Abstract
Plastids in Nicotiana tabacum are normally transmitted to the progeny by the maternal parent only. However, low-frequency paternal plastid transmission has been reported in crosses involving parents with an alien cytoplasm. Our objective was to determine whether paternal plastids are transmitted in crosses between parents with the normal cytoplasm. The transplastomic father lines carried a spectinomycin resistance (aadA) transgene incorporated in the plastid genome. The mother lines in the crosses were either (i) alloplasmic, with the Nicotiana undulata cytoplasm that confers cytoplasmic male sterility (CMS92) or (ii) normal, with the fertile N. tabacum cytoplasm. Here we report that plastids from the transplastomic father were transmitted in both cases at low (10−4-10−5) frequencies; therefore, rare paternal pollen transmission is not simply due to breakdown of normal controls caused by the alien cytoplasm. Furthermore, we have found that the entire plastid genome was transmitted by pollen rather than small plastid genome (ptDNA) fragments. Interestingly, the plants, which inherited paternal plastids, also carried paternal mitochondrial DNA, indicating cotransmission of plastids and mitochondria in the same pollen. The detection of rare paternal plastid transmission described here was facilitated by direct selection for the transplastomic spectinomycin resistance marker in tissue culture; therefore, recovery of rare paternal plastids in the germline is less likely to occur under field conditions.
Keywords: Nicotiana tabacum, organelle inheritance, plastid transformation, pollen transmission
DNA in a plant cell is found in three cellular compartments: the nucleus, plastids, and mitochondria. Genes encoded in the nucleus are inherited biparentally, according to Mendel's rules. In contrast, plastids and mitochondria may be inherited maternally, paternally, or from both parents. In most crops, the maternal parent transmits plastids, because plastids are excluded from the sperm cell or, even if not excluded, are left behind in synergid cells during fertilization (1–3). In Chlamydomonas reinhardtii, a unicellular alga, in which maternal (mating-type+) and paternal (mating-type−) chloroplasts fuse, only the maternal plastid genome (ptDNA) is inherited, because the maternal ptDNA is protected by methylation, whereas the nonmethylated paternal ptDNA is degraded (4). Once protocols for plastid transformation were developed (5, 6), localization of transgenes in the plastid genome was advocated as a means of transgene containment (7). Utility of plastid localization for containment became a hotly debated issue when the first herbicide-resistant transplastomic plants were obtained (8, 9). The reason for skepticism was the reported relatively high-frequency paternal ptDNA transmission in species in which plastids were assumed to be inherited strictly by the maternal parent. Paternal ptDNA transmission was detected in crosses by using streptomycin resistance (in 0.07–2.5%) (10, 11) and tentoxin resistance (0.5–2.5%) (12) in tobacco, pigment deficiency in petunia (2%) (13, 14), and atrazine resistance in foxtail or birdseed millet (0.03%) (15).
Common in these studies was utilization of alloplasmic substitution lines, in which plastids and mitochondria of one species were combined with the nuclear genome of another species. We therefore decided to test whether rare paternal plastid transmission occurs in Nicotiana tabacum if no alloplasmic substitution line is involved in the cross.
Here we report that paternal ptDNA is transmitted by pollen in crosses of plants with a normal cytoplasm in one of ≈10,000 seedlings. We have also shown that the entire plastid genome is transmitted by pollen rather than small ptDNA fragments, and that mitochondria (mitochondrial DNA) are always cotransmitted with the paternal ptDNA. Detection of rare paternal ptDNA transmission described here is biased by tissue culture selection, and the transgenic ptDNA is less likely to get into the germ line under field conditions.
Results
Paternal ptDNA Transmission to Alloplasmic Cytoplasmic Male Sterile (CMS) 92 Mother.
We first tested our protocols for paternal ptDNA transmission using an alloplasmic N. tabacum line, CMS92, as maternal parent. Line CMS92 was obtained by repeated backcrossing of Nicotiana undulata with N. tabacum that resulted in the replacement of the N. tabacum cytoplasm with the N. undulata cytoplasm. The ptDNA of paternal lines (Table 1) carried chimeric aadA genes in the trnV-3′rps12 intergenic region that confer spectinomycin and streptomycin resistance. Thus, seedlings that acquired a few copies of paternal ptDNA were expected to be spectinomycin- and streptomycin-resistant because of expression of aadA and male sterile, a CMS92 mitochondrial trait (12, 16) (Fig. 1A).
Table 1.
