Abstract
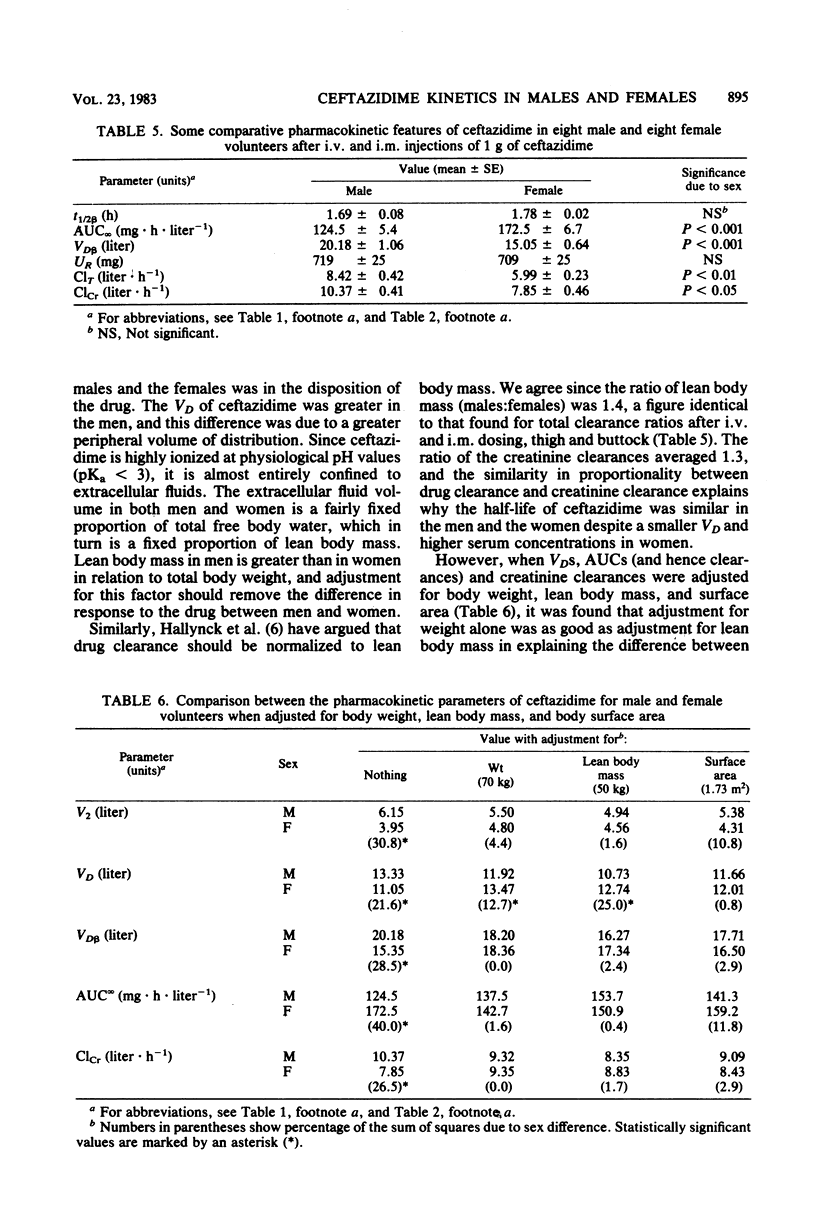
The pharmacokinetic behavior of ceftazidime was assessed after single bolus intravenous injections of 1 g to 12 male and 12 female volunteers. The kinetic handling of the drug was essentially identical in the two sexes, exhibiting two-compartment model characteristics. However, the peripheral compartment volume of distribution of ceftazidime was smaller in the females (mean 3.95 liters, compared with 6.15 liters), and this was attributed to a smaller extracellular fluid volume. Eight volunteers in each group also received single 1-g doses of ceftazidime into the vastus lateralis and gluteus maximus muscles. The time to peak concentration was longer in the women, and it was longer after injection into the gluteus maximus in both sexes, presumably because of differences in local blood flow. The bioavailability of ceftazidime may have been slightly reduced by delays in absorption. Again, body and renal clearances were similar for both sexes when allowance was made for differences in distribution volume.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Granneman G. R., Sennello L. T., Sonders R. C., Wynne B., Thomas E. W. Cefsulodin kinetics in healthy subjects after intramuscular and intravenous injection. Clin Pharmacol Ther. 1982 Jan;31(1):95–103. doi: 10.1038/clpt.1982.15. [DOI] [PubMed] [Google Scholar]
- Hallynck T. H., Soep H. H., Thomis J. A., Boelaert J., Daneels R., Dettli L. Should clearance be normalised to body surface or to lean body mass? Br J Clin Pharmacol. 1981 May;11(5):523–526. doi: 10.1111/j.1365-2125.1981.tb01163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding S. M., Eilon L. A., Harris A. M. Factors affecting the intramuscular absorption of cefuroxime. J Antimicrob Chemother. 1979 Jan;5(1):87–93. doi: 10.1093/jac/5.1.87. [DOI] [PubMed] [Google Scholar]
- Lüthy R., Blaser J., Bonetti A., Simmen H., Wise R., Siegenthaler W. Comparative multiple-dose pharmacokinetics of cefotaxime, moxalactam, and ceftazidime. Antimicrob Agents Chemother. 1981 Nov;20(5):567–575. doi: 10.1128/aac.20.5.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan C. H., Acred P., Harper P. B., Ryan D. M., Kirby S. M., Harding S. M. GR 20263, a new broad-spectrum cephalosporin with anti-pseudomonal activity. Antimicrob Agents Chemother. 1980 May;17(5):876–883. doi: 10.1128/aac.17.5.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedman A. J., Wagner J. G. CSTRIP, a fortran IV computer program for obtaining initial polyexponential parameter estimates. J Pharm Sci. 1976 Jul;65(7):1006–1010. doi: 10.1002/jps.2600650713. [DOI] [PubMed] [Google Scholar]
- Tjandramaga T. B., Van Hecken A., Mullie A., Verbesselt R., De Schepper P. J., Verbist L. Comparative pharmacokinetics of ceftazidime and moxalactam. Antimicrob Agents Chemother. 1982 Aug;22(2):237–241. doi: 10.1128/aac.22.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vukovich R. A., Brannick L. J., Sugerman A. A., Neiss E. S. Sex differences in the intramuscular absorption and bioavailability of cephradine. Clin Pharmacol Ther. 1975 Aug;18(2):215–220. doi: 10.1002/cpt1975182215. [DOI] [PubMed] [Google Scholar]


