the GABAergic cells inhibit the SGS cells that project to SGI and, in this way, attenuate their potential contributions to the generation of additional, unwanted, saccades during the course of a saccade. Because cells in SGS similar in morphology to those described here also project to the visual thalamus (1), the suppression of SGS activity also might contribute to the reduction of visual activity that has been observed in both the visual thalamus (8, 9, 19) and the cortex (20, 21). Although the suppression of the SGS cells that project to SGI may help prevent unwanted saccades from being triggered by saccade-induced movements of the visual field, the suppression of SGS cells that project to the visual thalamus might contribute to the prevention of visual perceptual blurring during the eye movement (6, 20). However, further experiments in which the projections of the inhibited SGS cells are identified by prelabeling them with a retrograde axonal tracer from the putative destinations of their axons and/or by filling them with biocytin will be required to establish their functions with certainty. Finally, other pathways from the superior colliculus to different regions of the thalamus may account for the perceptual stabilization of the visual field that occurs with each saccade (22). In conclusion, studies are necessary to identify with certainty the sources of input to the GABAergic SGI cells that project to SGS and also the efferent connections of the particular SGS cells that are inhibited by SGI. However, the evidence for an inhibitory pathway from SGI to SGS seems unequivocal and provides strong anatomical and physiological support for a circuit mechanism in the superior colliculus that utilizes inhibitory feedback from the command cells for saccades to suppress self-induced visual input.
Fig. 4.
Circuit model of the mechanism responsible for saccadic suppression. The model hypothesizes that the GABAergic neurons in SGI that project to SGS receive input from neighboring SGI premotor cells (B) and from SGS cells (C). The SGS cells (C) that receive this inhibitory feedback from SGI project to SGI and to visual relay nuclei in the dorsal thalamus.
Materials and Methods
GAD67-GFP Mice Express Intermediate Layer GABAergic Neurons That Project to the Superficial Layers.
The superior collicular slices were obtained from 17- to 22-day-old GAD67-GFP knockin mice (15) as described in the previous papers (23, 24). Mice were killed humanely by decapitation under deep ether anesthesia. Then the brain was quickly removed and submerged in an ice-cold Ringer's solution containing 234 mM sucrose/2.5 mM KCl/1.25 mM NaH2PO4/10 mM MgSO4/0.5 mM CaCl2/26 mM NaHCO3/11 mM glucose and bubbled with 95% O2/5% CO2, pH 7.4, for >5 min. Frontal slices (300 μm in thickness) of the superior colliculus were cut by using a Microslicer (DTK2000; Dosaka EM, Kyoto, Japan) and then incubated in a standard Ringer's solution containing 125 mM NaCl/2.5 mM KCl/2 mM CaCl 2/1 mM MgCl2/1.25 mM NaH2PO4/26 mM NaHCO3/25 glucose and bubbled with 95% O2/5% CO2, pH 7.4, for >1 h before the recording at room temperature (22–25°C). SGI GABAergic neurons were first identified by epifluorescence microscopy. After identification, whole-cell patch-clamp recording was performed on the neuron under the visual control of patch pipettes by using a patch-clamp amplifier (EPC-7; List, Darmstadt, Germany). The patch pipettes were filled with an internal solution containing 140 mM potassium gluconate/20 mM KCl/0.2 mM EGTA/2 mM MgCl2/2 mM Na2ATP/0.5 mM Na3GTP/10 mM Hepes/0.1 mM spermine, pH 7.3. Biocytin (5 mg/ml; Sigma, St. Louis, MO) was added in the internal solution to verify the location and morphology of recorded neurons. The resistance of electrodes was 4.0–7.0 MΩ in the bath solution. The liquid junction potential between the patch pipette solution and the standard Ringer's solution was estimated to be −10 mV, and the data were corrected according to this voltage. After recordings, the slices were fixed in 4% buffered paraformaldehyde solution for >2 days. They were then processed with the avidin–biotin peroxidase method (ABC kit; Vector Laboratories, Burlingame, CA), visualized with diaminobenzidine tetrahydrochloride (DAB; Dojin, Kumamoto, Japan), and intensified with nickel ammonium sulfate (3). The slices were counterstained with cresyl violet. Only cells with intact somas and proximal dendrites were drawn by using a camera lucida attached to a light microscope (Nikon, Tokoyo, Japan).
Photostimulation in the Intermediate Layer Evokes Inhibitory Responses in the Superficial Layers.
Briefly, coronal or parasagittal slices 200–300 μm thick were prepared from the superior colliculus of 20- to 30-day-old rats and mice. Slices were superfused at room temperature with oxygenated physiological saline (128 mM NaCl/2.6 mM KCl/1.35 mM MgCl2/2.6 mM CaCl2/1.04 mM NaH2PO4/27.02 mM NaHCO3/10.41 mM glucose) containing 100–150 μM carboxy-nitrobenzyl-2-caged glutamate (Molecular Probes, Eugene, OR). Recordings were made from 24 SGS cells. A 5–10 mM concentration of internal Na+ channel blocker QX-314 was included in the patch pipette. With QX-314, voltage-clamp recordings at membrane potentials as depolarized as −30 mV could be performed without generating Na+/K+ action potentials. The combination of high-chloride reversal potentials and low holding potentials increased the amplitude of IPSCs while reducing the amplitude of EPSCs that might otherwise occlude the inhibitory currents. The patch pipette solution contained 110 mM potassium gluconate/10 mM EGTA/4 mM MgCl2/4 mM ATP/0.4 mM GTP/20 mM Hepes, pH to 7.35 with KOH. To characterize the cell morphology, 0.5% biocytin was included in the pipette solution. The apparatus used for photostimulation was described by Helms et al. (4). In brief, UV light from a pulse laser was delivered through a microscope objective and focused on the slice. An electronic shutter was used to vary the duration of the light pulse (10 ms). The laser beam was aligned in the center of the objective and remained stationary in the field of view. A motorized microscope stage varied the distance between the site of photostimulation and the patch-clamped cell.