Paternal ptDNA transmission to CMS maternal parent detected by selection for spectinomycin resistance (500 mg/liter)
| Pollen donor | Protocol | No. of seedlings | No. of ptDNA transfer events | No. of mutants |
|---|---|---|---|---|
| Nt-pMHB10 | RM | 8,700 | 1 (PSpc1) | 0 |
| Nt-pMSK56 | RM | 2,046 | 1 (PSpc4) | 0 |
| Nt-pMSK56 | 6,590 | 0 | 0 | |
| Nt-pMHB10 | RMOP | 1,000 | 1 (PSpc66) | 2 |
| Nt-pMSK56 | RMOP | 2,610 | 2 (PSpc2, PSpc3) | 1 |
| Nt-pMSK56 | 18,420 | 0 | 0 | |
| Nt-pMSK56 | RM/RMOP | 128 | 0 | 0 |
| Nt-pMSK56 | RM/RMOP | 1,137 | 1 (PSpc7) | 3 |
| Nt-pMSK56 | RMOP/RMOP | 4,050 | 0 | 4 |
| Nt-pJEK6 | RMOP/RMOP | 3,178 | 4 (PSpc23, PSpc24, PSpc25, PSpc32) | 6 |
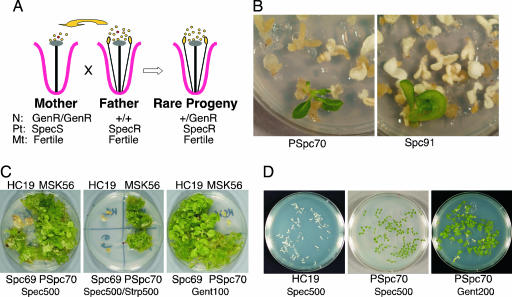
Fig. 1.
Transmission of paternal ptDNA in alloplasmic crosses. (A) Flower morphology of the male sterile N. tabacum CMS92 mother line and fertile Nt-pMSK56 transplastomic father line. Below are listed nuclear, plastid, and mitochondrial markers. (B) PSpc32 line has petaloid flowers and is male sterile, as the CMS92 mother line. (C) The PSpc4 (RM protocol) and PSpc2, PSpc3, and PSpc66 (RMOP protocol) paternal pollen transmission events. Arrows point to green resistant sectors. (D) Classification of spectinomycin-resistant lines. The aadA gene in paternal ptDNA confers streptomycin resistance to PSpc4 cells. Spontaneous spectinomycin-resistant mutant Spc93 is sensitive to streptomycin. Mother line N. tabacum CMS92 (sensitive to both drugs) and father line Nt-pMSK56 (resistant to both drugs) were controls. Leaf sections were cultured on RMOP medium containing 500 mg/liter spectinomycin or 500 mg/liter of both drugs.
Seedlings were screened for paternal ptDNA by testing them for the spectinomycin-resistance gene on a selective medium (500 mg/liter of spectinomycin). Four protocols were used. The seeds were surface sterilized and (i) germinated on plant growth [revised medium for plant maintenance in sterile culture (RM)] medium; (ii) germinated on callus induction [revised medium for organogenesis (shoot regeneration) of Nicotiana plumbaginifolia (RMOP)] medium; (iii) germinated on plant growth (RM) medium then transferred to callus induction (RMOP) medium (RM/RMOP protocol); (iv) germinated on callus induction medium and transferred onto callus induction medium (RMOP/RMOP protocol). For protocols i and ii plates were inspected for resistance for up to 4 mo. For protocols iii and iv, seedlings were germinated on the first selective medium (RM or RMOP) for 2–3 wk (200–300 seedlings per 10-cm Petri dish), then transferred to the RMOP medium (25 per 10-cm dish) and inspected for spectinomycin resistance for up to 4 mo. Results by paternal parent are listed in Table 1; cumulative data are in Table 2.
Table 2.
Rare paternal ptDNA transmission detected by selection for spectinomycin resistance (500 mg/liter)
| Maternal parent | Germinate/transfer | No. of seedlings | No. of ptDNA transfer events | No. of mutants | Frequency of ptDNA transfer | Frequency of mutants |
|---|---|---|---|---|---|---|
| CMS | RM | 17,336 | 2 | 0 | 1 × 10−4 | <6 × 10−5 |
| RMOP | 22,030 | 3 | 3 | 1 × 10−4 | 1 × 10−4 | |
| RM/RMOP | 1,265 | 1 | 3 | 8 × 10−4 | 2 × 10−3 | |
| RMOP/RMOP | 7,228 | 4 | 10 | 6 × 10−4 | 1 × 10-3 | |
| Fertile | RMOP | 31,897 | 0 | 1 | <3 × 10−5 | 3 × 10−5 |
| RM/RMOP | 1,350 | 0 | 6 | <6 × 10−4 | 4 × 10−3 | |
| RMOP/RMOP | 34,115 | 3* | 26 | 9 × 10−5 | 8 × 10−4 |
*PSpc64, PSpc68, and PSpc70.
Spectinomycin-sensitive seedlings or calli were white, whereas resistant sectors or calli were green on the selective medium (Fig. 1C). Resistance to spectinomycin could be due to expression of the Paternal aadA gene (lines designated PSpc and a number), or because of a new spontaneous point mutation in the 16S rRNA (lines designated Spc and a number). Seedlings carrying paternal plastids (aadA gene) were also streptomycin-resistant (17). In contrast, spontaneous spectinomycin-resistant mutants (Spc93) were streptomycin-sensitive, because mutations in the 16S rRNA that prevent spectinomycin binding do not confer cross resistance to streptomycin (Fig. 1D) (18, 19). Paternal ptDNA transfer events were recovered following each of the protocols (Table 1). The frequency of seedlings with paternal ptDNA was in the range of 1–6 × 10−4. The overall frequency, 10 events in 47,859 seedlings, corresponds to the frequency of 2 × 10−4.
Paternal pollen transmission has been confirmed by DNA gel-blot analysis indicating the regenerated plants carried the plastid genome of the donor N. tabacum paternal parent and not the maternal N. undulata ptDNA (see below). Plants regenerated from 9 of the 10 (exception was PSpc1) paternal ptDNA transmission lines have been purified to homoplasmy by one additional round of plant regeneration and transferred to the greenhouse. The flowers in each of the nine lines had the CMS92 petaloid phenotype of the maternal parent (Fig. 1B) and produced seed after fertilization with wild-type pollen.
In preliminary experiments, selection for streptomycin resistance (500 mg/liter) yielded two paternal ptDNA transmission events (PSt1 and PSt6). Because first selection on streptomycin medium yielded seedlings that often turned out to be sensitive in the second test, experiments to recover seedlings with paternal ptDNA by streptomycin selection were abandoned.
Paternal ptDNA Transmission in Cross with Normal Cytoplasm.
Testing of paternal ptDNA transmission between parents with the normal cytoplasm was carried out by using one transplastomic spectinomycin-resistant father, Nt-pMSK56, and mother lines, Nt-pHC18 or Nt-pHC19, that did not have any plastid genetic marker but carried a (nonsegregating) nuclear gentamycin-resistance gene. Therefore, seedlings acquiring the aadA gene from the paternal parent were expected to be resistant to spectinomycin, streptomycin, and gentamycin (Fig. 2A).
Fig. 2.
Transmission of paternal ptDNA between parents with normal cytoplasm. (A) Nuclear (N), plastid (Pt), and mitochondrial (Mt) genotypes of parental lines and exceptional hybrid with paternal ptDNA. (B) Identification of PSpc70 paternal ptDNA transmission event and Spc69 spontaneous spectinomycin-resistant mutant in RMOP/RMOP protocol. (C) Classification of spectinomycin-resistant lines. Paternal aadA gene confers streptomycin resistance to PSpc70. Spc69 spontaneous spectinomycin-resistant mutant is sensitive to streptomycin. Both lines are gentamycin-resistant. Nt-pHC19 and Nt-pMSK56 are parental lines. (D) Germination of seedlings on spectinomycin medium indicates that the PSpc70 paternal ptDNA transmission line is homoplastomic for the aadA transgene and segregates for the nuclear gentamycin resistance marker.
Three paternal ptDNA transfer events have been recovered in 34,115 seedlings following the RMOP/RMOP protocol (Fig. 2B), a frequency of 9 × 10−5 (Table 2). Transfer of paternal ptDNA as the source of spectinomycin resistance has been confirmed by demonstrating resistance to streptomycin and gentamycin (Fig. 2C). Hybrid origin of the PSpc70 line was also indicated by segregation of its seed progeny for gentamycin resistance (Fig. 2D).
Transmission of Entire ptDNA by Pollen.