Acknowledgments
We thank Katsuyuki Kaneda for comments on the manuscript. This research was supported by National Institutes of Health Grant EY08233 (to W.C.H.); Ministry of Education, Culture, Sports, Science and Technology of Japan Grants 13854029, 18019007, and 18200027 (to T.I.); and National Institute for Physiological Sciences fellowship (to T.S.).
Abbreviations
- EPSC
excitatory postsynaptic current
- IPSC
inhibitory postsynaptic current
- SGI
stratum grisium intermedium or intermediate gray layer
- SGP
stratum grisium profundum or deep gray layer
- SGS
stratum grisium superficiale or superficial gray layer.
Footnotes
The authors declare no conflict of interest.
References
- 1.May PJ. Progr Brain Res. 2006;151:321–378. doi: 10.1016/S0079-6123(05)51011-2. [DOI] [PubMed] [Google Scholar]
- 2.Lee PH, Helms MC, Augustine GJ, Hall WC. Proc Natl Acad Sci USA. 1997;94:13299–13304. doi: 10.1073/pnas.94.24.13299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Isa T, Endo T, Saito Y. J Neurosci. 1998;18:8496–8504. doi: 10.1523/JNEUROSCI.18-20-08496.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Helms MC, Özen G, Hall WC. J Neurophysiol. 2004;91:1706–1715. doi: 10.1152/jn.00705.2003. [DOI] [PubMed] [Google Scholar]
- 5.Goldberg ME, Wurtz RH. J Neurophysiol. 1972;35:542–559. doi: 10.1152/jn.1972.35.4.542. [DOI] [PubMed] [Google Scholar]
- 6.Volkmann FC. J Opt Soc Am. 1962;52:571–578. doi: 10.1364/josa.52.000571. [DOI] [PubMed] [Google Scholar]
- 7.Volkman FC. Vision Res. 1986;26:1401–1416. doi: 10.1016/0042-6989(86)90164-1. [DOI] [PubMed] [Google Scholar]
- 8.Zhu JJ, Lo F-S. Brain Res Bull. 1996;41:281–291. doi: 10.1016/s0361-9230(96)00201-8. [DOI] [PubMed] [Google Scholar]
- 9.Zhu JJ, Lo F-S. J Neurophysiol. 1998;80:1122–1131. doi: 10.1152/jn.1998.80.3.1122. [DOI] [PubMed] [Google Scholar]
- 10.Holt EB. Psychol Monogr. 1903;4:3–46. [Google Scholar]
- 11.Sherrington CS. Brain. 1918;41:323–343. [Google Scholar]
- 12.Volkman FC, Schick AML, Riggs LA. J Opt Soc Am. 1968;58:562–569. doi: 10.1364/josa.58.000562. [DOI] [PubMed] [Google Scholar]
- 13.Richmond BJ, Wurtz RH. J Neurophysiol. 1980;43:1156–1167. doi: 10.1152/jn.1980.43.4.1156. [DOI] [PubMed] [Google Scholar]
- 14.von Helmholtz H. A Treatise on Physiological Optics. Vol 3. New York: Dover; 1925. [Google Scholar]
- 15.Tamamaki N, Yanagawa Y, Tomioka R, Miyazaki J, Obata K, Kaneko T. J Comp Neurol. 2003;467:60–79. doi: 10.1002/cne.10905. [DOI] [PubMed] [Google Scholar]
- 16.Lee C, Rohrer WH, Sparks DL. Nature. 1988;332:357–360. doi: 10.1038/332357a0. [DOI] [PubMed] [Google Scholar]
- 17.Moschovakis AK, Karabelas AB, Highstein SM. J Neurophysiol. 1988;60:263–302. doi: 10.1152/jn.1988.60.1.263. [DOI] [PubMed] [Google Scholar]
- 18.Hall WC, Lee PH. Visual Neurosci. 1997;14:647–661. doi: 10.1017/s095252380001261x. [DOI] [PubMed] [Google Scholar]
- 19.Noda H. J Physiol (London) 1975;249:87–102. doi: 10.1113/jphysiol.1975.sp011004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Judge SJ, Wurtz RH, Richmond BJ. J Neurophysiol. 1980;43:1133–1155. doi: 10.1152/jn.1980.43.4.1133. [DOI] [PubMed] [Google Scholar]
- 21.Thiele A, Henning P, Kubischik M, Hoffmann K-P. Science. 2002;295:2460–2462. doi: 10.1126/science.1068788. [DOI] [PubMed] [Google Scholar]
- 22.Sommer MA, Wurtz R. Nature. 2006;444:374–377. doi: 10.1038/nature05279. [DOI] [PubMed] [Google Scholar]
- 23.Endo T, Yanagawa Y, Obata K, Isa T. Neurosci Lett. 2003;346:81–84. doi: 10.1016/s0304-3940(03)00570-6. [DOI] [PubMed] [Google Scholar]
- 24.Endo T, Yanagawa Y, Obata K, Isa T. J Neurophysiol. 2005;94:3893–3902. doi: 10.1152/jn.00211.2005. [DOI] [PubMed] [Google Scholar]