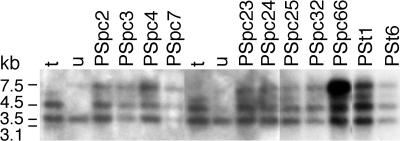
Transfer of ptDNA fragments to the nucleus has been shown during reproduction (20) and in somatic cells (21). Thus, transfer of paternal ptDNA may occur within an intact organelle or by a transformation-like process. In case of the transfer of organelles, we expected to find the entire paternal ptDNA, whereas transfer that involves transformation is likely to involve only ptDNA fragments. To distinguish between the two possibilities, we tested species-specific ptDNA restriction fragment length polymorphism (RFLP) markers in progeny to which paternal ptDNA has been transferred. Because the ptDNA sequence is available only for N. tabacum (22), first we identified RFLP markers that are suitable to distinguish the N. tabacum and N. undulata ptDNA. This was accomplished by digesting total leaf DNA of the two parental lines with a battery of eight restriction endonucleases that yield 100–400 ptDNA fragments each, then probing the blotted DNA (Fig. 3). We found polymorphic sites in five sets of genes in the large unique region (atpI-rpoC2, psbC-psaA, trnV-atpB, psbJ-psbE, and rps8-rps3), the insertion site of aadA in the inverted repeat (3′-rps12-rrn16-trnV), and one set of genes (psaC-ndhA) in the small unique region. Probing of total cellular DNA isolated from greenhouse-grown homoplastomic plants revealed only N. tabacum paternal ptDNA markers in the nine plants selected by spectinomycin resistance (PSpc2-PSpc66) and in two plants selected by streptomycin resistance (PSt1 and PSt6) (Table 3). We conclude, therefore, that the entire ptDNA is transmitted by pollen, most likely in intact organelles.
Fig. 3.
RFLP mapping of paternal ptDNA. DNA gel blot to identify RFLP markers in the CMS92 ptDNA. Lanes contain total cellular DNA of N. tabacum cv. Petit Havana (t), the CMS92 line (u), and transplastomic Nt-pMSK56 line (TP). The genes covered by the probe and restriction enzymes used for DNA digestion are listed on top. Fragment sizes are shown for N. tabacum.
Table 3.
RFLP markers in paternal ptDNA transmission lines
| Clones |
atpI-rpoC1 |
psbC-psaA |
trnV-atpB |
psbJ-psbE |
rps8-rps3 | 3′-rps12-rrn16 |
psaC-ndhA |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AseI | AluI | HpaII | AvaII | EcoRI | CIaI | EcoRI | HpaII | StyI | BamHI | HaeIII | HpaII | |
| PSpc2 | t | t | t | t | t | t | t | t | t | t | t | |
| PSpc3 | t | t | t | t | t | t | t | t | t | t | t | |
| PSpc4 | t | t | t | t | t | t | t | t | t | t | t | |
| PSpc7 | t | t | t | t | t | t | t | t | t | t | t | |
| PSpc23 | t | t | t | t | t | t | t | t | t | t | ||
| PSpc24 | t | t | t | t | t | t | t | t | t | t | ||
| PSpc25 | t | t | t | t | t | t | t | t | t | t | ||
| PSpc32 | t | t | t | t | t | t | t | t | ||||
| PSpc66 | t | t | t | t | t | t | t | |||||
| PSt1 | t | t | t | t | t | t | t | t | t | t | t | |
| PSt6 | t | t | t | t | t | t | t | t | t | t | ||
Cotransmission of Mitochondrial and Plastid DNAs.
Paternal transmission of plastids naturally triggers the question whether the nonselected mitochondria (mitochondrial DNA) is cotransmitted with the ptDNA from the father line. To answer this question, we first identified polymorphic restriction sites in the mitochondrial genome (mtDNA) using a mtDNA fragment containing the atp6 gene. Digestion of total cellular DNA with the SspI enzyme and probing with the atp6 probe revealed unique fragments in both the N. tabacum and N. undulata mitochondria. Probing of each of the 11 paternal ptDNA transfer lines revealed both N. tabacum- and N. undulata-specific fragments (Fig. 4), indicating that N. tabacum mitochondria were transmitted with the plastids by pollen.
Fig. 4.
Plants with paternal ptDNA have mtDNA from both parents. Note N. tabacum- (t) and N. undulata- (u) specific mtDNA fragments in parental lines and in plants obtained by spectinomycin (PSpc2–66) and streptomycin (PSt1, PSt6) selection. Total cellular DNA was probed with the 6.9-kb atp6 probe.
Discussion
Pollen Transmission of ptDNA.
We report here that pollen transmission of ptDNA occurs at a low frequency in crosses of N. tabacum lines carrying the normal cytoplasm. Thus, it appears that exclusion of paternal ptDNA fails at the frequency of 1 of 10,000 seedlings. Indeed, during male reproductive cell maturation, occasional plastids have been observed in tobacco sperm cells that could form the cytological bases of paternal pollen transmission (23). Alternatively, organellar inclusions in the sperm nucleus could provide a potential mechanism for transmitting organellar DNA into the next generation (24).
We normally evaluate seedling phenotype 2 wk after spreading 200–300 seeds on a selective (500 mg/liter) spectinomycin medium. Formation of resistant sectors due to expression of paternal aadA genes may take longer, 6–16 wk, and the sectors are relatively small. Thus, they are likely to remain undetected in a standard seed assay unless a large number of seedlings or seedling calli are cultivated for extended periods. Because of the lack of suitable genetic markers, transmission of paternal ptDNA by pollen thus far had been shown only in alloplasmic crosses, leaving open the possibility that paternal ptDNA transmission is due to the breakdown of normal control processes in the alloplasmic cross. Indeed, the frequency of paternal pollen transmission reported in alloplasmic crosses, in general, was significantly higher, 0.5–2.5% of seed progeny (10–14) than the value we found, 9 × 10−5, in the cross with the normal cytoplasm. However, the frequency of paternal pollen transmission in our alloplasmic cross was also relatively low, 2 × 10−4, and only ≈2-fold higher than the frequency in the cross between the normal parental lines. We believe this is due to our choice of tobacco line, N. tabacum cv. Petit Havana. Indeed, line-specific differences within a species are important; in Petunia, low-frequency paternal ptDNA transmission was detectable in only 5 of the 22 inbreds tested (25).
Protocols for Testing Paternal ptDNA Transmission.
The number of cell divisions in a developing plant is determined by the plant's developmental program. In contrast, tobacco cells in a culture can be grown indefinitely, so long as the cells are transferred to fresh medium at regular intervals. We were interested to find out whether the limitation of cell division in a seedling would interfere with the recovery of paternal ptDNA transfer events. Based on data in Table 2, it appears it is not necessary to callus the seedlings to identify paternal pollen transmission events when screening for a transgenic spectinomycin resistance marker. Although the frequency of paternal ptDNA transmission events was independent of the protocol, the number of spontaneous spectinomycin-resistant mutants was not; the two-step RMOP/RMOP protocol with repeated subculture on callus induction medium yielded 10 times more mutants than protocols, including only one subculture on callus induction medium (Table 2). Thus, longer culture and transfer onto fresh medium (more cell divisions) enabled recovery of more spontaneous mutants.
Cotransmission of Paternal Plastid and Mitochondrial DNA.
Taking advantage of species-specific plastid and mitochondrial RFLP markers in plants derived from the alloplasmic cross, we could address two important issues that are untraceable in the normal cross. First, we found by testing seven regions in the ptDNA, all RFLP markers derive from the paternal parent (Table 3). Thus, the entire ptDNA is transmitted, rather than small ptDNA fragments from the paternal parent, most likely in an intact plastid. There is no evidence for a transformation-like process that was reported for ptDNA transferred from the plastid to the nucleus (20, 21) or ptDNA recombination detected after chloroplast fusion (26). This conclusion is in agreement with earlier reports that failed to show any deviation from paternal ptDNA (10–13).
We also found that each of the alloplasmic lines that acquired paternal ptDNA also has the maternal CMS92 phenotype. Maintenance of maternal CMS phenotype and mtDNA restriction patterns led earlier investigators to the conclusion that the mtDNA is not transmitted together with the selected ptDNA (10–12). Interestingly, we found that each of the paternal ptDNA transfer lines has mixed mitochondrial DNA RFLP markers (Fig. 4). Thus, the mitochondria in these plants carry some of the N. tabacum mtDNA sequence in addition to the mitochondrial sequence causing the petaloid CMS92 phenotype (16, 27). Finding mixed parental mtDNA is not surprising. Mitochondria in plant cells may participate in a massive fusion cycle (28), and recombination of mtDNA following mitochondrial fusion is well documented (see, for example, ref. 29). Cotransfer of mtDNA and ptDNA in earlier studies may have been missed because of limited probing, or because it does not occur in all species combinations. Mitochondria are more frequent in sperm cells than plastids, and inclusion of both plastids and mitochondria in the cytoplasm or nucleus of exceptional sperm cells could provide a shared mechanism for transmitting both organelles into the next generation (23, 24).
Transgene Containment.
One of our objectives was to determine the absolute number of paternal ptDNA transmission events in a normal cross. When using a one-step protocol, we may have missed some paternal ptDNA transmission events because of nutritional limitation that prevented formation of a visible sector from each of the carrier cells. With the two-step protocol, we recovered significantly more spontaneous mutants but not significantly more transmission events; thus, the one event per ≈10,000 seedlings is the absolute number of ptDNA transmission events in N. tabacum cv. Petit Havana, the tobacco line we studied. The absolute number of ptDNA transmission events seen here is probably much higher than the number of events likely to appear under field conditions. The plastid genome is highly polyploid (30) and, when the cells divide, there is no exact duplication of the cytoplasm. Presence of ptDNA copies in the vegetative plant tissue depends on the paternal ptDNA copies being present in the shoot apex. Paternal ptDNA copies were clearly present in the original shoot apex of only two of the seedlings, PSpc3 and PSpc4 (Fig. 1C) of the 12 events that yielded plants. (Note that we disregard newly differentiated shoot apexes, such as PSpc66 in Fig. 1C.) Formation of rare sectors with a few copies of paternal ptDNA are biased by tissue culture selection; therefore, they are less likely to get into the germ line under field condition.
Transgene flow within crops and between crops and wild relatives is a genuine concern (31, 32). Experiments reported here are the first steps to design genetic screens that will lead to identification of nuclear genes controlling plastid pollen transmission. Understanding the genetic control of plastid inheritance will facilitate rational design of new transgenic crops in which escape of plastid transgenes by pollen can be minimized.
Materials and Methods
Plant Lines.
Mother lines with the normal cytoplasm were Nt-pHC18 or Nt-pHC19, N. tabacum cv. Petit Havana plants transformed with an aacC1 gentamycin resistance gene expressed in a cauliflower mosaic virus 35S cassette (33). In the Nt-pHC19 plants, an XbaI site is blunted downstream of the aacC1 coding region. CMS92 seed was kindly provided by Ezra Galun in the N. tabacum cv. Samsun background (16). The CMS92 mother line was an F1 hybrid of cv. Samsun and cv. Petit Havana. Father lines N. tabacum cv. Petit Havana Nt-pMHB10 (34), Nt-pMSK56 (35), and Nt-pJEK6 plants (36) carry transplastomic spectinomycin resistance (aadA) genes. The fertile mother line was manually emasculated before pollination.
Tissue Culture and Selection.
The RM plant growth medium was agar-solidified containing Murashige and Skoog salts (37) and 3% sucrose (pH 5.8). The RMOP medium is an RM medium containing benzyladenine (1 mg/liter), naphthaleneacetic acid, (0.1 mg/liter), and thiamine (1 mg/liter) (38). Antibiotics spectinomycin dihydrochloride and streptomycin sulfate were filter-sterilized and added after autoclaving. The seeds were sterilized by exposure to the vapor of 100 ml of 6% sodium hypochlorite (Clorox bleach) and 3 ml of concentrated HCl for 8 h. Seeds were counted with AlphaImager 2000 (Alpha Innotech, San Leandro, CA). General protocols for plant tissue culture have been described elsewhere (17, 39).
DNA Gel Blot Analysis.
The protocols for DNA isolation, digestion, and probing have been described (17, 39). The plastid DNA probes are listed in Table 4. Mitochondrial DNA was probed with a 6.9-kb PstI fragment containing the 3′-half of atp6 gene (40) generously provided by C. S. Levings, North Carolina State University, Raleigh, NC. For the complete N. tabacum mtDNA sequence, see GenBank accession no. BA00004 (41).
Table 4.
DNA probes to test N. tabacum and N. undulata (CMS92) ptDNA polymorphisms
| Region | Probe | Fragment, ptDNA | DNA source | Ref. |
|---|---|---|---|---|
| atpI-rpoC1 | SacI; 7.2 kb | SacI; 7.2 kb; 15662–22868 | pGS98 | 42 |
| psbC-psaA | PstI-SacII; 5.1 kb | PstI-SacII; 5.1 kb; 36830–42000 | pRB10 | 43 |
| trnV (trnA-Val(UAC)-atpB | EcoRI; 3.5 kb | Sal6; 49841–65310 | pBR322::Sal6 | 26 |
| psbJ-psbE | SalI-SpeI; 2.5 kb | SalI-SpeI; 65310–67693 | pRB1 | 44 |
| rps8-rps3 | SalI; 2.9 kb | SalI; 2.88 kb; 82754–85634 | pBR322::Sal10 | 26 |
| 3′-rps12-rrn16 | BamHI-ApaI; 2.1 kb | BglII-EcoRI; 101145–104081 | pPRV1 | 45 |
| psaC-ndhA | PCR fragment; 3.2 kb | Primers in ptDNA at: 119134–122304* | Tobacco leaf DNA | This paper |
*Primers: 5′-CTCGAACGTATCAATAAGCTAGAC-3′; 5′-CGTATGAGATGAAAATCTCACGTAC-3′.
Acknowledgments
We thank Arun K. Azhagiri and Kerry Ann Lutz for discussions. This research was supported by Award 2004-39454-15192 of the U.S. Department of Agriculture Biotechnology Risk Assessment Research Grant Program.
Abbreviations
- CMS
cytoplasmic male sterile
- mtDNA
mitochondrial genome
- ptDNA
plastid genome
- RM medium
revised medium for plant maintenance in sterile culture
- RMOP medium
revised medium for organogenesis (shoot regeneration) of Nicotiana plumbaginifolia
- RFLP
restriction fragment length polymorphism.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 6879.
References
- 1.Reboud X, Zeyl C. Heredity. 1994;72:132–140. [Google Scholar]
- 2.Mogensen HL. Am J Bot. 1996;83:383–404. [Google Scholar]
- 3.Hagemann R. Prog Bot. 2002;63:5–51. [Google Scholar]
- 4.Nishiyama R, Ito M, Yamaguchi Y, Koizumi N, Sano H. Proc Natl Acad Sci USA. 2002;99:5925–5930. doi: 10.1073/pnas.082120199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boynton JE, Gillham NW, Harris EH, Hosler JP, Johnson AM, Jones AR, Randolph-Anderson BL, Robertson D, Klein TM, Shark KB, Sanford JC. Science. 1988;240:1534–1538. doi: 10.1126/science.2897716. [DOI] [PubMed] [Google Scholar]
- 6.Svab Z, Hajdukiewicz P, Maliga P. Proc Natl Acad Sci USA. 1990;87:8526–8530. doi: 10.1073/pnas.87.21.8526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maliga P. Trends Biotech. 1993;11:101–107. [Google Scholar]
- 8.Daniell H, Datta R, Varma S, Gray S, Lee SB. Nat Biotechnol. 1998;16:345–348. doi: 10.1038/nbt0498-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bilang R, Potrykus I. Nat Biotechnol. 1998;16:333–334. doi: 10.1038/nbt0498-333. [DOI] [PubMed] [Google Scholar]
- 10.Medgyesy P, Pay A, Marton L. Mol Gen Genet. 1986;204:195–198. [Google Scholar]
- 11.Horlow C, Goujaud J, Lepingle A, Missonier C, Bourgin JP. Plant Cell Rep. 1990;9:249–252. doi: 10.1007/BF00232294. [DOI] [PubMed] [Google Scholar]
- 12.Avni A, Edelman M. Mol Gen Genet. 1991;225:273–277. doi: 10.1007/BF00269859. [DOI] [PubMed] [Google Scholar]
- 13.Cornu A, Dulieu H. J Hered. 1988;79:40–44. [Google Scholar]
- 14.Drepas A, Dulieu H. J Hered. 1992;83:6–10. [Google Scholar]
- 15.Wang T, Li Y, Shi Y, Reboud X, Darmency H, Gressel J. Theor Appl Genet. 2004;108:315–320. doi: 10.1007/s00122-003-1424-8. [DOI] [PubMed] [Google Scholar]
- 16.Galun E, Arze-Gonen P, Fluhr R, Edelman M, Aviv D. Mol Gen Genet. 1982;186:50–56. doi: 10.1007/BF00422911. [DOI] [PubMed] [Google Scholar]
- 17.Svab Z, Maliga P. Proc Natl Acad Sci USA. 1993;90:913–917. doi: 10.1073/pnas.90.3.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fromm H, Edelman M, Aviv D, Galun E. EMBO J. 1987;6:3233–3237. doi: 10.1002/j.1460-2075.1987.tb02640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Svab Z, Maliga P. Mol Gen Genet. 1991;228:316–319. doi: 10.1007/BF00282483. [DOI] [PubMed] [Google Scholar]
- 20.Huang CY, Ayliffe MA, Timmis JN. Nature. 2003;422:72–76. doi: 10.1038/nature01435. [DOI] [PubMed] [Google Scholar]
- 21.Stegemann S, Hartmann S, Ruf S, Bock R. Proc Natl Acad Sci USA. 2003;100:8828–8833. doi: 10.1073/pnas.1430924100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shinozaki K, Ohme M, Tanaka M, Wakasugi T, Hayashida N, Matsubayashi T, Zaita N, Chunwongse J, Obokata J, Yamaguchi-Shinozaki K, et al. EMBO J. 1986;5:2043–2049. doi: 10.1002/j.1460-2075.1986.tb04464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu HS, Russel SD. Planta. 1994;193:115–122. [Google Scholar]
- 24.Yu HS, Russell SD. Plant Cell. 1994;6:1477–1484. doi: 10.1105/tpc.6.10.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Derepas A, Dulieu H. J Hered. 1992;83:6–10. [Google Scholar]
- 26.Medgyesy P, Fejes E, Maliga P. Proc Natl Acad Sci USA. 1985;82:6960–6964. doi: 10.1073/pnas.82.20.6960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hanson MR, Bentolila S. Plant Cell. 2004;16(Suppl):S154–SI69. doi: 10.1105/tpc.015966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sheahan MB, McCurdy DW, Rose RJ. Plant J. 2005;44:744–755. doi: 10.1111/j.1365-313X.2005.02561.x. [DOI] [PubMed] [Google Scholar]
- 29.Boeshore ML, LIfshitz I, Hanson MR, Izhar S. Mol Gen Genet. 1983;190:459–467. [Google Scholar]
- 30.Bendich AJ. BioEssays. 1987;6:279–282. doi: 10.1002/bies.950060608. [DOI] [PubMed] [Google Scholar]
- 31.Ellstrand NC, Prentice HC, Hancock JF. Annu Rev Ecol Syst. 1999;30:539–563. [Google Scholar]
- 32.Riegel MA, Lamond M, Preston C, Powles SB, Roush RT. Science. 2002;296:2386–2388. doi: 10.1126/science.1071682. [DOI] [PubMed] [Google Scholar]
- 33.Carrer H, Staub JM, Maliga P. Plant Mol Biol. 1990;17:301–303. doi: 10.1007/BF00039510. [DOI] [PubMed] [Google Scholar]
- 34.Lutz KA, Bosacchi MH, Maliga P. Plant J. 2006;45:447–456. doi: 10.1111/j.1365-313X.2005.02608.x. [DOI] [PubMed] [Google Scholar]
- 35.Khan MS, Maliga P. Nat Biotechnol. 1999;17:910–915. doi: 10.1038/12907. [DOI] [PubMed] [Google Scholar]
- 36.Lutz KA, Knapp JE, Maliga P. Plant Physiol. 2001;125:1585–1590. doi: 10.1104/pp.125.4.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murashige T, Skoog F. Physiol Plant. 1962;15:473–497. [Google Scholar]
- 38.Sidorov V, Menczel L, Maliga P. Nature. 1981;294:87–88. [Google Scholar]
- 39.Lutz KA, Svab Z, Maliga P. Nat Protoc. 2006;1:900–910. doi: 10.1038/nprot.2006.118. [DOI] [PubMed] [Google Scholar]
- 40.Bland MM, Levings CS, III, Matzinger DF. Curr Genet. 1987;12:475–481. doi: 10.1007/BF00419555. [DOI] [PubMed] [Google Scholar]
- 41.Sugiyama Y, Watase Y, Nagase M, Makita N, Yagura S, Hirai A, Sugiura M. Mol Genet Genomics. 2005;272:603–615. doi: 10.1007/s00438-004-1075-8. [DOI] [PubMed] [Google Scholar]
- 42.Serino G, Maliga P. Plant Physiol. 1998;117:1165–1170. doi: 10.1104/pp.117.4.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bock R, Maliga P. Mol Gen Genet. 1995;247:439–443. doi: 10.1007/BF00293145. [DOI] [PubMed] [Google Scholar]
- 44.Bock R, Kössel H, Maliga P. EMBO J. 1994;13:4623–4628. doi: 10.1002/j.1460-2075.1994.tb06784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zoubenko OV, Allison LA, Svab Z, Maliga P. Nucleic Acids Res. 1994;22:3819–3824. doi: 10.1093/nar/22.19.3819. [DOI] [PMC free article] [PubMed] [Google Scholar]